Abstract
Background
In patients with stage 0–A (as per Milan criteria) hepatocellular carcinoma (HCC) image-guided ablation is less invasive and requires shorter hospitalization than resection, but long-term prognosis is poorer. This meta-analysis was conducted to investigate whether liver stiffness measurement (LSM) could be used to predict prognosis in HCC patients after tumor ablation.
Methods
A literature search was conducted for all studies published till July 2020 in PubMed, Web of Science, Cochrane Library and EMBASE. Studies were included if they investigated the association between pretreatment LSM and prognosis in HCC patients treated with ablation. Subgroup analysis, meta-regression, publication bias assessment and sensitivity were conducted.
Results
Eight studies (with a total of 1276 HCC patients) were included in this meta-analysis. All patients were treated with radiofrequency ablation. Pooled results showed that high pretreatment LSM were associated with poor overall survival (OS) (hazard ratio [HR] = 4.31, 95% confidence interval [CI]: 2.27–8.20, p < .001) and recurrence-free survival (RFS), regardless of whether LSM was considered as a categorical variable (HR = 2.63, 95% CI = 1.63–4.22, p < .001) or as a continuous variable (HR = 1.02, 95% CI = 1.01–1.04, p = .003). Among studies treating LSM value as a categorical variable, liver stiffness measured using acoustic radio force impulse (ARFI) or transient elastography (TE) was significantly associated with RFS, but not liver stiffness measured using two-dimensional shear wave elastography (SWE).
Conclusions
High baseline LSM value appears to be associated with poor prognosis in HCC patients treated with radiofrequency ablation.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the fourth most common cause of cancer-related deaths, and the incidence continues to rise in the United States [Citation1]. Due to scarcity of suitable donors for liver transplantation, resection and image-guided tumor ablation are the most widely used curative treatment for HCC [Citation2]. Although long-term prognosis is inferior with tumor ablation than with resection, ablation is usually recommended as the first-line treatment for HCC, especially many patients are unsuitable for resection owing to insufficient liver reserve function or systemic conditions [Citation3,Citation4].
The development of HCC is a complex multistep process, and the underlying mechanism remains unclear; however, it is known that liver fibrosis and cirrhosis play pivotal roles. Indeed, 2–5% patients with cirrhosis due to chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection develop HCC every year [Citation5]. Moreover, because cirrhosis impairs liver function, its presence appears to be an independent prognostic factor in HCC [Citation6–8].
The degree of liver fibrosis can be noninvasively evaluated by liver stiffness measurement (LSM) using magnetic resonance elastography (MRE) or ultrasound elastography, which includes the techniques of transient elastography (TE), shear wave elastography (SWE) and acoustic radio force impulse (ARFI) imaging. Several meta-analyses have demonstrated that high LSM value in patients with chronic liver diseases is associated with the increased risk of HCC and death [Citation9]. One meta-analysis that included 1942 patients who underwent hepatic resection found that the LSM value was associated with liver-related postoperative complications, such as post-hepatectomy liver failure, hepatic insufficiency and ascites [Citation10]. Several studies have indicated that the LSM value can predict overall survival (OS) or recurrence in patients with HCC after ablation [Citation11–13]; however, the results of these studies have not always been consistent.
Identifying HCC patients at high risk of recurrence and metastasis after ablation can help optimize management. To the best of our knowledge, no meta-analysis has examined prognostic value of LSM in patients treated with ablation. Therefore, we performed this study to determine whether baseline LSM value had prognostic value in HCC patients treated with tumor ablation.
2. Materials and methods
2.1. Search strategies
A comprehensive electronic search was performed using PubMed, EMBASE, Cochrane Library databases and Web of Science until 16 July 2020. Briefly, The following search terms were used: ‘liver’ or ‘hepat*’ AND ‘stiff*,’ ‘elasto*,’ ‘rigid*,’ ‘LS*,’ combined with ‘ablation*,’ ‘radiofrequency ablation,’ ‘microwave ablation*,’ ‘high intensity focused ultrasound ablation,’ ‘cryoablation,’ ‘hepatocellular carcinoma,’ ‘liver cancer*,’ The details of the search strategy are included in Supplementary Table 1. The references in the identified articles were also applied to trace other relevant studies.
