Abstract
Objectives
To construct a prognostic nomogram to predict the involution of the ablation zone for patients with low-risk papillary thyroid carcinoma (PTC) who underwent radiofrequency ablation (RFA) treatment.
Methods
Data from 204 patients with low-risk PTC without extrathyroidal extension or cervical lymph node or distant metastasis who underwent RFA treatment were collected from January 2018 to January 2019. Clinicopathological and imaging characteristics were analyzed. The prognostic factors associated with the involution of the ablation zone within 12 months after RFA were identified by logistic analysis, and the nomogram was established. Calibration curve and decision curve analysis were used to evaluate the nomogram performance.
Results
Of the 204 patients included in this study, the ablation zone in 78 (38%) patients did not completely disappear in the 12 months after RFA. Four variables, including sex (odds ratio [OR], 3.303; 95% confidence interval [CI], 1.418–8.418; p = 0.008), age (OR, 1.045; 95% CI, 1.012–1.081; p = 0.009), calcification size (OR, 1.666; 95% CI, 1.041–2.701; p = 0.035), and RFA energy (OR, 2.902; 95% CI, 1.333–6.683; p = 0.009), were found to be closely associated with ablation zone non-disappearance at 12 months after RFA by multivariate analysis. A nomogram model was constructed, and its accuracy was well validated (C-index = 0.787).
Conclusions
This study constructed and validated a risk model that could accurately predict the involution of the ablation zone after RFA for patients with PTC. This could provide clinicians with useful resource to guide patient counseling regarding tumor prognosis after RFA.
Introduction
Papillary thyroid carcinoma (PTC) or follicular epithelial cell-derived differentiated thyroid carcinoma, accounts for more than 90% of thyroid malignancies but has a mortality rate of less than 1% [Citation1–4]. Thyroidectomy is recommended as the first-line treatment method for PTC [Citation5,Citation6]. However, for low-risk patients (defined as having intrathyroidal PTC with no evidence of extrathyroidal extension, vascular invasion, or metastases) [Citation3], the necessity of thyroidectomy has become a focus of controversy both in China and abroad [Citation7,Citation8]. Active surveillance for low-risk papillary thyroid microcarcinoma (PTMC) was mentioned in the 2015 American Thyroid Association guidelines (ATA, 2015). However, it is still unclear which patients are suitable for long-term active surveillance and how to standardize the follow-up intervals and clinical indicators. Considering the rapidly increasing incidence of PTC in recent years worldwide [Citation8–11] and the clinical application of new treatments for this disease, researchers have investigated long-term outcomes of thermal ablation techniques [Citation12–16].
Radiofrequency ablation (RFA) is a heat-based percutaneous ablation technique that is most commonly used to treat benign thyroid nodules, recurrent thyroid cancers, and PTMC [Citation12,Citation17–22]. Accumulating evidence suggests that RFA has efficacy and safety similar to those of surgery for the treatment of primary and recurrent PTC.
Recently, a systematic review of the efficacy and safety of thermal ablation for treating primary PTMC reported a pooled rate of complete disappearance after treatment of 57% (nearly half) of the ablation zone [Citation12], they did not disappear during the mean follow-up period of 21.1 months. However, there have been only a few studies predicting the involution of the ablation zone after RFA treatment for patients with PTC. Therefore, this study aimed to construct a prognostic nomogram model to provide clinical evidence for physicians to predict the involution of the ablation zone after RFA in patients with low-risk PTC.
Materials and methods
Patients
This retrospective study was approved by the ethics committee of the First Medical Center of Chinese PLA General Hospital, and the requirement for patient consent was waived. Patients with low-risk PTC who had received RFA from January 2018 to January 2019, performed by the same physician (Y. K. L.) were eligible for the study. The physician had more than 20 years of clinical experience in the diagnosis and treatment of thyroid diseases in our center. RFA was performed with a bipolar generator (CelonLabPOWER; Olympus Surgical Technologies Europe, Hamburg, Germany) and an 18-gauge bipolar RF applicator with a 0.9 cm or 1.5 cm active tip (CelonProSurge micro 100-T09 or 100-T15, Olympus Surgical Technologies Europe, Hamburg, Germany).
The inclusion criteria were as follows: (1) conventional PTC confirmed by CNB; (2) all lesions ≤ 4 cm; (3) patient age ≥18 years; and (4) availability of clinical data, including tumor response evaluation and survival data. Patients were excluded for the following reasons: (1) history of thyroid surgery or radiotherapy; (2) history of other malignant tumors; (3) severe cardiopulmonary disease, coagulation disorder, or systemic infection; (4) evidence of extrathyroidal extension or cervical LN metastasis or distant metastases on ultrasound; and (5) a follow-up time less than 12 months. Patients were divided into two groups according to their ablation zone status at 12 months after RFA.
