Abstract
Purpose
This study aimed to analyze technical and clinical factors related to oncological outcomes in patients with localized prostate cancer (PC) who were treated with whole-gland high-intensity focused ultrasound (HIFU).
Materials and Methods
From 2007–2014, patients diagnosed with localized PC who underwent whole-gland HIFU were consecutively included retrospectively. Biochemical failure was defined according to the Phoenix ASTRO guidelines. The relationship between oncological outcomes and technical and clinical factors was evaluated.
Results
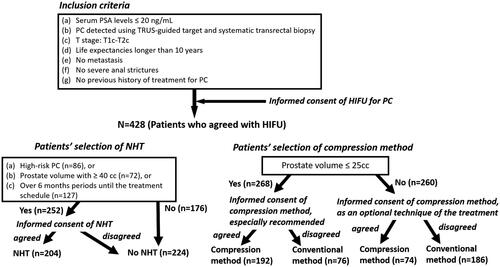
The study cohort included 428 patients. The median age was 67 years, and the median prostate-specific antigen level was 7.61 ng/mL. Patient risk classifications were low (n = 102), intermediate (n = 240), and high (n = 86). Biochemical disease-free survival rates of patients with HIFU for localized PC in the total, low-, intermediate-, and high-risk groups according to D’Amico risk groups over a median follow-up period of 5 years (range 9-144) were 68.4%, 80.4%, 65.6%, and 61.6%, respectively. In multivariate logistic regression analyses to predict biochemical failure of the treatment, neoadjuvant hormonal therapy (NHT) in the high-risk group (OR 0.225, p = 0.015), and compression method in the low- (OR 0.178, p = 0.030), intermediate- (OR0.291, p < 0.0001), and high-risk (OR 0.316, p = 0.049) groups were significant factors that reduced the risk of biochemical failure after treatment. There were no significant differences in complications between patients treated with compression and those treated conventionally.
Conclusions
NHT may potentially improve oncological outcomes for patients in the high-risk group, and compression methods can improve the oncological outcomes of whole-gland therapy with HIFU.
Introduction
High-intensity focused ultrasound (HIFU) is an extracorporeal ablative technology that delivers ultrasonic energy generated by a spherical transducer to pinpoint foci only millimeters wide, and only minor temperature changes are observed outside of the focal zone [Citation1]. In the analysis of long-term follow-up in HIFU for localized prostate cancer (PC), the biochemical disease-free survival (BDFS) in low-, intermediate-, and high-risk groups according to D’Amico risk classification [Citation2] with the Phoenix American Society for Therapeutic Radiology and Oncology (ASTRO) definition [Citation3] were reported as 95.0%, 80.9%, and 71.9% for 6 years [Citation4]; and 76%, 63%, and 57% for 8 years [Citation5], respectively. Further, the development of the HIFU device contributed to improving the oncological outcomes from 48.3% (1st generation Sonablate®) to 82.0% (3rd generation of the Sonablate®) in 6-year BDFS [Citation4].
However, in a systematic review and meta-analysis of current clinical evidence, the risk classification was associated with the BDFS in whole-gland therapy with HIFU [Citation6], and neoadjuvant hormonal therapy (NHT) benefited the patients in the high-risk group [Citation6]. The role of NHT related to HIFU has been unclear during long-term follow-up, and there are no preclinical data regarding this. In immunohistochemical analysis, NHT decreased the numerical density of microvessels in PC [Citation7]. Because the thermal effect of HIFU is degraded by blood flow [Citation8], the decreased effect on PC microvessels potentially contributes to the oncological outcomes of HIFU for PC. A previous study reported that NHT with HIFU treatment exhibits significant clinical benefits to intermediate- and high-risk patients [Citation9]. In terms of the technical aspects of HIFU, compared with conventional methods, intra-operative transrectal compression of the prostate inhibits prostatic swelling and intra-prostatic targeted point shift, and it leads to an increased volume of blood flow disappearance (89 vs. 96%; p = 0.001) after HIFU and improved biochemical disease-free survival (BDFS) in the medium-term follow-up (92.6 vs. 76.5%; p = 0.038) [Citation10]. Because prostate swelling negatively correlates with preoperative prostate volume (r=−0.484, p = 0.001), suggesting that a smaller prostate likely has more swelling [Citation11]; accordingly, the compression method may contribute to more efficacious treatment, especially in a smaller prostate. These results indicate that intraoperative compression has the potential to achieve precise whole-gland and lesion-targeted focal therapies.