Table 1. The main characteristics of studies included for LSM.
2.2. Study selection criteria
The inclusion criteria for the study were as follows:
Patients had been diagnosed as HCC according to the European association for the study of the liver guidelines [Citation3] or American Association for the Study of Liver Diseases practice guidelines [Citation4];
Studies involved the association of liver stiffness with prognostic outcome OS or recurrence-free survival (RFS);
Sufficient data were provided to calculate the hazard ratio (HR) and 95% confidence interval (CI);
Studies were not restricted by language and study size;
Articles were published in full texts, excluding the following:
Case reports, letters, conference abstracts, editorials, and reviews,
Studies with insufficient information to evaluate HRs and 95% CIs and
Studies with overlapping patients and data.
2.3. Data extraction and quality assessment
Two investigators independently selected the studies that fulfilled our inclusion criteria and extracted the relevant information. Disagreements were resolved by discussion with an independent expert. The following information was extracted: first author’s name, publication year, study design, country, patient age and sex, sample size, technique of ablation, Child–Pugh score, the etiology of HCC, technique of LSM, the cutoff of LSM, HRs for OS, RFS and 95% CIs. The Quality Assessment of Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of studies. This scale consists of three parameters: selection, comparability, and outcome assessment. NOS scores >6 are considered high-quality studies, which were assessed by two independent reviewers.
2.4. Statistical analysis
HRs with their 95% CIs from included studies were used to estimate the association of LSM and prognosis in patients with HCC after ablation therapies. Heterogeneity of pooled results was accessed by using Higgins I2 statistic. I2 > 50% was defined significant heterogeneity. A fixed-effect model or random-effect model was used according to the heterogeneity of the studies. The data were synthesized using a fixed-effect model with I2 < 50%. Otherwise, a random effect model was utilized. The sources of heterogeneity were evaluated by sensitivity, subgroup analysis, meta-regression. Sensitivity analysis was used to appraise the stability of the outcome. Funnel plots and Egger’s test were constructed to evaluate publication bias. All statistical tests were two-sided, and statistical significance was defined as p < .05. The pooled data were analyzed with STATA version 16.0 (Stata Corp., College Station, TX).
3. Results
3.1. Study selection and characteristics
The flowchart in shows the literature selection process. A total of 791 relevant records were initially retrieved from selected databases. After duplicates were removed, there remained 602 studies. Of these, 576 were excluded by screening titles and abstracts (because they were either conference abstracts, letters, reviews, case reports or irrelevant studies), leaving 26 potentially relevant full-text articles. Eventually, eight studies [Citation11–18] with a total of 1276 patients, were included in this meta-analysis. One of the included studies (Yoon et al. [Citation18]) compared the predictive value LSM evaluated by ARFI elastography versus TE; we treated this article as two separate studies – ‘Yoon 2018 AFFI’ and ‘Yoon 2018 TE’.
Figure 1. Flow chart of literature search and study selection. A total of 791 articles were initially retrieved. After carefully reviewed eight studies reporting liver stiffness measurement (LSM) were included in the analysis.
Although our search strategy did not specify the type of ablation therapy, all eight eligible studies had RFA as the ablation technique. Of the eight studies, five were prospective and three were retrospective observational studies. All eight studies used ultrasound-based techniques for measurement of liver stiffness: five studies [Citation11,Citation14–16,Citation18] used TE, two studies [Citation12,Citation17] used SWE and two studies [Citation13,Citation18] used ARFI. No studies that used MRE for measurement of liver stiffness and examined its association with prognosis had been reported until the day of our literature search. Notably, seven of the eight studies were from Asian countries (China and Korea); one study [Citation16] was from France. HBV was the most common cause of HCC in our studies, which is to be expected, given that chronic HBV infection is a leading cause of HCC in Eastern Asian countries. While three studies [Citation11,Citation15,Citation16] fitted LSM as a continuous variable in a multivariable model, the other five [Citation12–14,Citation17,Citation18] treated LSM as a categorical variable, using cutoff values identified by receiver operating characteristic curve analysis. All eight studies in this meta-analysis were identified as being of moderate quality with scores in the range of 5–6. summarizes the characteristics of the included studies.
