 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Radiofrequency ablation (RFA) has been recommended as the treatment for benign thyroid nodules (BTNs) by some guidelines. However, detailed follow-up instructions for thyroid function about the timing and affected populations after RFA are lacked due to insufficient researches. This 12-month prospective study aimed to evaluate the incidence and risk factors of thyroid dysfunction at different time points after RFA, especially within 1 week that previous studies didn’t concern.
Methods
Seventy-five euthyroid patients who underwent RFA for symptomatic BTNs were enrolled (ChiCTR-INR-16007884). The incidence of thyroid dysfunction within 1 week, at 1, 6, and 12 months after RFA was evaluated. The risk factors for different types of thyroid dysfunction in the different terms were further analyzed.
Results
Within 1 week after RFA, the incidence of thyroid dysfunction was as high as 36.00% unexpectedly, and only overt thyrotoxicosis and subclinical thyrotoxicosis occurred, which were significantly associated with the low-normal baseline thyrotropin (TSH) level (p = 0.001) and high ablation volume ratio (p = 0.008). From 1 to 12 months (the long term), the incidence dropped significantly and remained low (8.00–12.00%); and thyroid dysfunction presented as overt thyrotoxicosis, subclinical thyrotoxicosis, and subclinical hypothyroidism. The long-term thyrotoxicosis group had more cases with diabetes and lower baseline TSH levels. The long-term subclinical hypothyroidism group had more cases with positive thyroid peroxidase antibodies, higher baseline TSH levels, and higher ablation volume ratios.
Conclusions
After the RFA of BTNs, thyroid dysfunction was more likely to occur within 1 week and in populations with risk factors.
Introduction
The diagnosis of thyroid nodules (TNs) is increasingly common in clinical practice, with an incidence up to 50–60% in the general population [Citation1]. Approximately 85–93% of TNs are benign [Citation2]. Surgery is the main treatment strategy for benign TNs (BTNs) with clinical symptoms or discomfort [Citation1,Citation2]; however, some severe complications of the surgery, such as hypothyroidism, hypoparathyroidism, and recurrent laryngeal nerve injury, are of concern. In the last decades, abundant evidence has shown that radiofrequency ablation (RFA) is an effective and safe method for the treatment of BTNs [Citation3–10], which may also successfully reduce the complications of surgery. Some authoritative guidelines have already suggested RFA as a valid alternative to surgery in the treatment of symptomatic BTNs [Citation1,Citation11,Citation12].
In theory, RFA may lead to thyroid dysfunction because of the destruction of thyroid follicles [Citation13,Citation14]. Therefore, follow-up of thyroid function after RFA is recommended in the guidelines [Citation15–18]. However, the recommendations from guidelines lack detailed instructions, such as the specific time of follow-up or the kinds of patients needing more attention, which can confuse day-to-day clinical practice. For example, in the Chinese expert consensus [Citation15], follow-up in patients with malignant thyroid nodules and cervical lymphatic metastasis is mentioned, but patients with BTNs are not involved. The European Thyroid Association [Citation16] only recommends the follow-up at 3 months and doesn’t propose any long-term regular follow-up. The Austrian professional associations [Citation17] and the Korean Society of Thyroid Radiology (KSThR) [Citation18] have only made an vague recommendation that thyroid function should be monitored, without any details.
These vague recommendations in the different guidelines may be due to the controversial results from the previous studies, which have shown different results about the development of thyroid dysfunction at different time points (varying from 1 day to 12 months) after RFA [Citation19–29]; however, most have concluded that RFA would not cause severe outcomes related to thyroid dysfunction. Nonetheless, in the previous studies, the important time point of within 1 week after RFA has been ignored. Theoretically, follicle destruction caused by RFA leads to the release of thyroid hormone into the bloodstream. Thyroxine, the main form of thyroid hormone in the follicle, has a 1-week half-life period in the serum [Citation30]; thus, within 1 week after RFA should be an important monitoring period of thyrotoxicosis. Moreover, previous studies have revealed that only a proportion of patients developed thyroid dysfunction after RFA, which may suggest that only certain patients are at high risk. The identification of these patients will guide the effective follow-up and early detection of thyroid dysfunction. Although there were two descriptive studies focused on elderly patients or patients with the previous lobectomy who underwent RFA [Citation31,Citation32], neither of them was able to identify the risk factors.