In the present study, clinical and technical factors related to oncological outcomes were analyzed in a retrospective consecutive patient cohort with localized PC who were treated with whole-gland HIFU and followed up for 12 years. Although the present study is a retrospective cohort study, the results are expected to contribute to the understanding of the factors that affect the oncological outcomes of HIFU for localized PC, because there have been few reports of oncological outcomes over 10 years after HIFU.
Patients and methods
Patients
This study included patients who underwent whole gland HIFU as primary therapy for prostate cancer between 2007 and 2014 and who had (a) serum prostate-specific antigen (PSA) levels ≤20 ng/mL, (b) PC detected using transrectal ultrasound (TRUS)-guided target and systematic transrectal biopsy, (c) T stage: T1c-T2c, (d) life expectancy longer than 10 years, (e) no metastasis, (f) no severe anal strictures, and (g) no previous history of treatment for PC. These patients were informed of the procedure as an optional treatment. In addition to the standard treatment, those who underwent this procedure provided informed consent. NHT was recommended for patients who were diagnosed as being at high-risk PC, had a prostate volume of ≥40 cc, or had over 6 months until the treatment schedule. NHT was performed for patients who agreed to the use of NHT after informing them of the possibility of adverse effects of NHT and obtaining informed consent, for 6 to 12 months before HIFU (). The institutional review board approved this study (13 R-320).
Figure 1. Patient selection flow diagram / Patients who had (a) serum prostate-specific antigen (PSA) levels ≤ 20 ng/mL, (b) prostate cancer (PC) detected using transrectal ultrasound (TRUS)-guided target and systematic transrectal biopsy, (c) T stage: T1c-T2c, (d) life expectancy longer than 10 years, (e) no metastasis, (f) no severe anal strictures, and (g) no previous history of treatment for PC were informed of the procedure as an optional treatment. In addition to the standard treatment, those who underwent high-intensity focused ultrasound (HIFU) provided informed consent. Neo-adjuvant therapy (NHT) was recommended for patients who were diagnosed with high-risk PC or had prostate volume of ≥ 40 cc, or had over 6 months until the treatment schedule. NHT was performed for the patients who agreed to the use of NHT after they provided informed consent, which provided information regarding the possibility of adverse effects of NHT for 6 to 12 months before HIFU. Compression methods were recommended for patients with prostate volume ≤ 25 cc because of the ease of prostatic swelling during the treatment, and informed consent, which provided information regarding the possibility of rectal injury, was performed. For patients with prostate volume > 25 cc, the compression method was informed as an optional treatment technique. The compression method was performed for patients who agreed to undergo the novel treatment technique.
HIFU procedure
HIFU was performed by two surgeons (TU and SS) who had performed more than 100 procedures. In the treatment with HIFU, the third-generation Sonablate 500® TCM (SonaCare Medical, Indianapolis, IN, USA) was used, and details of the conventional procedure are mentioned in a previous report [Citation4]. For intra-operative compression of the prostate, the balloon was expanded by adding 80 to 280 ml of degassed water, and the distance from the rectal surface to the ultrasound transducer were set parallel at a distance of 20–30 mm depending on the prostate volume. During treatment, the degassed water was manually adjusted to the starting position by the physician. In the Sonablate 500® TCM, an automatic rectal wall position monitoring system was introduced that provides a real-time automatic alert of the distance from the probe to the rectal wall, preventing damage to the rectal wall and recto-urethral fistula [Citation12]. The distance from the rectal to ultrasound transducer was defined as the upper limit within 20 mm of the 4-cm transducer and 23 mm of the 3-cm transducer. Since 2007, we switched off the limitation of the automatic rectal wall position monitoring system for the intraoperative compression of the prostate under a research agreement with scientific officers and software engineers of SonaCare Medical. During the procedure, the reflectivity index system alerts physicians to a change in the TRUS image of the rectal wall during the treatment, allowing damage to the rectal wall to be observed [Citation12]. The compression method was recommended for patients with prostate volume ≤25 cc due to the ease of prostatic swelling during the treatment; after informing patients regarding the possibility of rectal injury, informed consent was obtained, and the procedure was performed. For patients with prostate volume > 25 cc, the compression method was informed as an optional treatment technique. The compression method was performed for patients who agreed with the novel treatment technique ().
