Abstract
Objective
To elucidate the efficacy and safety of MRgFUS in the treatment for refractory pain derived from medial knee OA.
Methods
Twenty patients with medial knee OA eligible for total knee arthroplasty were included in this prospective, non-controlled study (UMIN000010193). MRgFUS treatment was provided at the site of most severe tenderness around the medial femorotibial joint of each patient under real-time monitoring of temperature. The goal temperature of the targeted bone surface was 55 °C. Numerical rating scale (NRS) worst pain scores, Western Ontario and McMaster Universities osteoarthritis index (WOMAC) scores, EuroQol 5 dimensions index (EQ-5D) scores and pressure pain threshold (PPT) were evaluated before treatment (baseline) and at 1 week and 1, 3, 6, and 12 months post-treatment, respectively. Complications and adverse events were also assessed clinically and radiographically.
Results
Treatment response (a 50% or greater decrease in NRS score) was seen in 14 patients (14/19, 73.7%) at 12 months post-treatment. Mean NRS score rapidly decreased at 1 month after treatment and continued to decline through the following 12 months. At final follow-up, mean NRS score was 3.2 ± 1.9, significantly lower than at baseline (p = 0.0013). Mean WOMAC and EQ-5D scores also improved significantly from 1 month after treatment. Fifteen patients showed significant sustained increases in PPTs at the sites of most severe tenderness. No serious adverse events were observed during and after treatment.
Conclusions
MRgFUS treatments were effective not only for managing refractory pain, but also for improving physical functions without adverse events in elderly patients with medial knee OA.
Introduction
Chronic knee pain is a major symptom of knee osteoarthritis (OA), one of the most common musculoskeletal problems in the elderly. This symptom often leads to functional disability and impairs quality of life (QOL) [Citation1–3]. Refractory joint pain resulting from knee OA is known to involve various complicated factors [Citation1]. Among them, peripheral pain sensitization, representing a decrease in the pain threshold and an increase in responsiveness to suprathreshold stimuli of nociceptors, and subsequent central pain sensitization have been noted to be involved in knee OA [Citation4,Citation5]. In particular, peripheral pain sensitization has been reported as a major feature of localized and refractory persistent pain in OA. Once peripheral pain sensitization occurs, all normal joint movements and normal pressure sensations around the knee joint result in chronic persistent pain. Moreover, chronic persistent pain in knee OA contributes to the increased mechanical sensitivity of joint afferents, which normally do not respond to movement [Citation6,Citation7]. Mechanical sensitization causes functional disability due to aggravated knee pain during physical activity.
Various pharmacological and surgical treatments targeting these mechanisms have thus been conducted to alleviate severe knee pain, but no modalities have yet been shown to sufficiently control refractory knee pain [Citation8,Citation9]. Total knee arthroplasty (TKA) is a validated and reliable surgical intervention for alleviating the refractory chronic pain of knee OA [Citation10], but often cannot be performed due to unwillingness of the patient to undergo TKA or poor health condition in elderly fragile patients. In addition, some problems with TKA surgery can cause significantly more serious complications than conservative treatments, and chronic postsurgical pain occurs in 10–34% [Citation11]. Therefore, there is a need for advanced nonsurgical interventions to relieve refractory knee pain that are less invasive and more pain-relieving than traditional treatments, especially in the elderly.
High-intensity focused ultrasound (HIFU), which uses acoustic energy to ablate tissue focally, is an image-guided noninvasive novel treatment modality. Magnetic resonance image-guided focused ultrasound (MRgFUS) treatment enables accurate targeting and noninvasive real-time monitoring of temperature at the treatment site [Citation12,Citation13]. In recent years, the potential of palliative therapy for various pain conditions of bone and joint diseases has been demonstrated by accurately and reliably producing local bone denervation utilizing these crucial advantages of this system [Citation14–17]. In a previous preliminary study, we confirmed the feasibility of this treatment targeted to the tibial plateau for painful knee OA and reported the early pain-relieving effects on chronic knee pain, although the long-lasting effects of pain alleviation and improvement of physical function remain unclear [Citation16]. In addition, a tendency that severe tenderness of medial knee joints was likely to be more reduced in patients who responded to the treatment than in patients who did not respond to the treatment was reported by evaluation of pressure pain thresholds (PPTs) using a handheld algometer [Citation17]. PPTs are validated, reliable, and widely used as an additional objective method for assessing pain sensitization, involving the quantification of a patient’s response to a standardized pressure stimulation of nociceptors in patients with knee OA [Citation5,Citation18,Citation19]. We therefore hypothesized that FUS treatment at sites of severe tenderness caused by peripheral pain sensitization, rather than the tibial plateau, would reduce pain sensitization and relieve refractory chronic knee pain.
