Abstract
Objective
To compare the clinical outcomes of microwave ablation (MWA) and radiofrequency ablation (RFA) in the treatment of primary hyperparathyroidism (pHPT).
Method
This retrospective study included 104 pHPT patients treated by MWA or RFA between January 2015 and March 2020 in four centers. The clinical outcomes including effectiveness and complications were compared between the two groups. Ablation cure was defined as the reestablishment of normal values of serum calcium and intact parathyroid hormone (iPTH) at least more than 6 months. Clinical cure was defined as the reestablishment of normal values of serum calcium and iPTH throughout the entire follow-up period.
Results
A total of 77 patients underwent MWA (mean age, 55.5 ± 16.4 years) and 27 underwent RFA (mean age, 58.9 ± 15.6 years). During the follow-up (median, 18.7 months in the MWA group; 12 months in the RFA group), no difference was observed between ablation cure rates (88.3% vs. 88.9%, p = 1.000), clinical cure rates (87.0% vs. 82.3%, p = .880), recurrent pHPT (5.2% vs. 3.7%, p = .447), persistent pHPT (11.7% vs. 11.1%, p = 1.000) and complication rate (9.1% vs. 3.7%, p = .677). A maximum diameter less than 0.7 cm was an independent prognostic factor of uncured pHPT in ablation (hazard ratio, 0.1; 95% confidence interval: 0.02, 0.54; p = .007). Major complication – voice change encountered in five patients (6.5%) in the MWA group and in one patient (3.7%) in the RFA group.
Conclusion
Both RFA and MWA are safe and effective techniques for patients with pHPT, with comparable clinical outcomes.
Introduction
Primary hyperparathyroidism (pHPT) is a group of common mineral metabolism disorders and ranks third in endocrine diseases [Citation1,Citation2]. The global yearly incidence of pHPT is estimated to be between 1 and 4 per 1000 people, increases with age and shows a sex bias, with studies reporting a female-to-male ratio of approximately 3–4:1 [Citation3].
The central target organs of abnormally increased parathyroid hormone (PTH) are bone and kidney, which can cause a series of clinical symptoms such as osteoporosis with related fracture, nephrolithiasis, reduced renal function, neurocognitive features and even hypercalcemia crisis [Citation3]. Although the most common clinical presentation of pHPT has changed from symptomatic to asymptomatic in recent years, nearly 20–30% of patients with asymptomatic pHPT are at risk for disease progression, which requires treatment [Citation4,Citation5].
Current treatment guidelines recommend surgical resection as the preferred treatment for patients with pHPT [Citation6]. In recent years, thermal ablation (i.e., microwave ablation (MWA), radiofrequency ablation (RFA), laser ablation (LA) and high intensity focused ultrasound (HIFU)) has been suggested as an alternative to surgery for pHPT in senile patients with comorbidities who are unfit for surgery or in young female patients who are anxious about the possibility of scar formation [Citation7–15]. Indeed, a previous study indicated that the cure rate was similar between surgical resection and MWA for pHPT [Citation11]. RFA is also one of the thermal ablation techniques that has been preliminarily applied for pHPT. Although it was only reported in a few patients, the short-term efficacy was promising. According to reported results, both MWA and RFA are encouraging modalities for pHPT [Citation9–13]. Nevertheless, data from a direct comparison of MWA and RFA for the treatment of pHPT are lacking. The aim of the present study was to evaluate the clinical outcomes of MWA vs. RFA in the treatment of pHPT.
Materials and methods
Study design and patients
This was a retrospective study. Between January 2015 and March 2020, data from all consecutive patients who were referred to four hospitals (China-Japan Friendship Hospital, Zhejiang Provincial People’s Hospital, Yantai Affiliated Hospital of Binzhou Medical College and the First Affiliated Hospital of Baotou Medical College) for the treatment of pHPT were reviewed. The number of patients received ablation in each center was 76, 26, 11 and 8, respectively. Only patients who underwent MWA or RFA for pHPT were included. Patients with recurrent pHPT, misdiagnosed secondary hyperparathyroidism (sHPT) or a history of surgery were excluded. The study protocol was approved by the Human Ethics Review Committee of participating hospitals (zryyec/2015/27-2). All patients provided written informed consent for treatment, and informed consent for participation in the current study was waived.