Image analysis
At 14 days to 28 days after the treatment, contrast-enhanced MRI was performed using a 1.5-Tesla magnet (Signa HD; GE Healthcare, Little Chalfont, UK) with an eight-channel cardiac coil for the evaluation of blood flow in the prostate. The details of postoperative contrast-enhanced magnetic resonance imaging (MRI) were detailed in previous literature [Citation10]. The blood flow disappearance was evaluated on the final dynamic post-contrast image, and the volume of blood flow disappearance was measured using a three-dimensional (3 D) manually reconstructed, segmented MRI by two experienced radiologists using ZIO Station® (AMIN, Tokyo, Japan).
Oncological outcomes
Serum PSA levels were measured every 3 months during follow-up. Biochemical failure was defined according to the Phoenix ASTRO definition (PSA nadir + 2 ng/mL) [Citation3].
Complications and erectile function
Complications were evaluated every 3 months after treatment using the Common Terminology Criteria for Adverse Events v5.0 (CTCAE). Erectile function was analyzed using the International Index of Erectile Function-5 (IIEF-5) for 12 months after treatment in patients who manifested erectile function before treatment.
Statistical analysis
All statistical analyses were performed using IBM SPSS® Statistics version 26 (IBM, Armonk, NY, USA). The differences in patient characteristics, volume of blood flow disappearance, complications, and IIEF-5 were analyzed using the Mann–Whitney U test for quantitative data and using the chi-squared test for categorical variables such as T stage, Gleason score, and D’Amico risk classification. Survival curves were based on the Kaplan-Meier method, and the log-rank test was used for univariate comparisons. Univariate and multivariate analyses using logistic regression identified significant risk factors for biochemical failure. Statistical significance was set at p < 0.05.
Results
Our study included a total of 428 patients. details the patient characteristics. The median age was 67 years (range: 45–85 years), and the median PSA level was 7.61 ng/mL (range: 1.36–19.20 ng/mL). Clinical stages were T1c, n = 285; T2a, n = 61; T2b, n = 36; and T2c, n = 46; Gleason scores were 3 + 3: n = 158, 3 + 4: n = 142, 4 + 3: n = 73; and 4 + 4: n = 38; and risk classifications were low: n = 102, intermediate: n = 240, and high: n = 86. The median follow-up period was 5-years (9–144 months). Perioperative data are presented in . The overall and cancer-specific survival rates were 98.9 and 99.5%, respectively. The BDFS of the patients in the total, low-, intermediate-, and high-risk groups according to the D’Amico risk groups were 68.4%, 80.4%, 65.6%, and 61.6%, respectively (). shows the characteristics of patients who were treated with and without neo-adjuvant hormonal therapy. Among the patients who were treated with and without ne-adjuvant hormonal therapy, age and serum PSA value were significantly higher and prostate volume was significantly lower in those treated with neo-adjuvant therapy than in those without neo-adjuvant therapy; however, there was no significant difference in Gleason score, number of patients in the risk groups, and compression method. shows the characteristics of patients who were treated with and without the compression method. Among the patients who were treated with and without compression methods, prostate volume, number of patients in the Gleason score groups and risk groups, and follow-up periods were significantly different; however, there was no significant difference in age, serum PSA value, T stage, and number of patients with NHT. Because of the different numbers in the groups, BDFS was evaluated using multivariate logistic regression analyses to predict the biochemical failure of treatment by the D’Amico risk groups in patients treated with and without NHT and compression methods. BDFS in patients with and without NHT showed