The purpose of this study was to elucidate the therapeutic efficacy and safety of MRgFUS for the treatment of refractory pain derived from the medial knee OA in a prospective, noncontrolled manner.
Materials and methods
This study (Clinical Trial ID: UMIN000010193) was conducted in accordance with the international standards for clinical trials using a prospective, noncontrolled design. The study protocol was approved by the institutional review board at Kochi Medical School, Kochi University. All patients provided written, informed consent before entry into the study and were volunteers who did not receive other treatment options.
Patient selection
Patients suffering from refractory medial knee pain who had been diagnosed radiographically with knee OA who had been referred with indications for surgical treatment to Kochi University Hospital from May 2013 to May 2016 were recruited to this study.
Inclusion criteria that patients had to meet in order to participate in this study were as follows: age 60 years or older; chronic medial knee pain refractory to other conservative treatments (e.g., physical therapy performed by a physical therapist, regular use of acetaminophen or nonsteroidal anti-inflammatory drugs, and intra-articular injection with corticosteroids or hyaluronic acid) for more than 6 months; worst numerical rating scale (NRS) pain score more than 4; medial knee OA of grade 3 or 4 on the Kellgren-Lawrence classification [Citation20]; the presence of tenderness at the medial knee joint; target area clearly visible on noncontrast magnetic resonance image (MRI); and accessible to a focused ultrasound device.
Exclusion criteria were as follows: patients with other knee diseases such as rheumatoid arthritis, infection or both fresh and previous fracture; patients with lateral knee pain or with locking of knee; cortical bone surface of the targeted site less than 10 mm from the skin; patients receiving dialysis, having unstable cardiac status, severe hypertension, active uncontrolled diseases such as infection, hematological diseases, neurological diseases, severe neurovascular diseases, severe coagulation disorders or using anticoagulant/platelet drugs; patients unable to express subjective pain during treatment; patients having contraindications for MRI due to implanted metallic devices, claustrophobia or obesity. Patients with severe underlying medical conditions were also excluded because of expected difficulty with more than one-year follow-up assessment and to avoid the occurrence of additional other pathological medical problems.
Patients who participated in this study were initially assessed for eligibility in the following manner. In advance, conventional conservative therapies that had not yet been performed for the knee pain were attempted. Local anesthetic injection was performed at the site of tenderness, the planned target of the focused ultrasound energy delivery (so-called sonication), to exclude the pain-alleviating effects of local anesthesia during treatment. After these conservative treatments, cases showing no improvement were enrolled and evaluated for clinical outcome measures and medical conditions.
All patients continued the same conservative treatments that they were receiving at the time of providing informed consent, to reflect actual clinical conditions. If acute exacerbation of knee pain occurred due to any reason, use of additional painkillers except injection materials was permitted, depending on the situation. However, these additional treatments were restricted to being performed within 48 h before visiting the clinic for post-treatment assessment. After the planned MRgFUS treatment was finished, patients were allowed to receive surgical treatment such as TKA freely. Cases that received any other surgical treatments, had a serious illness, or required hospitalization for some reason were not included in the analysis.
MRgFUS treatment
All interventions were conducted in the outpatient clinic at Kochi University Hospital, using the MRgFUS system (ExAblate® 2100 conformal bone system; InSightec, Haifa, Israel) under MRI scanner guidance (Signa EXCITE 3.0-T MRI; GE Healthcare, Milwaukee, WI, USA). In this series, the medial knee joint space and the medial tibial condyle of the enrolled patient were carefully pressed from the ventral side to the dorsal side with the examiner's (MK) finger to search the site where the patient felt severe pain. Then, we defined the site of the lowest PPT with measurement of a handheld algometer as the site of most severe tenderness. In advance, MRI was performed by attaching a detectable marker to that site. MRgFUS treatment was planned within approximately 2 cm radius area centered on the bone surface just below the marker on MRI as the target area of sonication.
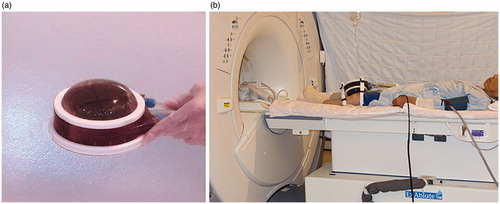
Patients underwent local anesthesia with 10 ml of 0.75% ropivacaine around the periosteum at the target area and were positioned supine on the MRI table. No sedation was used throughout the treatment. The conformal sonication transducer has a semipermeable membrane-covered ball that circulates cold degassed water on the irradiated side to cool the skin during treatment. That transducer was firmly secured to the medial side of the knee with a strap so that the center of that ball was above the site of tenderness ().