3.2. Predictive value of LSM as a categorical variable
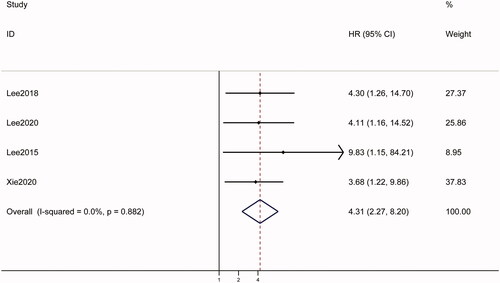
Among the five studies [Citation12–14,Citation17,Citation18] that treated LSM as a categorical variable, four [Citation12–14,Citation17] examined the association between baseline LSM and OS. Pooled analysis of data from the four studies showed that high baseline LSM was significantly associated with poor OS (HR = 4.31, 95% CI: 2.27–8.20, p < .001), with no heterogeneity (I2 = 0.0%; ). Since there was no heterogeneity, subgroup analyses were not conducted.
Figure 2. Forest plots of the relationship between LSM and survival outcomes in hepatocellular carcinoma patients treated with RFA in categorical group. A fixed-effect model was used to evaluate the impact of LSM on OS. The pooled result indicated that high LSM was associated with poor OS. LSM: liver stiffness measurement; HR: hazard ratio; CI: confidence interval.
All five studies [Citation12–14,Citation17,Citation18] evaluated the relationship between pretreatment LSM and RFS. Pooled analysis revealed that high baseline LSM was significantly associated with poor RFS (HR = 2.63, 95% CI = 1.63–4.22, p < .001), with high heterogeneity (I2 = 65.2%; ). To identify the origins of heterogeneity, subgroup analyses were performed after stratifying the study population by country of study and technique used. As shown in , studies using ARFI and TE found significant association between LSM and RFS, while studies using 2D-SWE found no significant association. LSM was significantly associated with RFS in studies from both Korea (HR = 1.09, 95% CI: 1.42–5.74, p = .003) and China (HR = 2.18, 95% CI: 1.28–3.72, p = .004).
Figure 3. Forest plots of the relationship between LSM and survival outcomes in hepatocellular carcinoma patients treated with RFA in categorical group. A random-effect model was used to evaluate the impact of LSM on RFS. The pooled result indicated that high LSM was associated with poor RFS. LSM: liver stiffness measurement; RFS: recurrence-free survival; HR: hazard ratio; CI: confidence interval; HR: hazard ratio.
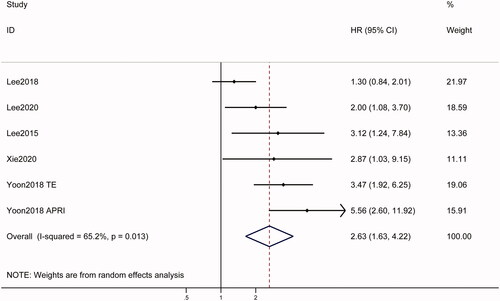
Table 2. Results of subgroup analysis.
3.3. Predictive value of LSM as a continuous variable
Of the three studies [Citation11,Citation15,Citation16] that considered LSM as a continuous variable, only one (Rekik et al. [Citation16]) examined the association between LSM and OS. In that study (from France) high LSM value assessed by TE was associated with poor OS in 159 cirrhosis patients with HCC (HR = 1.02, 95% CI: 1.01–1.04, p < .001).