no significant difference in total (74.9 and 58.3%, p = 0.194) and in low- (84.5 and 76.1%, p = 0.446) and intermediate-risk groups (71.6 and 55.1%, p = 0.469), but not in the high-risk group (79.0 and 43.7%, p = 0.027) (). In patients diagnosed with biochemical failure and no failure, there was a significant difference in the rate of compression method (37.8 vs. 69.4%, p < 0.0001) (). A comparison of patients who were diagnosed with biochemical failure and no failure after treatment according to D’Amico risk groups, there was a significant difference in the rate of NHT in the high-risk group (33.3 vs. 63.2%, p = 0.024) and compression methods in the low- (43.8 vs. 81.4%, p = 0.001), intermediate- (35.9 vs. 65.3%, p < 0.0001), and high-risk groups (38.9 vs. 64.7%, p = 0.049) (). In multivariate logistic regression analyses to predict the biochemical failure of the D’Amico risk groups, NHT in the high-risk group (OR 0.225: 95% CI, 0.068-0.750, p = 0.015) and compression method in the low- (OR 0.178: 95% CI, 0.058-0.549, p = 0.030), intermediate- (OR0.291, 95% CI, 0.159-0.532, p < 0.0001), and high-risk (OR 0.316: 95% CI, 0.100-0.996, p = 0.049) groups were significant factors that reduced the risk of biochemical failure after treatment (). BDFS in patients with and without compression was significantly different in total (78.6 and 51.3%, p < 0.0001) and in the low- (88.2 and 59.3%, p = 0.002), intermediate- (77.2 and 49.5%, p < 0.0001), and high-risk groups (67.1 and 48.9%, p = 0.006) (). Twelve-year BDFS in patients without NHT and with or without compression was significantly different in total (64.9 and 49.9%, p < 0.0001) and in low- (93.2 and 62.3%, p = 0.027), intermediate- (59.5 and 53.7%, p = 0.013), and high-risk groups (50.1 and 30.0%, p = 0.047) (). Twelve-year BDFS in the patients treated with NHT with and without compression methods showed a significant difference in total (87.0 and 52.5%, p < 0.0001) and in low- (85.2 and 58.3%, p = 0.051), and intermediate-risk groups (86.7 and 43.9%, p < 0.0001), but not in the high-risk group (84.1 and 72.6%, p = 0.056) (). Rates of the volume of disappearance of blood flow in each patient were significantly higher in those diagnosed as having non-recurrence than in the low- (89 vs. 74%, p < 0.0001), intermediate- (88 vs. 68%, p < 0.0001), and high-risk groups (90 vs. 64%, p < 0.0001) (). Furthermore, rates of the volume of disappearance of blood flow were significantly higher in patients treated with the compression method than in those treated with the conventional method in patients with recurrence and non-recurrence in low- (p < 0.0001 and p = 0.001), intermediate- (p < 0.0001 and p < 0.0001), and high-risk (p < 0.0001 and p = 0.003) groups ().
Figure 2. Biochemical disease-free survival of the patients who were treated with high-intensity focused ultrasound over a median follow-up period of 5 years / The biochemical disease-free survival of the patients who were treated with high-intensity focused ultrasound for localized prostate cancer in the total, low-, intermediate-, and high-risk groups according to D’Amico risk groups were 68.4, 80.4, 65.6 and 61.6%, respectively, over a median follow-up period of 5 years.
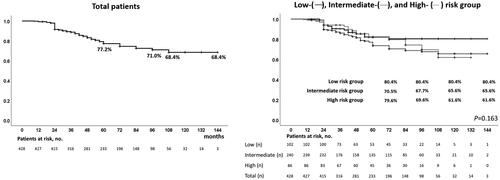
Figure 3. Biochemical disease-free survival of patients treated with high-intensity focused ultrasound with and without neo-adjuvant hormonal therapy over a median follow-up period of 5 years / In patients treated with high-intensity focused ultrasound for localized prostate cancer, disease-free survival in patients with and without neoadjuvant hormonal therapy showed no significant difference in total (74.9 and 58.3%, p = 0.194), low- (84.5 and 76.1%, p = 0.446), and intermediate-risk groups (71.6 and 55.1%, p = 0.469), but there was a significant difference in the high-risk group (79.0 and 43.7%, p = 0.027) over a median follow-up period of 5 years.