Therefore, we conducted this prospective study to evaluate the occurrence of thyroid dysfunction after the RFA of BTNs in 75 patients. The follow-up time points were within 1 week, at 1, 6, and 12 months after RFA. Moreover, the risk factors were analyzed to identify patients who would be prone to the development of thyroid dysfunction after RFA.
Materials and methods
Study design
This prospective study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (No. [2015]2-215) and was registered at www.chictr.org.cn (ChiCTR-INR-16007884). All patients signed informed consent forms before participating in the study. This study had two parts: changes in thyroid function (Study 1) and analysis of risk factors for thyroid dysfunction (Study 2). The flowchart is shown in .
Figure 1. Flowchart of the study design. BTNs: benign thyroid nodules; RFA: radiofrequency ablation; FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyrotropin.
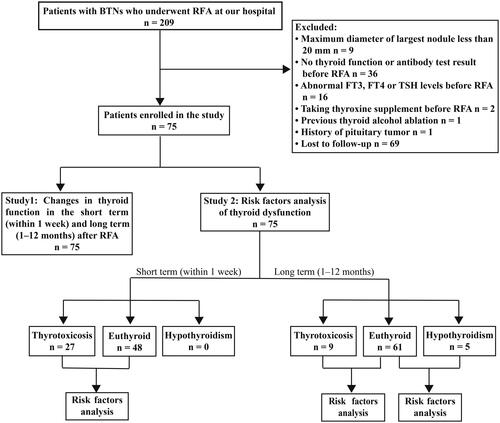
The inclusion criteria were: (1) the presence of benign nodules (Bethesda class II on cytology); (2) having at least one nodule with the maximum diameter ≥ 20 mm; (3) the presence of local symptoms or cosmetic concerns; (4) serum free triiodothyronine (FT3), free thyroxine (FT4), and thyrotropin (TSH) levels within normal ranges; (5) not taking thyroxine supplement, iodine-131 or anti-thyroid medications before RFA; (6) no previous thyroid alcohol and thermal ablation; (7) with thyroid antibody test results (thyroid peroxidase antibody, TPOAb and thyroglobulin antibody, TgAb) before RFA; and (8) 12 months follow-up. The exclusion criteria were: (1) the presence of massive calcification; (2) suspected malignancy; (3) hypothalamus and pituitary diseases; and (4) pregnancy.
Pre-ablation assessment
The cosmetic score was assessed using the scale described by the KSThR [Citation33]. The nodule and thyroid lobe volume was calculated using the following formula: The laboratory examinations included TSH (0.35–4.94 μIU/ml), FT3 (2.63–5.70 pmol/L), FT4 (9.01–19.05 pmol/L), TPOAb (<5.61 IU/ml), and TgAb (<4.11 IU/ml).
Ablation volume ratio and initial ablation ratio
The ablation volume ratio was defined as: (total ablation volume/total thyroid volume) × 100% [Citation34], as shown in . The total thyroid volume included only the left and right lobes. If the ablated nodule was in the isthmus, the nodule volume was added to the total thyroid volume. For those patients with the previous thyroidectomy, the volume of residual thyroid was used.
Figure 2. (A) Ablation volume ratio = (ablation volume of nodule a + ablation volume of nodule b + ablation volume of nodule c)/total thyroid volume × 100%. (B) Initial ablation ratio = ablation volume of the nodule/total volume of the nodule × 100%. When the treated nodule achieves complete ablation, the initial ablation ratio is 100%.