Figure 1. (a) Conformal sonication transducer with semipermeable membrane-covered ball. (b) Patient with transducer secured to the medial side of the left knee with a strap.
Coronal, sagittal, and axial unenhanced T2-weighted MR images were obtained and loaded into the MRgFUS workstation to allow accurate three-dimensional planning and targeting of the lesion. At this time, the position of the transducer was adjusted so that the center of the transducer was located at the site of most severe tenderness. If the location proved unsuitable, the procedure for confirmation of a suitable transducer position by MRI was repeated, while changing the placement position.
The outline of the bone surface as well as skin and the target area to be treated were carefully drawn on the planning images in coronal and axial views. After determining the target area, several parameters, including the required energy level, number of sonications, and the direction of the pathway of the sonication, were automatically optimized. The size of one sonication on the bone surface was set to 1 cm in diameter. The ultrasound beam was angled to avoid the joint cavity and popliteal neurovascular bundle, and the sonications judged to be unnecessary for irradiation were omitted from the schedule.
Initially, a low-energy test sonication was performed to ensure the safety and accuracy of the procedure. Therapeutic sonications then began with higher energy to achieve ablation. Each sonication session lasted more than 20 s and was repeated after a cooling period of several minutes between sonications. Throughout treatment, the location of each sonication and the temperature elevation in the tissue adjacent to the target area were monitored in real time, using proton resonance frequency shift thermometry [Citation21,Citation22] (). The goal temperature of the bone surface in the target area was 55 °C, and treatment parameters such as energy, duration and direction of sonication, or spot size were modified in response to the monitoring, with additional sonications added as needed (). Patients were allowed to interrupt the sonication at any time by pushing the stop button if they felt pain associated with sonication. Investigators interrupted sonication on an external console if the tissue temperature around the treatment site obviously exceeded 60 °C during the procedure.
Figure 2. Real-time monitoring on the MRgFUS console display demonstrating sonication of 770 J (27 W, 28 s). Current sonication spot (green circle) and ultrasound beam pathway (light blue area) are shown on the axial-view of MRI.
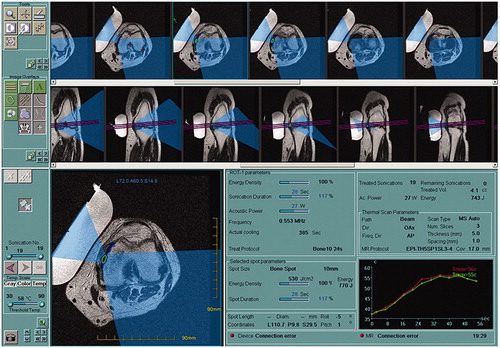
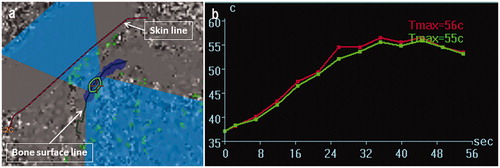
Figure 3. (a) The thermal evaluation screen displaying the results of the last performed sonication. The operator can confirm the results of individual sonication sessions after each sonication or after a series of the treatments. Note the successful previous sonication area is mapped in the dark blue. (b) Temperature elevation is monitored by two curves indicated mean (green) and maximum (red) temperature at the area of the cross cursor in the left screen.
Clinical assessments
Numerical rating scale (NRS) pain scores, Western Ontario and McMaster Universities osteoarthritis index (WOMAC) score [Citation23], and EuroQol 5 Dimension (EQ-5D) index [Citation24] were utilized as patient-reported outcome measures to confirm clinical therapeutic effects. Furthermore, PPT was measured to evaluate the local therapeutic effect on pain at the treatment site.