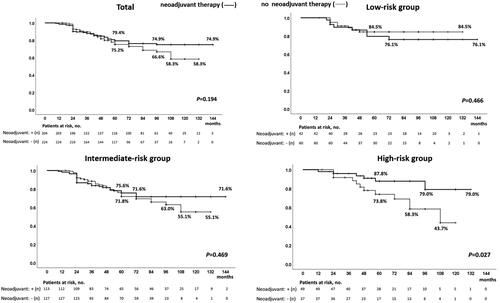
Figure 4. Biochemical disease-free survival of patients treated with high-intensity focused ultrasound with and without the compression method over a median follow-up period of 5 years / In patients treated with high-intensity focused ultrasound for localized prostate cancer, disease-free survival rates in patients treated with and without compression were significantly different in total (78.6 and 51.3%, p < 0.0001) and in low- (88.2 and 59.3%, p = 0.002), intermediate- (77.2 and 49.5%, p < 0.0001), and high-risk groups (67.1 and 48.9%, p = 0.006) over a median follow-up period of 5 years.
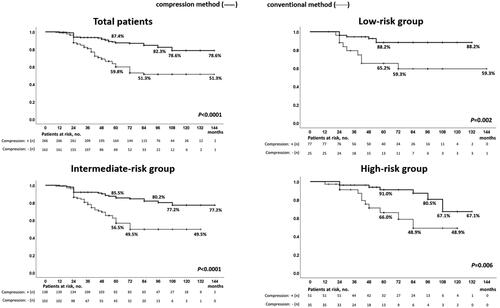
Figure 5. Biochemical disease-free survival of patients treated with high-intensity focused ultrasound without neo-adjuvant hormonal therapy with and without the compression method over a median follow-up period of 5 years / In patients treated with high-intensity focused ultrasound for localized prostate cancer, biochemical disease-free survival in patients without neo-adjuvant hormonal therapy with and without compression methods were significantly different in total (64.9 and 49.9%, p < 0.0001) and in low- (93.2 and 62.3%, p = 0.027), intermediate- (59.5 and 53.7%, p = 0.013), and high-risk groups (50.1 and 30.0%, p = 0.047) over a median follow-up period of 5 years.
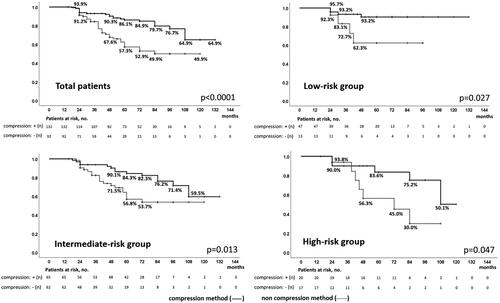
Figure 6. Biochemical disease-free survival of patients treated with high-intensity focused ultrasound with neo-adjuvant hormonal therapy with and without the compression method over a median follow-up period of 5 years / In patients treated with high-intensity focused ultrasound for localized prostate cancer, biochemical disease-free survival in patients who underwent neoadjuvant hormonal therapy with and without compression was significantly different in total (87.0 and 52.5%, p < 0.0001) and in low- (85.2 and 58.3%, p = 0.051), and intermediate-risk groups (86.7 and 43.9%, p < 0.0001), but not in the high-risk group (84.1 and 72.6%, p = 0.056) over a median follow-up period of 5 years.
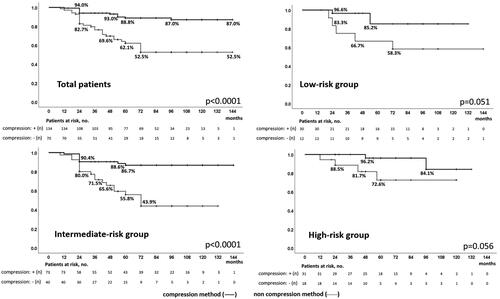
Figure 7. Rates of three-dimensional measured volume of disappearance of blood flow in each prostate gland / The rates of three-dimensional measured volume of disappearance of blood flow were significantly higher in patients diagnosed with non-recurrence than in the low- (89 vs. 74%, p < 0.0001), intermediate- (88 vs. 68%, p < 0.0001), and high-risk groups (90 vs. 64%, p < 0.0001). Furthermore, the rates of the three-dimensional measured volume of disappearance of blood flow were significantly higher in patients treated with the compression method than with the conventional method in patients with recurrence and non-recurrence in the low- (p < 0.0001 and p = 0.001), intermediate- (p < 0.0001 and p < 0.0001), and high- (p < 0.0001 and p = 0.003) risk groups.