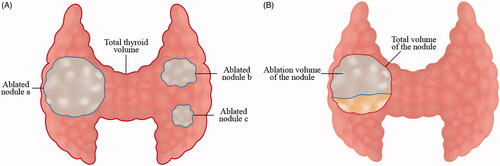
The initial ablation ratio (IAR) was calculated as follows: [Citation35,Citation36], which is shown in . The ablation volume of the nodule was defined by the disappearance of enhancement compared with the initial nodule on contrast-enhanced ultrasound (CEUS) immediately after RFA. The total volume of the nodule was measured before RFA. When the treated nodule achieves complete ablation, the initial ablation ratio is 100%.
RFA treatment
All procedures were performed under ultrasound guidance by two physicians. The RFA device comprised the VRS01 RFA system (STARmed, Korea) and internally cooled 18 G electrodes with 0.7–1cm active tips.
Lidocaine was used for local anesthesia. For the nodules adjacent to critical structures (e.g., recurrent laryngeal nerve), a 5% glucose solution was injected to create a distance of at least 3 mm between the nodule and surrounding structures. The electrode was inserted into the deepest portion of the target nodule with a trans-isthmic or lateral approach, and then, a ‘moving-shot technique’ was performed [Citation33]. After the initial ablation, CEUS assisted in the determination of whether there was a residual part. And if there was a residual part in the nodule, complementary ablation would be applied.
Follow-up
Clinical, biochemical, and ultrasound examinations were performed within 1 week, at 1, 6, and 12 months after RFA. The short term was defined as within one week and the long term was defined as from 1 to 12 months.
The primary outcome was the incidence of thyroid dysfunction at each time point. Thyroid dysfunction included thyrotoxicosis (overt and subclinical) and hypothyroidism (overt and subclinical). Thyroid dysfunction was defined biochemically as follows: (1) overt thyrotoxicosis: TSH < 0.35 μIU/ml, FT4 > 19.05 pmol/L and/or FT3 > 5.70 pmol/L; (2) subclinical thyrotoxicosis: TSH < 0.35 μIU/ml, normal FT4 and FT3; (3) overt hypothyroidism: TSH > 4.94 μIU/ml, FT4 < 9.01 pmol/L; and (4) subclinical hypothyroidism: TSH > 4.94 μIU/ml, normal FT4 [Citation37,Citation38].
Other secondary outcomes included changes in FT3, FT4, and TSH levels, changes in nodule volume and cosmetic score, and the complications rate. The volume reduction ratio (VRR) was calculated with the following equation:
Statistical analysis
The normality of the data distribution was checked using the Shapiro-Wilk test. The data with the normal distribution were expressed as means ± standard deviations, and the data with the non-normal distribution were expressed as medians and interquartile ranges (IQRs). The changes in FT3, FT4, and TSH levels over time from baseline were assessed by Wilcoxon signed-rank test. The incidence of thyroid dysfunction at different time points was reported using percentage and 95% confidence intervals (CIs) and compared by χ2 test. In the analysis of risk factors for thyroid dysfunction in 1 week–12 months or thyrotoxicosis within 1 week, the variables that were significant in the univariate binary logistic regression analysis were evaluated further in the multivariate analysis using a forward stepwise method. According to the regression equation, the prediction probability of each patient was calculated. The cutoff point for prediction probability was evaluated by the receiver operating characteristic (ROC) curve and Youden’s index. In the analysis of risk factors for different types of thyroid dysfunction in 1–12 months, Mann–Whitney U test and t-test were used for the comparison of continuous variables, and χ2 test and Fisher’s exact test were used for categoric variables. All statistical analyses were performed using SPSS, version 21.0. The difference was considered to be statistically significant when p < 0.05.
Results
From January 2016 to April 2020, 209 consecutive patients with BTNs underwent RFA at our hospital. After excluding 134 patients (), 75 patients with 114 BTNs, whose baseline characteristics are shown in , were analyzed. The median maximum nodule diameter at baseline was 26.00 mm (IQR, 16.25–40.00 mm), and median nodule volume was 3.80 ml (IQR, 0.94–10.53 ml). The median IAR was 100%, and there were 107 (93.86%) ablated nodules with an IAR of 100%. After RFA, the nodule volume decreased persistently compared to baseline, and the VRR at 12 months was up to 93.91% (). The cosmetic scores also decreased significantly ().