Imaging
Routine ultrasound (US) and contrast-enhanced ultrasound (CEUS) were performed in all patients by using a LOGIQ E9 scanner (GE Healthcare, Chicago, IL)/iU22 US scanner (Philips, Amsterdam, Netherlands) with a 6–15 MHz linear probe/5–12 MHz linear probe, and the US contrast agent was Sonovue (Bracco, Milan, Italy). All patients also underwent technetium 99-m-labeled sestamibi single-photon emission computed tomography (99mTc-sestamibi SPECT) for localization of parathyroid nodules prior to the ablation procedure.
The diagnosis of pHPT nodules on routine US was based on the following criteria: (1) enlarged hypoechoic parathyroid glands with clearly defined margins and (2) no suspicion of lymph node metastasis.
Ablation procedure
Before ablation, (i) intravenous fluid resuscitation and pharmacological management (calcitonin, bisphosphonate and furosemide) were used in patients who presented with a hypercalcemic crisis and (ii) patients with vitamin D deficiency were supplemented with 1000–2000 IU vitamin D per day [Citation16].
The patient was placed in a supine position. The ablation site was routinely sterilized and draped with sterile towels. After local anesthesia with 1% lidocaine, a hydrodissection technique was used to prevent unexpected thermal injury to adjacent important structures (e.g., the esophagus, trachea, carotid artery and recurrent laryngeal nerve (RLN)). First, under the guidance of US, an 18-gauge needle was punctured and the needle tip was placed between the target parathyroid nodule and its surrounding structures. Then, 0.9% saline (for the MWA group) or sterilized water (for the RFA group) was injected to establish a liquid isolation zone more than 0.5 cm in distance between the parathyroid and adjacent structures. Then, a mixture of 2% lidocaine and normal saline (1:3) was administered near the periparathyroid capsule for local anesthesia. For ablation, a cooled MWA antenna (16 G or 17 G) with a 3-mm active tip (Intelligent Basic Type Microwave Tumor Ablation System, KY-2000, Kangyou Medical, Nanjing, China; or Nanjing ECO Microwave System, Nanjing, China) or a 17 G radiofrequency electrode with a 7-mm active tip (VIVA; STARmed, Goyang, South Korea) was used. After the antenna or electrode was inserted into the target nodule under US guidance, a multipoint ablation technique was adopted to complete the ablation procedure [Citation16]. Power outputs of 30 W and 35 W were usually applied in MWA and RFA, respectively. At each point, the radiation time was 15–25 s in MWA or when the impedance was reduced in RFA. The ablation procedure is shown in . For patients with bilateral pHPT nodules, the contralateral side ablation was only performed when vocal cord movement was normal on US and no voice change after one side was ablated; otherwise, the procedure was stopped and the second session was suspended until RLN function recovered.
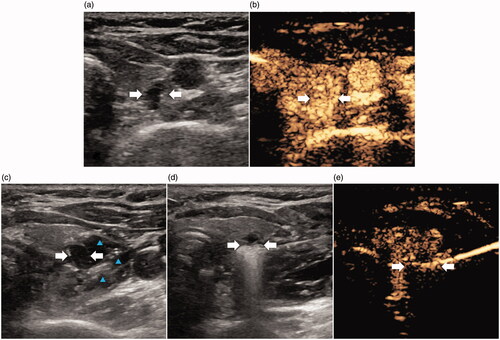
Figure 1. Images show ablation of pHPT nodule in a 47-year old female. (a) A hypoechoic pHPT nodule with sharp boundary (arrows) was behind superior right lobe of thyroid on US. (b) Uniform hyperenhancement of pHPT nodule (arrows) in arterial phase on CEUS. (c) Establishment of hydrodissection (blue arrow) around pHPT nodule (arrows). (d) Ablation procedure of pHPT nodule (arrows): hyperechoic area emerging inside nodule. (e) After ablation, non-enhancement area covered pHPT nodule (arrows) on CEUS. CEUS: contrast-enhanced US; pHPT: primary hyperparathyroidism; US: ultrasound.
Follow-up and outcomes
US examination and blood biochemistry (e.g., serum intact parathyroid hormone (iPTH), phosphorus, calcium and alkaline phosphatase (ALP)) were conducted 24 h before ablation and at 2 h, one day, seven days, 1 month, 3 months, 6 months and then at 6-month intervals after ablation. Since the shape of the pHPT nodule was similar to an ellipsoid, the following sphere volume formula was used to calculate nodule volume: V=πabc/6 (V, the volume; a, the largest diameter; b and c are the other two perpendicular diameters). The volume reduction rate (VRR) was formulated as follows: VRR=(initial volume – final volume)×100/initial volume (%).