NRS pain scores to evaluate the intensity of knee pain are most commonly scored on a scale of 0 to 10, with 0 being ‘no pain’ and 10 being ‘the worst pain imaginable’. Peak pain intensity in the affected knee during the previous 24 h was measured at each assessment visit using a 0–10 NRS. Response to treatment was defined as a 50% or greater decrease in NRS score, as recommended by the Outcome Measures in Rheumatology Clinical Trials and Osteoarthritis Research Society International (OMERACT-OARSI) [Citation25]. The percentage of responders was calculated at each assessment point after treatment. The WOMAC is a disease-specific purpose-built instrument to evaluate the self-reported health status of patients with knee OA. WOMAC has three discrete domains: pain (five questions, subscale score 0–20); stiffness (two questions, 0–8); and physical function (17 questions, 0–68). The minimum score is 0 (best score) and the maximum score is 96 (worst score). The EQ-5D index is a generic instrument for describing and valuing health-related quality of life states, consisting of the five dimensions of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Responses obtained for the EQ-5D index were converted to EQ-5D scores based on the Japanese value sets, indicated on a scale from 0 (Dead) to 1.0 (Full health). Among the enrolled patients who allowed us to evaluate local therapeutic effects, PPTs were assessed at target sites with the most severe tenderness using a handheld algometer (Commander; J Tech Medical Industries, Heber City, UT, USA) with a 1-cm2 rubber probe. The PPT was defined as the first point at which the patient perceived the pressure as slight pain. PPTs were measured four times by a single examiner (MK) at each evaluation point, and the average of the two values remaining after excluding the maximum and minimum values was used for analysis. With reference to the anatomical landmarks, measurement sites were located at the same site at all assessment visits before and after treatment. Prior to the pretreatment assessment, high intra-rater reliability was confirmed in each patient.
All clinical outcome measures and medical conditions were assessed in the outpatient clinic at pretreatment (baseline), and 1 week, and 1, 3, 6, and 12 months after treatment. Twelve months later, a five-year follow-up was conducted to confirm the condition of the patient. Worst NRS scores were also investigated at final follow-up.
Radiographical assessments
To evaluate the safety of treatment, all complications and adverse events were assessed and reported during patient visits. Radiological assessments were performed at pretreatment, and 3 months, 6 months and 12 months post-treatment by X-ray, plain MRI, and CT. On MRI and CT, changes in image signals at the sonication site were investigated at each assessment point. On the frontal-plane X-ray, femorotibial angle (FTA), representing the intersection of the anatomical axes of the femur and tibia [Citation26], was compared before and 12 months after treatment.
Statistical analysis
Demographic data and clinical variables are presented as the mean, standard deviation, range, and percent in the text and figures. Friedman test, followed by Dunn’s post hoc test, was used to evaluate the time course of worst NRS pain scores, WOMAC scores, EQ-5D scores, and PPT at every assessment point. Wilcoxon signed-rank test was used for the comparison of differences between pre- and post-treatment femorotibial angle. Significant differences were recognized at the level of p < 0.05. Statistical analyses were conducted using GraphPad Prism version 7 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Clinical characteristics
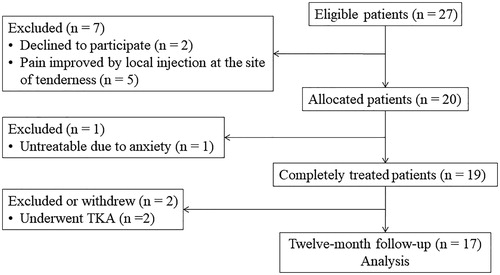
Twenty-seven patients met the eligibility to participate in this study (). Of these, five patients who showed improvement of medial knee pain with local anesthetic injection and two patients who needed treatment for other illnesses were excluded. Finally, 20 patients (15 women, 5 men) with a mean age of 78 ± 6 years (range, 60–88 years) were enrolled in this study, all of whom complained of the worst knee pain on standing-up and walking. Mean duration of hospital visit for knee pain was 71 ± 48 months (range: 9–122 months). Mean body mass index was 25.4 ± 3.9 kg/m2. Fifteen patients were diagnosed as OA grade IV and five as OA grade III, according to the Kellgren–Lawrence classification.
Of the remaining 20 patients, 19 completed a single procedure of MRgFUS treatment for medial knee pain. The remaining patient withdrew from the study before treatment due to anxiety, and was thus excluded from analysis of therapeutic effects. Of the remaining 19 patients, two underwent TKA because pain relief within 3 months after treatment was unsatisfactory. Seventeen patients completed all assessments of NRS pain scores, WOMAC scores, and EQ-5D scores until 12 months. Mean follow-up for NRS scores was 34 ± 13 months (range: 18–59 months) after treatment. Withdrawal factors at final follow-up were hospitalization for treatment of cancer in three patients, osteoporotic fracture in three, and other medical disorders in five. Fifteen of the 19 patients were able to complete all measurements of PPT at the sites of most severe tenderness.
Settings for MRgFUS treatment in this study were as follows: mean setup time for preparing the system before treatment, 62 ± 16 min (range: 45–105 min); and mean FUS treatment time, 59 ± 19 min (range: 27–97 min). Mean number of sonications was 16.0 ± 3.8 (range: 10–23) per patient and mean sonication energy was 729 ± 215 J (range: 288–1356 J) per sonication. Of the 19 patients who completed treatment, eight patients used the stop button due to sonication pain; of these, five patients used it once, and the remaining three used it 4–6 times.