Table 1. Baseline characteristics.
Table 2. Treatment efficacy within 12 months after RFA.
The complications rate was 4.00% (3/75). Three patients experienced voice changes after RFA, and they all recovered without any sequelae.
Study 1: changes in thyroid function in the short term and long term after RFA
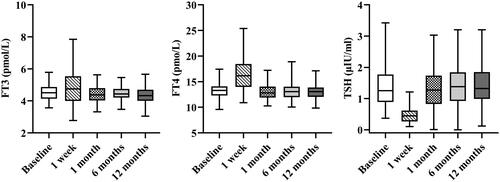
The changes in thyroid function indexes were different in the different terms after RFA. In the short term (within 1 week), serum FT3 (4.79 vs. 4.50 pmol/L, p = 0.013) and FT4 (16.32 vs. 13.39 pmol/L, p < 0.001) levels rose significantly, and TSH level (0.51 vs. 1.27 μIU/ml, p < 0.001) dropped significantly compared to baseline (). On the contrary, in the long term (at 1, 6, and 12 months), there were no significant differences in FT3, FT4, and TSH levels from baseline (all p > 0.05).
Figure 3. Changes in FT3, FT4, and TSH before and after RFA. FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyrotropin; RFA: radiofrequency ablation.
Types and incidence of thyroid dysfunction also varied in the different terms after RFA. In the short term, only overt thyrotoxicosis and subclinical thyrotoxicosis occurred, and the incidence was as high as 36.00% (95% CI: 24.88–47.12%) (), which was significantly higher than that at all the other time points in the long term (all p < 0.05). And in the long term, overt thyrotoxicosis, subclinical thyrotoxicosis, and subclinical hypothyroidism occurred, and the incidence of thyroid dysfunction, 8.00% (95% CI: 1.72–14.28%), 12.00% (95% CI: 4.47–19.53%), and 12.00% (95% CI: 4.47–19.53%) at 1, 6, and 12 months, respectively (), were comparable (p > 0.05). The dynamic thyroid function changes in all patients within 12 months are presented in Supplementary Figure 1.
Figure 4. Incidence of different types of thyroid dysfunction within 12 months after RFA. RFA: radiofrequency ablation.
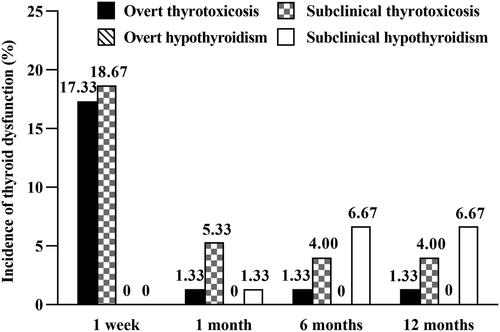
These results showed that within 1 week, there was a peak in thyroid dysfunction, and only thyrotoxicosis occurred. Then in the long term, the incidence of thyroid dysfunction dropped and remained low.
In the short term, three patients with overt thyrotoxicosis and one patient with subclinical thyrotoxicosis had thyrotoxicosis-related symptoms, such as palpitations, fatigue, heat intolerance, and tremors. Only the patient developing subclinical thyrotoxicosis took medication for 1 month to control the palpitations. In the long term, one patient developed Grave’s disease at 6 and 12 months, with palpitations, irritability, heat intolerance, and tremors; one patient developing subclinical hypothyroidism at 1 month was asymptomatic and took a thyroxine supplement from 2 months after RFA.
Study 2: risk factors analysis of thyroid dysfunction after RFA
Among the 75 patients, 35 cases developed thyroid dysfunction and 40 cases were euthyroid from 1 week to 12 months. The thyroid dysfunction group had higher total ablation volumes (p = 0.026) and ablation volume ratios (p = 0.007) than the euthyroid group. In the multivariate analysis, only the ablation volume ratio (OR = 1.037, 95% CI: 1.010–1.065, p = 0.007) was shown to be an independent risk factor ().