The ablation cure was defined as the reestablishment of normal values of serum calcium and iPTH at least more than 6 months after MWA/RFA. Clinical cure was defined as the reestablishment of normal values of serum calcium and iPTH throughout the entire follow-up period after MWA/RFA. Adverse events were classified into two categories: persistent pHPT and recurrent pHPT. Persistent pHPT referred to a failure to achieve normocalcemia/iPTH within 6 months and recurrent pHPT referred to the recurrence of hypercalcemia and/or an elevated iPTH level 6 months after MWA [Citation6].
According to the reporting standards of the Society of Interventional Radiology [Citation17], to compare the safety of the RFA and MWA groups, complications, including voice change and hemorrhage, and side effects such as pain, fever, numbness in the peri-ablation and follow-up periods were recorded.
Statistical analysis
The SPSS software package (version 20.0, IBM, Armonk, NY) was used to perform the statistical analysis. Continuous data are presented as the mean ± standard deviation or the median and 25–75% interquartile range (IQR) if the data did not fit a normal distribution. The Wilcoxon rank sum test or t test was used for continuous variables, and the χ2 test or Fisher exact test was used for categorical variables, as appropriate. Clinical cure curves were plotted by the Kaplan–Meier method using R-Studio software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria). All tests were two sided, with p<.01 considered to indicate a statistically significant difference.
Results
Patients
A total of 121 consecutive patients underwent MWA (n = 89) or RFA (n = 32) during the study period; 104 (86.0%) of 121 patients met the eligibility criteria (). Among the 104 patients, 77 (74.0%) underwent MWA and 27 (26.0%) underwent RFA. The baseline data were balanced between these two groups ().
Figure 2. Patient flowchart. MWA: microwave ablation; RFA: radiofrequency ablation; pHPT: primary hyperparathyroidism; PTX: parathyroidectomy.
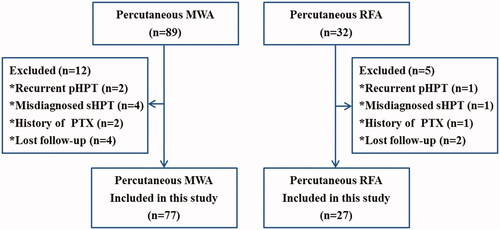
Table 1. Baseline characteristics of patients underwent MWA and RFA.
Thermal ablation
In the MWA group, 77 patients (with 86 parathyroid nodules) received 80 treatment sessions (74 were treated with one session and three were treated with two sessions). All patients in the RFA group underwent one session. Of the three patients who underwent two sessions in the MWA group, two patients received additional ablation due to elevated serum iPTH/calcium and hyperenhanced residual abnormal parathyroid tissue on CEUS three months after the first ablation. One older patient with large hyperplastic parathyroid nodules (volume: 37.4 cm3) and high levels of serum ALP (447 IU/L) and iPTH (572 pg/mL) presented with a hypercalcemic crisis. She received two-session ablation within 3 months to avoid severe hypocalcemia. The ablation time for the MWA group was significantly longer than that for the RFA group (p<.001), while there was no difference in energy between these two groups (p = .02) ().
Table 2. Comparison of treatment parameter and clinical efficacy between MWA and RFA group.
Therapeutic efficacy evaluation at follow-up
There was no significant difference in the ablation cure rates between the MWA group and RFA group (88.3% vs. 88.9%, p = 1.000). The median follow-up was 18.7 months (IQR: 9.6–42.3 months) in the MWA group and 12.0 months (IQR: 6.1–34.1 months) in the RFA group (p = .017). The rates of achieving normal levels of iPTH, calcium and phosphorus were comparable between the MWA group and RFA group at each follow-up time point (). Recurrent and persistent pHPT were not significantly different between the two groups (recurrent pHPT, MWA 5.2% (4/77) vs. RFA 3.7% (1/27), p = .448; persistent pHPT, MWA 11.7% (9/77) vs. RFA 11.1% (3/27), p = 1.000). The clinical cure curve of the thermal ablation is shown in . There were no differences in the clinical cure rates between the MWA and RFA groups (87.0% vs. 82.3% at 12 month, p = .88). During the 20-month follow-up period, the clinical cure rates of the MWA and RFA groups were still above 80%.