Clinical assessments
Regarding the safety of MRgFUS, no serious treatment-related complications such as skin burns, bleeding, sensory disturbance, neuropathy or fractures were encountered. As minor complications, sonication pain was observed in eight patients (42.1%) and transient exacerbation of pain for several days after treatment was reported by six patients (31.6%). In the former cases, by interrupting sonication with the stop button, the scheduled treatment could be completed without any other complications. In the latter cases, additional administration of oral acetaminophen alleviated the pain.
presents the analyses of clinical outcome data. According to OMERACT-OARSI, responders among the 19 patients who completed treatment with MRgFUS comprised 13 patients (68.4%) at 3 and 6 months, and 14 patients (73.7%) at 1 and 12 months after treatment. Mean worst NRS pain scores in 17 patients (excluding 2 patients who underwent TKA) showed a significant decrease from 1 month, continuing to decline at 12 months, and remaining significantly lower at final follow-up (mean, 3.2 ± 1.9; p = 0.0013) compared to baseline. A peak reduction in mean worst NRS pain score of 4.3 (59.3%) was observed at 12 months, while peak reduction in mean worst NRS pain score was 5.0 (67.0%) in 14 responders at that time. During the one-year follow-up period, five patients had an NRS pain score of 0 for knee pain, one of whom reported that knee pain had completely disappeared. As a result, two of 17 patients were able to reduce their pretreatment medications and 6 did not necessarily require intra-articular injections for knee pain. Mean WOMAC scores, similar to worst NRS pain scores, improved significantly from 1 month after treatment. In particular, in the subscale evaluation, significant improvements were observed not only in the pain subscale, but also in the stiffness subscale and the physical function subscale. Mean EQ-5D scores also showed significant improvements from 1 month after treatment, but no significant difference was apparent at 12 months. Of the 15 patients for whom PPT was measured, nine showed the lowest PPT at the level of the joint space and six at the level of the proximal medial tibial condyle. In all 15 patients, the planned sonications were performed on the site of most severe tenderness. Mean PPTs of the treated sites increased from 153 ± 44 kPa at baseline to 248 ± 65 kPa (p = 0.0031) at 1 month, and remained with a significant increase of 264 ± 74 kPa (p = 0.0003) at 12 months. All patients evaluated showed elevated PPT, regardless of changes in knee pain ().
Figure 5. Mean pressure pain thresholds inside the treated knee pre- (baseline) and post-treatment. Error bar represents standard deviation of the mean. *p < 0.005, †p < 0.0005 versus baseline
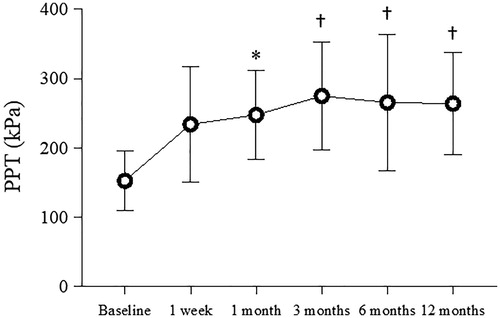
Table 1. Clinical outcomes and analysis.
Radiographical assessments
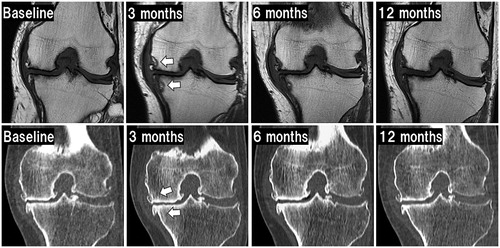
Radiological findings similar to were observed at the sonication sites on MRI in all 17 patients at 3 months and tended to decrease from 6 months post-treatment. T2 fat saturation on MRI post-treatment showed no high-intensity areas on the ablated cortex bone. Osteosclerotic reactions appeared in 8 (47.1%) of 17 patients on CT. Despite changes in MRI and CT findings, no radiological findings of fracture or segmental collapse of the treated bone, OA progression, osteonecrotic change, or changes in alignment of the affected lower extremity were seen during follow-up. Mean FTAs did not differ significantly between 185 ± 5° pretreatment and 185 ± 6° at 12 months post-treatment.
Figure 6. Radiological changes after treatment. Images in the upper row show T1-weighted MRI. Images in the lower row show CT pretreatment (baseline) and post-treatment. Arrows on MRI indicate low intensity changes at the margin of the sonication site. These image findings gradually diminished in this case. Arrows on CT indicate slight osteosclerotic changes at the sonication site at 3 months or later.