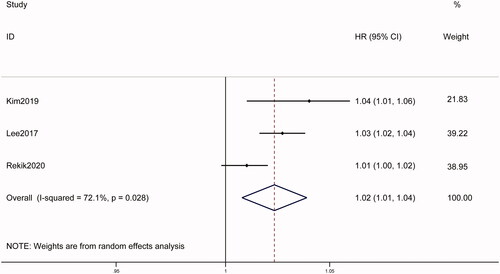
All three studies reported the association between LSM and RFS. Pooled data showed that high baseline LSM was associated with poor RFS (HR = 1.02, 95% CI: 1.01–1.04, p = .003), with significant heterogeneity (I2 = 72.1%; ). Subgroup analysis was performed after stratifying by geographic region; stratification by technique was not done as all studies used TE. The results found that substantial heterogeneity drop to 0 and the pooled HR was 1.03 (95% CI: 1.02–1.04, p < .001) in Korea, indicating that population involved in different studies may be the source of heterogeneity.
Figure 4. Forest plots of the relationship between LSM and survival outcomes in hepatocellular carcinoma patients treated with RFA in continuous group. A random-effect model was used to evaluate the impact of LSM on RFS. The pooled result indicated that high LSM was associated with poor RFS. LSM: liver stiffness measurement; RFS: recurrence-free survival; HR: hazard ratio; CI: confidence interval; HR: hazard ratio.
3.4. Sensitivity analysis and meta regression
We also performed sensitivity analyses for OS and RFS to determine whether an individual study influenced the results. In studies treating LSM as a categorical variable, the combined HRs (and 95% CIs) for OS and RFS were not significantly altered when any one study was excluded. However, among studies treating LSM as a continuous variable, when Lee et al. [Citation15] was excluded, LSM was no longer significantly associated with RFS, indicating that the study had a significant effect on the polled results.
In studies treating LSM as a categorical variable, the funnel plot for RFS indicated no obvious publication bias (). Egger’s test also ruled out publication bias (p = .144). The funnel plot for OS was not done as there were limited studies. For the same reason, publication bias was not evaluated among the studies treating LSM as a continuous variable.
Figure 5. Funnel plots for assessing publication bias in survival outcomes. The funnel plots of the association between LSM and RFS were basically symmetrical. LSM: liver stiffness measurement; RFS: recurrence-free survival.
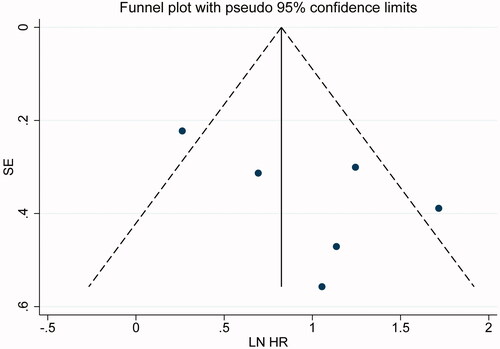
Multivariate meta-regression showed that no significant influencing factor for pooled HRs on OS or RFS indicated by LSM, regardless of regardless of variable type (data are not shown).
4. Discussion
In this meta-analysis, we pooled the data of eight studies – with a total of 1276 HCC patients treated with ablation – to explore the prognostic value of baseline LSM. We found that high pretreatment LSM, as a categorical variable, was predictive of poor OS and RFS. High pretreatment LSM, as a continuous variable, was predictive of poor RFS. Only one study reported that LSM, as a continuous variable, was associated with poor OS.
Liver stiffness reflects the degree of hepatic fibrosis, inflammation and portal pressure [Citation19,Citation20]. Previous studies have demonstrated that LSM has prognostic value in patients with decompensated cirrhosis, is a predictor of mortality in patients with chronic liver disease, and a predictor of HCC development and of postoperative complications in HCC patients undergoing hepatectomy [Citation9,Citation10,Citation21]. In this study, we confirmed that high LSM value (as a categorical variable) was a predictor of poor OS after RFA. The study from France (Rekik et al. [Citation16]), which considered LSM value as a continuous variable, found high LSM value to be associated with poor OS. Even though the variable in its continuous form provides more information, the categorized form is of more practical use for clinicians. Notably, all studies treating LSM as a categorical variable were from Asia, with high proportion of hepatitis B-positive individuals; the study from France treated LSM as a continuous variable and had a high proportion of hepatitic C-positive patients. Although the number of studies is limited, LSM has been previously shown to have prognostic value in Western patients with HCV infection [Citation21].