Figure 3. Clinical cure rate curves. MWA: microwave ablation; RFA: radiofrequency ablation; M: month.
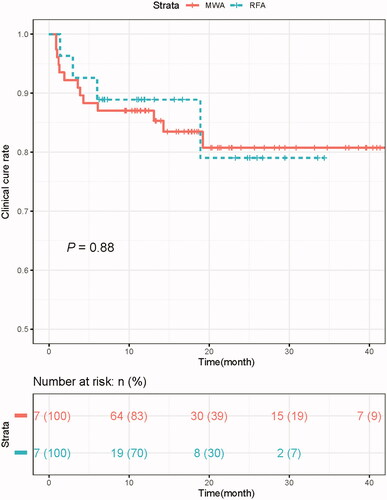
Table 3. The rates of achieving the normal level for iPTH, serum calcium and phosphorus in MWA group and RFA group after treatment.
In the MWA group, nine patients did not receive ablation cures. The levels of serum iPTH and calcium increased in three patients, while in the other six patients, only serum iPTH increased but serum calcium was normal. Of the three patients who had not been cured in the RFA group, only serum iPTH levels were increased, and serum calcium levels were normal. The follow-up imaging examination indicated (i) the ablation zone covered the soft tissue other than the target pHPT nodule in five cases in the MWA group; (ii) adjacent lymph nodes rather than the pHPT nodules were ablated in two cases with Hashimoto's thyroiditis in the MWA group; and (iii) local residual hyperplastic parathyroid tissues in ablation areas were found in three cases (two cases in the RFA group and one case in the MWA group). In the other two patients with uncured pHPT (one case in MWA group and the other in RFA group), there were no new parathyroid nodules found in the imaging examination within six months.
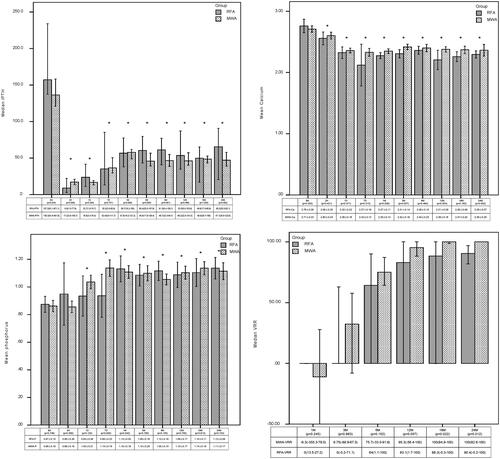
Serum iPTH, calcium and phosphorus were compared before ablation vs. each follow-up point in the two groups, all of which showed significant decreases in the two groups (all p<.01). There were no significant differences in the median iPTH, mean calcium or phosphorus between the RFA and MWA groups during the observation time (all p>.01). Although there were differences in the biochemical indicators at some follow-up points (iPTH 2 h; serum calcium, 3 months, 12 months; serum phosphorus, seven days), they were all within the normal range of clinical measurement values. The VRR of the ablation zone significantly increased 3–6 months after ablation. The median nodular VRRs showed no significant difference between these two groups (all p>.01) ().
Figure 4. The comparison of the serum iPTH, calcium, phosphorus, VRR between the RFA and MWA groups at each follow-up period. iPTH: intact parathyroid hormone; MWA: microwave ablation; RFA: radiofrequency ablation; H: hour; D: day; M: month; BA: before ablation; Ca: calcium; P: phosphorus; VRR: volume reduction rate. *p<.001 vs. before ablation.
Risk factors associated with patient outcome
The maximum diameter of pHPT nodules was an independent prognostic factor of uncured pHPT in ablation (hazard ratio, 0.1; 95% confidence interval: 0.02, 0.54; p = .007) (). According to the ROC analysis, a maximum diameter less than 0.7 cm resulted in the highest Youden index values of uncured pHPT. Among the 13 patients with pHPT nodules smaller than 0.7 cm, eight (61.5%) patients did not receive ablation cure. If cases with pHPT nodules less than 0.7 cm were excluded, the cure rates of thermal ablation in MWA and RFA were 92.3% (60/65) and 92.3% (24/26), respectively.
Table 4. Analyses of prognostic factors for patients who did not received ablation cure.