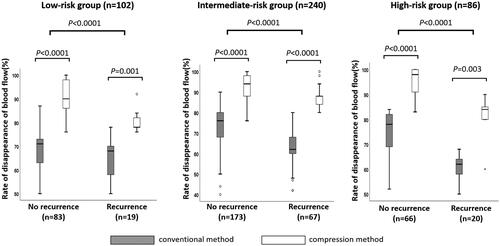
Table 1. Patient characteristics.
Table 2. Perioperative data.
Table 3. Comparison of characteristics of patients treated with and without neo-adjuvant hormonal therapy.
Table 4. Comparison of characteristics of patients treated with and without compression method.
Table 5. Comparison of characteristics of patients diagnosed as having biochemical failure and no failure after the treatment.
Table 6. Comparison of characteristics of patients diagnosed as having biochemical failure and no failure after the treatment according to D’Amico risk groups.
Table 7. Univariate and multivariate logistic regression analyzes to predict biochemical failure after the treatment according to D’Amico risk groups.
lists the complications. There were no significant differences in urethral stricture (grade 2: p = 0.930, grade 3: p = 0.974), epididymitis (p = 0.522), incontinence (p = 0.635), bladder neck contraction (p = 0.582), rectourethral fistula (p = 0.728), and erectile function (p = 0.466) between the patients who were treated with compression and those treated with the conventional method. Regarding longitudinal changes in the IIEF-5 score in all patients (n = 428) () and the patients who had erectile function before treatment over 12 months after treatment (n = 179), there were no significant differences in IIEF-5 scores at pretreatment and at 3, 6, and 12 months after treatment between the patients who were treated with compression and those treated with the conventional method ().
Table 8. Complications in patients treated with the compression and conventional methods.
Table 9. IIEF-5 score in the all patients over 12 months after the treatment (n = 428).
Table 10. IIEF-5 score in patients with erectile function before the treatment over 12 months after the treatment (n = 179).
Discussion
In a previous report, intra-operative compression of the prostate suppressed prostate swelling during HIFU, which has the potential to contribute to the accurate treatment [Citation10]. By means of prostate compression, prostatic pressure inside the fibrous prostatic capsule can be increased [Citation13]. Because the major reason for prostatic swelling is rapidly induced diffuse stromal edema caused by HIFU in the pathological analysis [Citation14], compression of the prostate would suppress the edema due to the highly increased intra-prostatic pressure. In previous reports, intraoperative increases in prostate volume (21 vs. 5.3%; p = 0.044), intra-prostatic targeted point shift (4 mm vs. 2 mm, p = 0.040 in the transition zone; 3 mm vs. 0 mm; p = 0.001 in the peripheral zone), and the volume occupied by the non-enhanced area (89 vs. 96%; p = 0.001) were significantly suppressed in the compression method compared with the conventional method, and the BDFS in patients treated with the compression method was significantly higher compared with those treated with the conventional method (92.6 vs. 76.5%; p = 0.038) [Citation10]. In the present study, the rates of blood flow disappearance in each patient were significantly higher in the patients who were diagnosed as having non-recurrence than those in the low-, intermediate-, and high-risk groups. Furthermore, there was a significant difference in the BDFS in the total and the low-, intermediate-, and high-risk groups in patients with and without compression methods. In multivariate logistic regression analyses to predict biochemical failure, compression methods reduced the risk of biochemical failure after treatment in the low-, intermediate-, and high-risk groups. Although the present study was retrospective, these results indicate that the compression method has the potential to improve treatment efficacy of whole-gland therapy with HIFU. Furthermore, BDFS in patients undergoing NHT using the compression method and without NHT using the conventional method were 67.1 and 30.0% in the high-risk group, respectively. Therefore, the combination of NHT and compression methods has the potential to improve oncological outcomes in patients in the high-risk group. After focal therapy, there was a difference in the detection of csPC from 0% to 26.5% in follow-up biopsy from the treated area in recent studies [Citation15–22]; this could be due to influences and differences in treatment techniques during the studies. The compression method has the potential to improve precise lesion-target focal therapy with HIFU because the intra-prostatic target point shift is suppressed.