Discussion
This study is the first report to examine the efficacy and safety of MRgFUS for refractory chronic pain in medial knee OA. A long-lasting and significant improvement of physical function was seen according to WOMAC scores and NRS scores of worst knee pain during more than 12 months from early post-treatment without major complications, despite being restricted to elderly patients with severe knee OA eligible for TKA.
The early pain-relieving effect of FUS treatment was similar to previous reports of bone metastases, lumbar facet joint OA, or osteoid osteoma [Citation14,Citation15]. The mechanism of this pain alleviation in the early stage is most likely to involve local degeneration of nociceptors and nerve fibers caused by the thermal denaturation of the sonication area. Protein denaturation occurs when the temperature reaches over 55 °C for a few seconds [Citation15,Citation27–30] and that was the target temperature in this study. Heating these peri-articular tissues of the medial femorotibial joint beyond the threshold of protein denaturation is considered to result in localized denervation of the rich neural network causing peripheral pain sensitization. However, because evaluation of thermal denaturation of nerve terminals following irradiation is difficult, PPTs widely used for assessing peripheral pain sensitization were used as a substitute for the evaluation of nerve ending degeneration [Citation5,Citation18,Citation19]. In all enrolled patients, lower PPTs were observed on the site of most severe tenderness at pretreatment, and this is also a general finding in OA knees [Citation31]. After sonications to the bone surface just below and around that site, PPTs on the site of most severe tenderness were markedly increased in all patients, meaning that patients felt less pain by pressure stimulation, since localized denervation was obtained on sonicated areas. As a result, the response rate at which refractory knee pain was reduced by 50% or more remained at 73.7%, even at 12 months after treatment, and PPT also remained elevated. This proves that localized thermal denervation of sites where the PPT was lowest, that is, sites that were the most sensitive to pressure stimulation, can reduce refractory pain of medial knee OA in a minimally invasive manner over the long term. The site of most severe tenderness thus seems to represent the preferred target for FUS treatment. Furthermore, the response rate in this study tended to be higher than in our previous reports. The reason might be that most patients with the lowest PPT located at the medial knee joint in this study were irradiated with sonications mainly on osteophytes of both the medial femoral condyle and medial tibial plateau, whereas the main therapeutic target in the previous report was osteophytes of only the medial tibial plateau, even if severe tenderness was present at the level of the knee joint, based on a previous report that sensory nerve invasion containing substance P and calcitonin gene-related peptide was seen in tibial osteophytes of OA patients [Citation32]. Pathological structures such as osteophytes and joint space narrowing are thought to have little to do with OA pain [Citation1,Citation4,Citation33]. However, the periosteum and synovium surrounding the osteophytes may be responsible for the sensitization of nociceptive nerve terminals. Therefore, when severe tenderness is present at the level of the knee joint, irradiating both femoral and tibial osteophytes may offer effective inhibition of the peripheral pain sensitization involved in refractory knee pain.
Radiofrequency denervation for OA knee pain has been reported based on principles similar to thermal ablation [Citation34]. Compared with radiofrequency (RF) denervation, MRgFUS treatment shows some advantages. Closed-loop, real-time spatial and thermal monitoring allow safer and more accurate sonications to the target area of the medial knee joint without the hypesthesia often observed in RF denervation when the most peripheral terminals of the sensory nerve are ablated. Moreover, the efficacy of RF denervation is highly technique-dependent, because identifying target nerves for relieving knee pain is difficult. In fact, the therapeutic results of RF denervation conducted at our facility were lower and more short-term than the results of MRgFUS treatment [Citation35], similar to other reports of RF denervation for OA knee pain [Citation34]. On the other hand, some disadvantages include the huge initial cost of MRgFUS systems and the long time required for treatment, in comparison to RF denervation techniques. In addition, patients with contraindications for MRI, such as claustrophobia and anxiety-related symptoms, cannot undergo the treatment.
We believe that the long-term analgesic effects on chronic knee pain are important in combination with thermal denervation of FUS treatment and subsequent nonpharmacological therapies including physical and exercise therapy. Although these therapies are well known as core treatments that are effective and safe for all patients with knee OA [Citation8], severe knee pain with peripheral sensitization of knee OA suppresses the physical activity of the patient and often makes these core treatments difficult to carry out. In fact, patients enrolled in this study had also reduced physical activity because they were refractory to conventional pharmacological treatments such as oral non-steroidal anti-inflammatory drugs and intra-articular steroid injections. Also, in the elderly, long-term use of pharmacological treatments is likely to result in systemic toxicity [Citation8,Citation9]. Among the patients in this study, MRgFUS treatment with the goal of thermal denervation of the neural network causing peripheral pain sensitization appears to have resulted in improved physical function and quality of life. This is because compliance with physical and exercise therapy was improved in responders. This treatment thus has the potential as an innovative, alternative, nonsurgical treatment for pain relief and maintenance of physical function among patients with knee OA if conventional pharmacological treatments prove ineffective.