Complications
Major complication – voice change encountered in five patients (6.5%) in the MWA group and in one patient (3.7%) in the RFA group. Five patients’ voices recovered completely within 6 months after ablation. One patient’s voice change was relieved by medications, including corticosteroids and physiotherapy, but they still experienced persistent voice impairment throughout the follow-up period. Two (2.6%) out of 77 patients in the MWA group exhibited minor complications – hematomas, which recovered within one week without sequelae. Side effects (fever, headache and numbness), hypocalcemia and transient hypoparathyroidism all rapidly recovered within 1 month without any specific therapy. There were no significant differences in posttreatment complications, side effects, transient hypoparathyroidism or hypocalcemia between the MWA and RFA groups (complications, 9.1% vs. 3.7%, p = .677; side effects, 22.1% vs. 29.6%, p = .442; transient hypoparathyroidism, 37.7% vs. 66.7%, p = .013; hypocalcemia, 2.6% vs. 7.4%, p = .276) ().
Discussion
MWA and RFA are the most recent and exciting advances among thermal ablation techniques and have relatively more applications in the treatment of pHPT [Citation9–14,Citation18,Citation19]. They share several common advantages in treating pHPT, such as minimal invasiveness, good tolerance and good repeatability. The internally cooled RF electrode and MW antenna also help prevent overheating and lead to greater energy efficiency. However, the two ablation modalities differ substantially in the basic mechanism of energy deposition. RF ablation uses the flow of current through conducting electrodes within body tissue, while MWA uses an electromagnetic field around an insulated and electrically independent antenna, which may lead to many differences between the two modalities including ablation zone, treatment time and session [Citation9–14,Citation18,Citation19].
The present multicenter study enrolled 104 pHPT patients with 114 parathyroid nodules and had a median follow-up of 12 months to compare the possible differences between MWA and RFA in the treatment of pHPT. The results show that there is no difference in the ablation cure rate of the two methods (MWA: 88.3%, RFA: 88.9%). During the follow-up period, there was no difference in the clinical cure curve of the two methods. After 20 months of follow-up, the clinical cure rate was still higher than 80%. In addition, the same incidence of persistent and recurrent pHPT was observed in both the MWA and RFA groups. This shows that although the basic mechanism is different between the two methods, both kinds of energy can inactivate pHPT nodules, and the medium-term treatment effectiveness is promising. The result is similar to previous reports in the literature [Citation10,Citation12,Citation20].
Compared with the surgical resections reported in the literature with a cure rate of 95%, the cure rate of MWA and RFA is slightly lower [Citation21,Citation22]. The reasons might be as follows: first, ablation of the small target pHPT nodules failed. Statistical analysis shows that the diameter of pHPT nodules less than 0.7 cm is an independent risk factor for failure to achieve ablation cure. When establishing hydrodissection, the hypoechoic manifestations of pHPT nodules and the isolation fluid are similar; the nodular-like surrounding tissues change, and surrounding structure displacements will easily misguide the operator to false target pHPT. In fact, even during open surgery, some small parathyroid nodules may be missed [Citation23]. Therefore, some guidelines suggest that hyperplastic parathyroid nodules with a diameter of 1 cm or more are an indication for surgical resection [Citation24,Citation25]. At present, guidelines for thermal ablation for pHPT have not been established. Nevertheless, if cases that with small pHPT nodules (maximum diameter <0.7 cm) were excluded, the cure rate of thermal ablation could reach 92.3%, which is comparable to that of surgery. The second reason for failure to achieve ablation cure is incomplete ablation, which has also been reported in previous studies [Citation11,Citation14,Citation26]. According to this study, we recommend that complete ablation should involve hypoechoic hyperplastic nodules and the surrounding normal parathyroid tissue, which will become clearer after the establishment of hydrodissection.
After ablation, serum iPTH, calcium and phosphorus were significantly improved, and the volume of the ablation zone began to decrease significantly after 3–6 months. There was no difference in the above parameters in the MWA and RFA groups at each follow-up time point. A previous study comparing MWA and RFA in benign thyroid nodules demonstrated that a larger VRR can be achieved in the RFA group than those in the MWA group at 6 months and later follow-up [Citation27]. In the present study, there was no difference in VRR between the MWA and RFA groups after 3 months. This might be attributed to the relatively small size of the pHPT nodule (median maximum diameter: 1.3–1.6 cm). The low-power, short radiation time and multiple point ablation strategy might help to minimize the carbonization of the ablation zone caused by the high core temperature of MWA (RFA vs. MWA; 110 °C vs. 150 °C) and promote absorption [Citation28,Citation29]. Therefore, the absorption of the ablation zone of the two groups was similar.