HCC recurrence after treatment can be classified by timing as early recurrence (occurring within 2 years after treatment) or as late recurrence (occurring ≥2 years after treatment); it can further be classified by site as local recurrence or distant recurrence. Early recurrence and local recurrence are associated with the characteristics of the tumor and the tumor microenvironment, whereas late recurrence and distant recurrence are more likely to be ‘de novo’ carcinogenesis and to be associated with underlying liver disease and uncontrollable cirrhosis [Citation22–24]. Previous studies have indicated that necroinflammatory activity caused by chronic hepatitis B and hepatitis C and consequent severe liver fibrosis lead to local recurrence after RFA, and that LSM could be a reliable predictor of relapse [Citation15,Citation25,Citation26]. Our results for RFS were consistent with these previous studies. Subgroup analysis after stratification by country showed LSM (as categorical variable) to be significantly associated with RFS in Korea and China, where HBV is the leading etiology of HCC. Interestingly, subgroup analysis based on technique revealed liver stiffness measured by ARFI and TE to be significantly associated with RFS, but not liver stiffness measured by 2D-SWE. In the 2D-SWE group, the results of RFS differ from OS, which may attribute to better tumor characteristic at baseline and antiviral therapy in Lee et al. [Citation12] compared with Xie and Yu [Citation17]. LSM value, as a continuous variable, was found to predict RFS in this study also.
Optimal treatments have been recommended for each stage of HCC (e.g., resection/ablation for early stage, transarterial chemoembolization for intermediate stage, and systemic therapy or immunotherapy for advanced stage), but recurrence and disease progression after treatment is common and long-term survival remains poor, with 5-year survival rates of <20% [Citation1]. Pretreatment identification of high-risk patients by LSM may help improve prognosis by allowing clinicians to select individualized treatment strategies.
Although the prognostic value of liver stiffness in chronic liver disease is now recognized, there is still no consensus on the best modality for its measurement. Three methods – TE, 2D-SWE and AFRI – are based on ultrasound elastography. TE and 2D-SWE apply shear stress on the target tissue via a mechanical vibrator [Citation27]. Unlike TE, ARFI elastography can be applied to overweight patients or those with serious complications, such as heart failure and ascites; additionally, it can be performed during ultrasonographic examination for the RFA procedure [Citation18]. Previous meta-analyses have shown that these methods are comparably efficient in identifying cirrhosis and liver fibrosis in patients with chronic liver disease or in predicting postoperative complications in HCC patients undergoing hepatectomy [Citation9,Citation10]. Although our results showed liver stiffness measured by 2D-SWE to be significantly associated with OS but not RFS, the small number of studies and heterogeneity in the population may explain this result.
There is still no consensus on whether LSM should be applied as a continuous or categorical variable for prognosis prediction. Our meta-analysis demonstrated that LSM as a continuous variable can be a predictor of RFS. The optimal cutoff for categorizing LSM value remains unclear. The range of cutoff values in the studies included in our meta-analysis was very small, but was generally consistent with previous studies. Notably, the cutoff values in our meta-analysis were all from Asian studies; the ideal cutoff value in Western populations needs further investigation.
There are several limitations in our study. First, although we sought to include patients treated by any type of ablation, only RFA was used in the eight studies in this meta-analysis. This may have induced a selection bias. It also limits the generalizability of our results. Second, seven of the eight studies were from Asian countries; only one was from a Western country (France). Our results may not be generalizable to Western countries where HCV infection is more common that HBV infection. Third, although most patients in our study had no prior treatment history, a few patients had received other treatments. This heterogeneity of treatment history may have compromised the robustness of our results. Fourth, even though multiple methods were adopted to explore the effect of heterogeneity on RFS, several heterogeneities remain unexplained. Last, because of the small number studies included in this meta-analysis, statistical tests were not performed for funnel plot asymmetry.
5. Conclusion
High baseline LSM value appears to be a predictor of poor prognosis in HCC patients treated with RFA. The technique used for LSM and the geographic region may affect the predictive value of LSM. Further large high-quality studies – with patients receiving different types of ablation therapies – are needed to confirm the prognostic value of LSM.