Regarding the safety of MRgFUS treatment, this modality appears desirable as an outpatient non-surgical intervention because of the absence of serious adverse events related to the treatment and the short treatment time. As minor complications, sonication pain during treatment and temporary exacerbation of knee pain for several days after treatment were observed. Sonication pain is the most common complaint associated with this treatment for any disease [Citation36]. Although general anesthesia, epidural anesthesia, intravenous opioids and sedation can be beneficial when alleviating sonication pain, the use of those forms of anesthesia may cause serious tissue damage at the sonication area and disrupt patient feedback on pain. To avoid serious thermal tissue damage in this study, patients were allowed to press a button to interrupt sonication if they perceived pain. In addition, the operator interrupted a sonication at the console if the temperature rose excessively during real-time monitoring. Only the use of local anesthesia with ropivacaine on the periosteum and soft tissues above the bone in the target area, without intravenous opioids or sedation, was sufficient to allow completion of all scheduled sonications.
Changes in signal intensity occurred in all cases at the treatment site on MRI. These phenomena are likely due to the thermal and mechanical (nonthermal) effects of focused ultrasound [Citation37]. Ultrasound energy is generally absorbed by cortical bone, and the increase in local temperature generated by the ultrasound causes thermal tissue damage. In fact, in the present study, the occurrence of a bone marrow lesion (BML) on the bone surface layer at the irradiation site was confirmed in addition to soft tissue on the bone surface. This likely contributed to the temporary enhancement of pain immediately after treatment; significant improvement in pain tended to be delayed in FUS treatment for knee OA compared with other diseases. Fortunately, this BML reduced in size on MRI over time. In a previous study, we reported no bone necrosis in a histopathological evaluation of irradiated bone in two patients who underwent TKA following FUS [Citation16]. In the present study, we set the target temperature of 55 °C during sonication lower than the target temperature of 60 °C in the previous study, which may have further limited the damage to bone marrow. Thermal denaturation of nerve endings causing peripheral sensitization due to an increase in the temperature of tissue only on the bone surface at the site of tenderness should be ideal. However, the transducer of the ExAblate 2100 Conformal Bone System was a better fit for the extremities and was preferable for FUS treatment of knee OA compared with that of the ExAblate 2000 system. On the other hand, the ExAblate 2100 system has a frequency half as low as that of the ExAblate 2000 system, meaning that even with low-intensity ultrasonic irradiation, the ultrasonic waves easily penetrate deep into the knee bone and are more likely to affect the bone marrow on the bone surface. Nevertheless, the fact that hardly any changes were observed in bone marrow directly below the osteophytes suggests that ultrasonic energy had less effect on bone marrow, because osteophytes absorb ultrasonic energy more than the surrounding bone. This implies that irradiation targeting osteophytes is effective in terms of safety as well as pain alleviation. Interestingly, slight osteosclerotic changes at the sonication area, similar to bone formation after the treatment of bone metastases [Citation28,Citation36], were observed in 7 of the 19 patients on CT. Although the mechanisms underlying osteosclerotic change in the treated area remain unknown, non-thermal effects of FUS treatment might affect new bone formation. Further basic research of the treated bone marrow would be necessary to assess this phenomenon.
Some limitations of this study are as follows. First, this study was a prospective case series from a single institute, including a small number of patients without a control group. Hence, placebo effects of MRgFUS treatment could not be eliminated because of the impossibility of performing sham sonication utilizing the ExAblate 2100 conformal bone system. However, the elderly patients enrolled in this study revealed significant pain alleviation for a long period from the early stage after treatment on areas of severe tenderness, along with a significant increase in pressure pain threshold. In addition, this therapeutic effect also produced significant improvements in the physical activity of patients eligible for TKA. We therefore believe that MRgFUS has potential as an alternative treatment for the refractory persistent pain of medial knee OA. Second, relatively long-term follow-up after treatment confirmed the absence of treatment-related complications such as progression of joint deformity or varus deformity of the knee. However, further investigation is needed to clarify the safety of this treatment for younger individuals, as changes in MRI signal intensity were observed on the superficial layer of bone at the sonication site. Finally, since significant improvements in physical function are the result of outcomes reported by patients, further analysis of actual physical functions such as walking ability, muscle strength, and ROM is needed.