In a prior systematic review and meta-analysis of the current clinical evidence, neoadjuvant hormonal therapy (NHT) benefited patients in the high-risk group [Citation6]. NHT has been reported to reduce the volume of the prostate and PC and be useful in treating the micro-metastasis in PC [Citation23]. Several studies reported the benefit of long-term hormonal therapy in oncological outcomes for the radiotherapy and surgery in patients with high-risk features [Citation24,Citation25]. In a retrospective analysis of the effect of NHT followed by robotic-assisted radical prostatectomy for intermediate- and high-risk PC, biochemical recurrence was lower in patients who underwent NHT than in those without NHT during a median follow-up of 44.5 months (87.5 vs. 54.17%, p = 0.0243), and the hazard ratio of BDFS was 0.404 (95% CI: 0.203-0.803, p = 0.0047) in patients who underwent NHT [Citation25]. Based on NHT duration, BDFS was significantly large in patients who underwent NHT for 4–12 months than for 2–3 months [Citation25]. Immunohistochemical analysis showed a significant decrease in the numerical density of microvessels of PC and non-tumor tissue in the patients who received NHT compared with that of patients without NHT [Citation7]. HIFU cause destruction within prostate tissue via thermal and mechanical effects [Citation1, Citation26]. The thermal effect is degraded by regional prostatic blood flow [Citation8]. A vascular reduction effect of NHT might decrease the blood flow and improve the thermal effects for PC. In the present study, 12-year BDFS in patients with and without NHT was significantly different in the high-risk group. In multivariate logistic regression analysis, NHT was a significant factor that reduced the risk of biochemical failure in the high-risk group. Based on previous reports and the present results, the role of NHT in the reduction of blood flow in PC and treatment of micro-metastasis would have the potential to contribute to the oncological outcomes for patients in the high-risk group.
Complications were evaluated based on the difference between compression and conventional methods. There were no significant differences in urethral stricture, epididymitis, incontinence, bladder neck contraction, rectourethral fistula, or erectile function between the two methods. The risk of temporal transrectal compression is considered to be harmless for patients, because fecal urgency occurs when rectal capacity is at a median of 550 ml in a rectal retention test involving an enema [Citation27]. However, the setting of the target area should be undertaken carefully to prevent rectal ablation in the compression method, because the target area is close to the rectal wall due to the thickness of the rectal wall and peri-rectal fat between the prostate and rectum. In longitudinal changes of the IIEF-5 in the patients, there were no significant differences in IIEF-5 scores at 12 months after treatment between the patients who were treated with compression and those treated with the conventional method. These results show that there was no significant difference in the effect of compression and conventional methods on erectile function.
This study had some limitations. First, the present study was a single-institutional retrospective consecutive case series analysis, and a higher percentage of patients in the high-risk group received NHT than in the other groups according to the study design. A multi-institutional randomized prospective study will contribute to the analysis of the oncological effects of NHT and compression methods in whole-gland ablation with HIFU for localized PC. Second, the Phoenix ASTRO definition of PSA failure was based on the analysis of the PSA response after radiotherapy. However, oncological outcomes in patients with localized PC treated with HIFU were evaluated according to the definition in most previous studies, and we used the definition for the evaluation. Third, there was a difference in the duration of NHT in the present study. Therefore, the most effective duration of NHT for oncological outcomes after HIFU was unclear in the present study. A randomized prospective study is needed to evaluate the usefulness of the NHT in terms of duration.
Conclusions
In the present study, compression methods have the potential to improve the oncological outcomes of whole-gland therapy with HIFU, which has the potential to contribute to precise lesion-target focal therapy with HIFU, because this method suppresses intraoperative prostatic swelling and target shift during HIFU. NHT has the potential to contribute to oncological outcomes in patients in the high-risk group. A multi-institutional randomized prospective study will reveal the oncological effects of NHT and compression methods in whole-gland ablation with HIFU for localized PC.
Acknowledgements
The authors thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- Madersbacher S, Pedevilla M, Vingers L, et al. Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res. 1995;55(15):3346–3351.
- D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974.
- Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974.
- Uchida T, Tomonaga T, Kim H, et al. Improved outcomes with advancements in high intensity focused ultrasound devices for the treatment of localized prostate cancer. J Urol. 2015;193(1):103–110.