Acknowledgments
The authors thank the patients and their families.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the LEASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
- Ucar F, Sezer S, Ginis Z, et al. APRI, the FIB-4 score, and Forn’s index have noninvasive diagnostic value for liver fibrosis in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2013;25:1076–1081.
- Wang Q, Blank S, Fiel MI, et al. The severity of liver fibrosis influences the prognostic value of inflammation-based scores in hepatitis B-associated hepatocellular carcinoma. Ann Surg Oncol. 2015;22( 3):S1125–S1132.
- Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61(1):292–302.
- Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(12):1573–1584. e1571–1572; quiz e1588–1579
- Huang Z, Huang J, Zhou T, et al. Prognostic value of liver stiffness measurement for the liver-related surgical outcomes of patients under hepatic resection: a meta-analysis. PLoS One. 2018;13(1):e0190512.
- Kim R, Jeong WK, Kang TW, et al. Intrahepatic distant recurrence after radiofrequency ablation of hepatocellular carcinoma: relationship with portal hypertension. Acta Radiol. 2019;60(12):1609–1618.
- Lee DH, Lee JM, Yoon JH, et al. Liver stiffness measured by two-dimensional shear-wave elastography: prognostic value after radiofrequency ablation for hepatocellular carcinoma. Liver Cancer. 2018;7(1):65–75.
- Lee PC, Chiou YY, Chiu NC, et al. Liver stiffness measured by acoustic radiation force impulse elastography predicted prognoses of hepatocellular carcinoma after radiofrequency ablation. Sci Rep. 2020;10(1):2006.
- Lee SH, Kim SU, Jang JW, et al. Use of transient elastography to predict de novo recurrence after radiofrequency ablation for hepatocellular carcinoma. Onco Targets Ther. 2015;8:347–356.
- Lee YR, Park SY, Kim SU, et al. Using transient elastography to predict hepatocellular carcinoma recurrence after radiofrequency ablation. J Gastroenterol Hepatol. 2017;32(5):1079–1086.
- Rekik S, Allaire M, Mumana A, et al. Transient elastography predicts survival after radiofrequency ablation of hepatocellular carcinoma developing on cirrhosis. J Gastroenterol Hepatol. 2020;35(1):142–150.
- Xie X, Yu Y. Prognosis value of liver stiffness measurements by 2D-SWE in primary HBV-positive hepatocellular carcinoma following radiofrequency ablation. Transl Cancer Res Tcr. 2020;9(4):2518–2526.
- Yoon JS, Lee YR, Kweon YO, et al. Comparison of acoustic radiation force impulse elastography and transient elastography for prediction of hepatocellular carcinoma recurrence after radiofrequency ablation. Eur J Gastroenterol Hepatol. 2018;30(10):1230–1236.
- Jansen C, Moller P, Meyer C, et al. Increase in liver stiffness after transjugular intrahepatic portosystemic shunt is associated with inflammation and predicts mortality. Hepatology. 2018;67(4):1472–1484.
- Piecha F, Mandorfer M, Peccerella T, et al. Pharmacological decrease of liver stiffness is pressure-related and predicts long-term clinical outcome. Am J Physiol Gastrointest Liver Physiol. 2018;315(4):G484–G494.
- You MW, Kim KW, Shim JJ, et al. Impact of liver-stiffness measurement on hepatocellular carcinoma development in chronic hepatitis C patients treated with direct-acting antivirals: a systematic review and time-to-event meta-analysis. J Gastroenterol Hepatol. 2020;36:601–608. https://doi.org/10.1111/jgh.15243
- Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38(10):977–981.
- Kim YS, Rhim H, Cho OK, et al. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59(3):432–441.
- Poon RT, Fan ST, Ng IO, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231(4):544–551.
- Berzigotti A, Reig M, Abraldes JG, et al. Value of transient elastography measured with fibroscan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2015;261(4):e105.
- Jung KS, Kim SU, Choi GH, et al. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan(R)). Ann Surg Oncol. 2012;19(13):4278–4286.
- Jeong WK, Lim HK, Lee HK, et al. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014;33(3):149–160.