In conclusion, MRgFUS treatments for refractory pain of medial knee OA were effective not only for achieving long-lasting pain alleviation, but also for improving physical functions without any adverse events. Further, the significant and sustained increase in PPTs suggests that therapeutic sonications were involved in long-lasting suppression of peripheral pain sensitization resulting from localized thermal degeneration at the sites of most severe tenderness. MRgFUS application represents a promising and advanced nonsurgical alternative treatment for refractory chronic pain in elderly patients with medial knee OA eligible for TKA. Further improvements in FUS devices and treatment procedures need to be established for pain management in knee OA.
Acknowledgements
The authors thank Mr. Yair Bauer, Mr. Ori Atar, and Mr. Michio Morikawa for their technical support of the MRgFUS system.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973.
- Dawson J, Linsell L, Zondervan K, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology (Oxford). 2004;43(4):497–504.
- Mili F, Helmick CG, Moriarty DG. Health related quality of life among adults reporting arthritis: analysis of data from the Behavioral Risk Factor Surveillance System, US, 1996-99. J Rheumatol. 2003;30(1):160–166.
- Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(7):1043–1056.
- Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6(10):599–606.
- Grigg P, Schaible HG, Schmidt RF. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol. 1986;55(4):635–643.
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60(6):2180–2195.
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589.
- Mushtaq S, Choudhary R, Scanzello CR. Non-surgical treatment of osteoarthritis-related pain in the elderly. Curr Rev Musculoskelet Med. 2011;4(3):113–122.
- Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597–1606.
- Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435.
- Cline HE, Schenck JF, Hynynen K, et al. MR-guided focused ultrasound surgery. J Comput Assist Tomogr. 1992;16(6):956–965.
- Hynynen K, Darkazanli A, Unger E, et al. MRI-guided noninvasive ultrasound surgery. Med Phys. 1993;20(1):107–115.
- Huisman M, ter Haar G, Napoli A, et al. International consensus on use of focused ultrasound for painful bone metastases: current status and future directions. Int J Hyperthermia. 2015;31(3):251–259.
- Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain-case series of an innovative new technique. Eur Radiol. 2012;22(12):2822–2835.
- Izumi M, Ikeuchi M, Kawasaki M, et al. MR-guided focused ultrasound for the novel and innovative management of osteoarthritic knee pain. BMC Musculoskelet Disord. 2013;14(1):267.
- Namba H, Kawasaki M, Izumi M, et al. Effects of MRgFUS treatment on musculoskeletal pain: comparison between bone metastasis and chronic knee/lumbar osteoarthritis. Pain Res Manag. 2019;2019:4867904.
- Wessel J. The reliability and validity of pain threshold measurements in osteoarthritis of the knee. Scand J Rheumatol. 1995;24(4):238–242.
- Wylde V, Palmer S, Learmonth ID, et al. Test-retest reliability of Quantitative Sensory Testing in knee osteoarthritis and healthy participants. Osteoarthr Cartil. 2011;19(6):655–658.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
- Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814–823.
- Blackwell J, Krasny MJ, O'Brien A, et al. Proton resonance frequency shift thermometry: a review of modern clinical practices. J Magn Reson Imaging. 2020. DOI:10.1002/jmri.27446
- Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840.
- EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
- Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil. 2004;12(5):389–399.
- Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56(4):1204–1211.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294.
- Gianfelice D, Gupta C, Kucharczyk W, et al. Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound. Radiology. 2008;249(1):355–363.
- Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2002;8 (Suppl 1):S100–S12.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800.
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049.
- Suri S, Gill SE, Massena de Camin S, et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–1428.
- Arendt-Nielsen L. Joint pain: more to it than just structural damage? Pain. 2017;158 Suppl 1:S66–S73.
- Jamison DE, Cohen SP. Radiofrequency techniques to treat chronic knee pain: a comprehensive review of anatomy, effectiveness, treatment parameters, and patient selection. J Pain Res. 2018;11:1879–1888.
- Ikeuchi M, Ushida T, Izumi M, et al. Percutaneous radiofrequency treatment for refractory anteromedial pain of osteoarthritic knees. Pain Med. 2011;12(4):546–551.
- Rodrigues DB, Stauffer PR, Vrba D, et al. Focused ultrasound for treatment of bone tumours. Int J Hyperthermia. 2015;31(3):260–271.
- Furusawa Y, Hassan MA, Zhao QL, et al. Effects of therapeutic ultrasound on the nucleus and genomic DNA. Ultrason Sonochem. 2014;21(6):2061–2068.