- Crouzet S, Chapelon JY, Rouviere O, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol. 2014;65(5):907–914.
- He Y, Tan P, He M, et al. The primary treatment of prostate cancer with high-intensity focused ultrasound: A systematic review and meta-analysis. Medicine. 2020;99(41):e22610:
- Bakarev MA, Levin VP, Kachesov IV, et al. Status of the microcirculatory network as a factor of prognosis and evaluation of therapeutic efficiency in prostate cancer treated by high-intensity focused ultrasound in combination with androgen deprivation. Bull Exp Biol Med. 2018;165(5):682–687.
- Wiart M, Curiel L, Gelet A, et al. Influence of perfusion on high-intensity focused ultrasound prostate ablation: a first-pass MRI study. Magn Reson Med. 2007;58(1):119–127.
- Sumitomo M, Hayashi M, Watanabe T, et al. Efficacy of short-term androgen deprivation with high-intensity focused ultrasound in the treatment of prostate cancer in Japan. Urology. 2008;72(6):1335–1340.
- Shoji S, Hashimoto A, Nakamoto M, et al. Morphological analysis of the effects of intraoperative transrectal compression of the prostate during high-intensity focused ultrasound for localized prostate cancer. Int J Urol. 2015;22(6):563–571.
- Shoji S, Uchida T, Nakamoto M, et al. Prostate swelling and shift during high intensity focused ultrasound: implication for targeted focal therapy. J Urol. 2013;190(4):1224–1232.
- Shoji SM, Scionti S. High intensity focused ultrasound (HIFU) treatment of prostate cancer. In: Truls Bjerklund Johansen, David J. Breen, Damian Greene, Vladimir Mouraviev, editors. Handbook of focal therapy for prostate and renal cancer. UK: JP Medical LTD; 2016, p. 241–254.
- Walz J, Burnett AL, Costello AJ, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57(2):179–192.
- Shoji S, Tonooka A, Hashimoto A, et al. Time-dependent change of blood flow in the prostate treated with high-intensity focused ultrasound. Int J Urol. 2014;21(9):942–945.
- Ahmed HU, Dickinson L, Charman S, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol. 2015;68(6):927–936.
- Feijoo ER, Sivaraman A, Barret E, et al. Focal high-intensity focused ultrasound targeted hemiablation for unilateral prostate cancer: a prospective evaluation of oncologic and functional outcomes. Eur Urol. 2016;69(2):214–220.
- van Velthoven R, Aoun F, Marcelis Q, et al. A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(1):79–83.
- Rischmann P, Gelet A, Riche B, et al. Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur Urol. 2017;71(2):267–273.
- Guillaumier S, Peters M, Arya M, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74(4):422–429.
- Ganzer R, Hadaschik B, Pahernik S, et al. Prospective multicenter phase ii study on focal therapy (hemiablation) of the prostate with high intensity focused ultrasound. J Urol. 2018;199(4):983–989.
- Shoji S, Hiraiwa S, Uemura K, et al. Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: prospective analysis of oncological and functional outcomes. Int J Clin Oncol. 2020;25(10):1844–1853.
- Abreu AL, Peretsman S, Iwata A, et al. High Intensity Focused Ultrasound Hemigland Ablation for Prostate Cancer: Initial Outcomes of a United States Series. J Urol. 2020;204(4):741–747.
- Köllermann J, Feek U, Müller H, et al. Nondetected tumor (pT0) after prolonged, neoadjuvant treatment of localized prostatic carcinoma. Eur Urol. 2000;38(6):714–720.
- Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497–2504.
- Hu JC, Hung SC, Ou YC. Assessments of neoadjuvant hormone therapy followed by robotic-assisted radical prostatectomy for intermediate- and high-risk prostate cancer. Anticancer Res. 2017;37(6):3143–3150.
- Chapelon JY, Ribault M, Vernier F, et al. Treatment of localised prostate cancer with transrectal high intensity focused ultrasound. Eur J Ultrasound. 1999;9(1):31–38.
- Fox M, Thumshirn M, Fruhauf H, et al. Determinants of fecal continence in healthy, continent subjects: a comprehensive analysis by anal manometry, rectal barostat and a stool substitute retention test. Digestion. 2011;83(1-2):46–53.