Major and minor complications, side effects, hypocalcemia and transient hypoparathyroidism were not significantly different between the RFA and MWA groups. Voice changes after ablation are the most common major complication. The incidences of the two groups were 6.5% (MWA) and 3.7% (RFA). Transient hoarseness occurred in five patients (4.8%). This rate was slightly higher than that reported for parathyroidectomies (3.9%) and thermal ablation of thyroid nodules (1.5%) [Citation28,Citation29]. However, compared with the incidence of permanent RLN injury in thyroidectomy or parathyroidectomy, the rate of permanent nerve palsy hoarseness was lower (thermal ablation vs. surgery: 1.3% vs. 3.9%) [Citation11]. The reason for this might be attributed to the anatomic sites of the parathyroid glands. Some parathyroid nodules were too close to the RLN which was very sensitive to thermal stimulation. Efficient hydrodissection technology could effectively reduce thermal stimulation to the RLN. Additionally, accurate puncture and ablation monitored by US could help protect the RLN against thermal injury.
There are still a few limitations that should be mentioned. First, as a retrospective study, there may be selection bias in the present study, and thus additional prospective studies are needed to establish more definitive results. Second, the number of RFA cases was relatively small, and the long-term efficacy of MWA and RFA for pHPT remains to be verified. Third, since biopsy was not recommended, no pathological results were obtained.
Conclusion
Both RFA and MWA are safe and effective techniques for selected patients with pHPT due to their favorable cure rate and low incidence of complications. There was no difference between these two methods in terms of the clinical outcome of pHPT. The small size of the pHPT nodule was a risk factor leading to operative failure for both MWA and RFA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Fraser WD. Hyperparathyroidism. Lancet. 2009;374(9684):145–158.
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet. 2018;391(10116):168–178.
- Gasser RW. Clinical aspects of primary hyperparathyroidism: clinical manifestations, diagnosis, and therapy. Wien Med Wochenschr. 2013;163(17–18):397–402.
- Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93(9):3462–3470.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569.
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016;151(10):959–968.
- Hamdy NA. Parathyroid gland: is parathyroidectomy safe and beneficial in the elderly? Nat Rev Endocrinol. 2009;5(8):422–423.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Korkusuz H, Wolf T, Grunwald F. Feasibility of bipolar radiofrequency ablation in patients with parathyroid adenoma: a first evaluation. Int J Hyperthermia. 2018;34(5):639–643.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):835–840.
- Wei Y, Peng L, Li Y, et al. Clinical study on safety and efficacy of microwave ablation for primary hyperparathyroidism. Korean J Radiol. 2020;21(5):572–581.
- Ye J, Huang W, Huang G, et al. Efficacy and safety of US-guided thermal ablation for primary hyperparathyroidism: a systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):245–253.
- Ha EJ, Baek JH, Baek SM. Minimally invasive treatment for benign parathyroid lesions: treatment efficacy and safety based on nodule characteristics. Korean J Radiol. 2020;21(12):1383.
- Appelbaum L, Goldberg SN, Ierace T, et al. US-guided laser treatment of parathyroid adenomas. Int J Hyperthermia. 2020;37(1):366–372.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- Xu SY, Wang Y, Xie Q, et al. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J. 2013;54(7):e137–e140.
- Sormaz IC, Poyanli A, Acar S, et al. The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602.
- Wu W, Zhou Q, Xu S, et al. Two-year changes of biochemical profiles and bone mineral density after percutaneous ultrasound-guided microwave ablation for primary hyperparathyroidism. Endocrine. 2021;71(2):476–483.
- Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253(3):585–591.
- Singh ON, Rodriguez-Gutierrez R, Maraka S, et al. Outcomes of parathyroidectomy in patients with primary hyperparathyroidism: a systematic review and meta-analysis. World J Surg. 2016;40(10):2359–2377.
- Alhefdhi A, Schneider DF, Sippel R, et al. Recurrent and persistence primary hyperparathyroidism occurs more frequently in patients with double adenomas. J Surg Res. 2014;190(1):198–202.
- National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10(1):98–109.
- Bucy D, Pollard R, Nelson R. Analysis of factors affecting outcome of ultrasound-guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. Vet Radiol Ultrasound. 2017;58(1):83–89.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554.
- Ahmed M, Brace CL, Lee FJ, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369.
- Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012;2012:919650.