Table 3. Risk factors analysis of all types of thyroid dysfunction in 1 week–12 months after RFA.
Considering that the risk factors may be different in different types of thyroid dysfunction and the different terms after RFA, the following analysis was divided into three parts.
1. Risk factors of thyrotoxicosis in the short term (within 1 week)
In the short term, only overt thyrotoxicosis and subclinical thyrotoxicosis (n = 27) occurred. The thyrotoxicosis group had lower baseline TSH levels (p = 0.001), higher total ablation volumes (p = 0.003), and higher ablation volume ratios (p = 0.019) than the euthyroid group. In the multivariate analysis, the low-normal baseline TSH level (OR = 0.178, 95% CI: 0.065–0.484, p = 0.001) and high ablation volume ratio (OR = 1.044, 95% CI: 1.011–1.077, p = 0.008) were independent risk factors (). Therefore, a model was generated to predict thyrotoxicosis: p = 1/[1+e−(0.145−1.727×X1 + 0.043×X2)], where X1 was the TSH value (μIU/ml) and X2 was the ablation volume ratio (%). The ROC analysis showed that the area under the curve for predictive probability was 0.83 and the best cutoff point was 0.44, with a sensitivity of 74.07% and a specificity of 81.25%.
Table 4. Risk factors analysis of thyrotoxicosis within 1 week after RFA.
2. Risk factors of thyrotoxicosis in the long term (1–12 months)
In the long term, nine patients developed overt thyrotoxicosis or subclinical thyrotoxicosis. The thyrotoxicosis group had more cases with a history of diabetes (22.22 vs. 1.64%, p = 0.042), and lower baseline TSH levels (0.90 vs. 1.27 μIU/ml, p = 0.028) than the euthyroid group ().
Table 5. Analysis of different types of thyroid dysfunction in 1–12 months after RFA.
3. Risk factors of subclinical hypothyroidism in the long term (1–12 months)
In the long term, five patients developed subclinical hypothyroidism, and no patients developed overt hypothyroidism. The subclinical hypothyroidism group showed more cases with the positive TPOAb (60.00 vs. 16.39%, p = 0.048), higher baseline TSH levels (3.42 vs. 1.27 μIU/ml, p < 0.001), and higher ablation volume ratios (54.57 vs. 35.92%, p = 0.034) than the euthyroid group ().
Discussion
In this prospective study, we focused mainly on the occurrence and risk factors of thyroid dysfunction in euthyroid patients with BTNs after RFA. The incidence of thyroid dysfunction was as high as 36.00% within 1 week, which then decreased during 1–12 months. Moreover, some risk factors were also found.
Generally, most researchers believe that it’s rare to develop thyroid dysfunction after RFA in euthyroid patients at long-term follow-up (1–12 months). Previous studies have reported 0.36–5.26% of overt thyrotoxicosis [Citation19–26] and 0.07% of overt hypothyroidism [Citation27], which was similar to our study. The reported cases of subclinical thyroid dysfunction were much rarer, with only one case of subclinical thyrotoxicosis (1/237, 0.42%) being reported in an Austrian study [Citation22]. However, a significantly higher incidence was found in our study (subclinical thyrotoxicosis, 4.00–5.33%; subclinical hypothyroidism, 1.33–6.67%). The possible reason is that the previous researchers didn’t pay much attention to subclinical dysfunction since that it might be transient and the patients didn’t show any related symptoms. However, as is known and has been pointed out in many guidelines [Citation37–42], thyroid dysfunction, not only overt but also subclinical, is associated with cardiovascular events and mortality. Therefore, timely follow-up and recognition of both overt and subclinical thyroid dysfunction are of clinical importance. Therefore, we suggest strongly the regular monitoring of thyroid function after RFA.
Different from a few studies on the short-term thyroid dysfunction (1.27–3.70% of thyrotoxicosis and 7.41% of hypothyroidism at 1 day) [Citation28,Citation29], we reported a significantly higher incidence (36.00%) of thyroid dysfunction within 1 week after RFA, and all cases were thyrotoxicosis. There are some possible explanations. First, the definitions of thyroid dysfunction were not completely the same. In our study, we defined overt thyrotoxicosis as FT3/FT4 increase plus TSH decrease, and overt hypothyroidism as FT4 decrease plus TSH increase. Second, we emphasized the complete ablation of BTNs, so the ablation volume ratio (which is proved to be related to thyroid dysfunction in our study) may be larger than that in previous studies. Third, the coagulative necrosis caused by RFA may lead to a continuous release of thyroid hormone. As a result, the incidence of thyroid dysfunction at 1 day may be lower than that within 1 week. This finding emphasized the ‘within 1 week’ as a critical time point to detect thyroid dysfunction, especially thyrotoxicosis which can aggravate the cardiovascular-related symptoms. Thus, we suggest highly that this time point should be added in the follow-up plan after RFA, especially in patients with a history of cardiovascular diseases so that a preventive therapy can be implemented in time. In our study, one patient with a solid BTN (the maximum diameter was 52.80 mm) received RFA. Her preoperative electrocardiograph showed frequent multifocal ventricular premature beats (bigeminy). Within 1 week after RFA, she developed subclinical thyrotoxicosis and presented with significant palpitations. This patient was transferred to the cardiologist and took medication for 1 month to control the symptom.
It is a strength of our study that the possible risk factors of thyroid dysfunction were further analyzed to help determine which kinds of patients need more attention after RFA. We verified the high ablation volume ratio as the risk factor for all types of thyroid dysfunction, and further analyzed the different types of thyroid dysfunction in different terms.
For thyrotoxicosis in the short term after RFA, the low-normal baseline TSH level was an independent factor (OR = 0.178, 95% CI: 0.065–0.484, p = 0.001). The decreased baseline TSH, although in the normal range, might indicate that patients were already at the limit of the thyroid capacity, and was likely to decrease to a much lower level after RFA. The high ablation volume ratio was another risk factor (OR = 1.044, 95% CI: 1.011–1.077, p = 0.008). As expected, larger nodule ablation means that more follicles were destructed, leading to a greater release of thyroid hormone and an occurrence of thyrotoxicosis.
Although detailed statistical analysis couldn’t be completed because of the low incidence of long-term thyroid dysfunction, the findings of our study still indicate some underlying factors. The history of diabetes and low-normal baseline TSH level may be the underlying risk factors for long-term thyrotoxicosis. The positive TPOAb, high-normal baseline TSH level, and high ablation volume ratio may be related to the long-term subclinical hypothyroidism. The positive TPOAb indicates inflammation, and RFA may promote this inflammation and accelerate the follicles’ destruction, resulting in subclinical hypothyroidism.
Consistent with previous studies, RFA was effective in reducing benign thyroid nodule volume, although we reported a higher VRR (93.31%) at 12 months compared with previous studies [Citation43] that reported a VRR of 72.40%. We speculated that the higher VRR might be due to the smaller baseline nodule volume (3.80 vs. 17.20 ml) and the complete ablation in our study. Most (93.86%) of nodules in our study achieved complete ablation, and we believe that the complete ablation can result in better VRR.
Our study has some limitations. First, although iodine intake is associated with thyroid function, the related data were not collected. However, the city where we conducted the prospective study is classified as an adequate iodine intake region, with a reported iodine concentration in the drinking water of 6.55 μg/L and household salt of 29.1 mg/kg [Citation44]. We consider this national information may compensate largely for this limitation. Second, whether the thyrotoxicosis after RFA was caused by hyperthyroidism or inflammation and whether the nodules were toxic nodules when the baseline TSH level was low-normal, couldn’t be confirmed in this study. Further studies collecting more indexes, such as the TSH receptor antibody, inflammatory factors, and radionuclide examination, are needed. Third, the cases of thyrotoxicosis within 1 week might be insufficient for the multivariate analysis of the three variables, and the analysis results of risk factors need to be further verified in future studies with larger sample sizes.
In summary, euthyroid patients could develop thyroid dysfunction after the RFA of BTNs. Therefore, thyroid function should be monitored. The follow-up within 1 week should be a critical follow-up time point. The TSH level and TPOAb before RFA and ablation volume ratio during RFA may be related to thyroid dysfunction after RFA.
Supplemental Material
Download PDF (90.9 KB)Acknowledgments
We would like to thank Mr. Lianxiong Yuan for his assistance in the data analysis.
Disclosure statement
No potential competing interest was reported by the author(s).
Additional information
Funding
References
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocr Pract. 2016;22(5):622–639.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. Am J Neuroradiol. 2015;36(7):1321–1325.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Wang JF, Wu T, Hu KP, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J. 2017;130(11):1361–1370.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Chen F, Tian G, Kong D, et al. Radiofrequency ablation for treatment of benign thyroid nodules: a PRISMA-compliant systematic review and meta-analysis of outcomes. Medicine. 2016;95(34):e4659.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016;17(3):370–395.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):375–381.
- Branovan DI, Fridman M, Lushchyk M, et al. Morphological changes induced by bipolar radiofrequency ablation in thyroid nodules – a preclinical ex vivo investigation. Eur Endocrinol. 2016;12(2):85–88.
- Holmer C, Lehmann KS, Knappe V, et al. Bipolar radiofrequency ablation for nodular thyroid disease-ex vivo and in vivo evaluation of a dose-response relationship. J Surg Res. 2011;169(2):234–240.
- Thyroid Cancer Committee of China Anti-Cancer Association, Interventional Ultrasound Committee of Chinese College of Interventionalists, Tumor Ablation Committee of Chinese College of Interventionalists, et al. Chinese expert consensus on thermal ablation for thyroid benign nodes, microcarcinoma and metastatic cervical lymph nodes (2018 edition). China Cancer. 2018;27(9):670–672.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Dobnig H, Zechmann W, Hermann M, et al. Radiofrequency ablation of thyroid nodules: “good clinical practice recommendations” for Austria: an interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr. 2020;170(1–2):6–14.
- Kim JH, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label, trial (LARA trial). Thyroid. 2020;30(6):847–856.
- Ben HA, Ghanassia E, Espiard S, et al. Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules? Int J Hyperthermia. 2019;36(1):666–676.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Dobnig H, Amrein K. Monopolar radiofrequency ablation of thyroid nodules: a prospective Austrian single-center study. Thyroid. 2018;28(4):472–480.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33(8):911–919.
- Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab. 2017;61(2):173–179.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460–466.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014; 2014:934595.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16(4):361–367.
- Wang TH, Yan JQ, Zheng Y, et al. Physiology. 3rd ed. Beijing: People's Medical Publishing House; 2015. p. 565.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19(3):219–225.
- Ha EJ, Baek JH, Lee JH, et al. Radiofrequency ablation of benign thyroid nodules does not affect thyroid function in patients with previous lobectomy. Thyroid. 2013;23(3):289–293.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13(2):117–125.
- Lang BHH, Woo YC, Chiu KW. High-intensity focused ablation (HIFU) of single benign thyroid nodule rarely alters underlying thyroid function. Int J Hyperthermia. 2017;33(8):875–881.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Bernardi S, Cavallaro M, Colombin G, et al. Initial ablation ratio predicts volume reduction and retreatment after 5 years from radiofrequency ablation of benign thyroid nodules. Front Endocrinol. 2020;11:582550.
- Chinese Society of Endocrinology. Guidelines for diagnosis and treatment of thyroid diseases in China-hyperthyroidism. Chin J Intern Med. 2007;46(10):876–882.
- Chinese Society of Endocrinology. Guidelines for diagnosis and treatment of hypothyroidism in adults. Chin J Endocrinol Metab. 2017;33(2):167–180.
- Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200–1235.
- Biondi B, Bartalena L, Cooper DS, et al. The 2015 European Thyroid Association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. Eur Thyroid J. 2015;4(3):149–163.
- Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. 2020;30(12):1759–1770.
- Shan Z, Chen L, Lian X, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid. 2016;26(8):1125–1130.