Data collection
Clinical details including patient demographic information (age and sex), nodule characteristics, RFA parameters, and follow-up data were collated for each patient. The ultrasound imaging characteristics of each nodule referred to its location, size, aspect ratio, echo, composition, calcification, and blood flow distribution. According to the anatomical structure of the thyroid, the tumor location was recorded as left lobe, right lobe, or isthmus. The largest diameter was recorded as the tumor size. Aspect ratio of tumors with a greater height than width was classified as >1, and otherwise as ≤1. Calcification was divided into four categories: absent, punctate calcification (<1 mm), plaque calcification (1–2 mm), and large calcification (>2 mm). Blood flow pattern was categorized into four levels according to the Adler blood flow grading system [Citation23]: absent, 1–2 punctate or short blood flow, 3–4 punctate or 1-vessel blood flow, and more than 1 colored blood flow. RFA parameters included output power, duration, and energy. Follow-up data included complications, volume reduction rate (VRR) at each interval, and disease progression. Complications included pain, hematoma, and hoarseness, recorded in detail with degree and duration. VRR was calculated as VRR = ([initial volume − final volume] × 100%)/initial volume. Disease progression was defined as local tumor progression, local tumor recurrence, contralateral newly growth tumor, or lymph node or distant metastases.
Statistical analysis
A binary variable was designated to represent ablation zone disappearance at 12 months after RFA. The ablation zone disappearance within 12 months was assigned a value of 0, whereas the ablation zone non-disappearance was assigned a value of 1. Univariate unconditional logistic regression was used to assess the association of different covariates with 12-month ablation zone status. Statistical significance was then evaluated using the Wald test (p < 0.1). Multivariate logistic regression models were developed with statistically significant covariates by forward and backward methods. In the construction of multivariate models, significance of each covariate was also evaluated by the Wald test (p < 0.05). According to the type of covariates (categorical or continuous), collinearity was verified using the t test, Fisher exact test, analysis of variance, or variance inflation factors.
The performance and accuracy of the nomogram were evaluated by consistency index (C-index) and calibration curve. A C-index > 0.5 indicated a positive correlation with the predictive ability of the nomogram. In addition, decision curve analysis was performed to confirm the clinical usefulness of the model compared with unique factors. SPSS version 24.0 (IBM Corp., Armonk, NY, USA) and R 4.0.3 software (http://www.r-project.org/) were used in the statistical analyses with the ‘rms’ and ‘car’ packages.
Results
Patient characteristics and treatment efficacy
The patient characteristics are presented in . A total of 204 patients (158 female and 46 male) with low-risk PTC ≤4 cm diagnosed from January 2018 to January 2019 were enrolled in this study. Of all patients, the mean age was 43.25 ± 10.47 years (range 21–72 years). The mean size of the primary tumors was 0.8 ± 0.47 cm (range 0.3–3.8 cm). According to the analysis, eventually, 126 (62%) patients experienced ablation zone disappearance within 12 months after RFA, 76 (37%) patients displayed ablation necrosis with no active tumor cells, and 2 (1%) patients displayed ablation necrosis with active tumor cells present on CNB, which were performed at 6 months or later after RFA [Citation24]. In the group where the ablation zone did not disappear, there were 10 tumors presenting with 1–2 mm calcification and 2 cases with calcification larger than 2 mm, which was higher than that in the group with complete disappearance. There was only one patient in the group with complete disappearance having local tumor recurrence after RFA. Complications were observed in one patient who experienced voice change and two that experienced moderate pain.
Table 1. Clinical characteristics.
Identification of independent prognostic factors for complete ablation zone disappearance
We performed univariate and multivariate logistic regression analyses to identify independent prognostic factors for complete ablation zone disappearance within 12 months after RFA. The results of univariate analysis showed that sex (odds ratio [OR], 2.343; 95% confidence interval [CI], 1.140–5.143; p = 0.026), age (OR, 1.026; 95% CI, 0.998–1.005; p = 0.071), tumor size (OR, 6.295; 95% CI, 2.981–14.441; p < 0.01), calcification type (OR, 1.9; 95% CI, 1.271–2.9; p < 0.01), Color Doppler flow imaging (OR, 1.405; 95% CI, 0.99–2.013; p = 0.058), RFA power (OR, 1.833; 95% CI, 1.423–2.424; p < 0.01), RFA duration (OR, 1.009; 95% CI, 1.005–1.013; p < 0.01), and RFA energy (OR, 3.717; 95% CI, 2.278–6.457; p < 0.01) were related to the ablation zone status at 12 months after RFA (). The multivariate logistic regression analysis to further assess the most closely related factors showed that sex (OR, 3.303; 95% CI, 1.418–8.418; p < 0.01), age (OR, 1.045; 95% CI, 1.012–1.081; p < 0.01), calcification type (OR, 1.666; 95% CI, 1.041–2.701; p = 0.035), and RFA energy (OR, 2.902; 95% CI, 1.333–6.683; p < 0.01) were independent factors predicting ablation zone status at 12 months after RFA ().
Table 2. Univariate analysis of ablation zone completely disappearance in training cohort.
Table 3. Multivariate analysis of ablation zone completely disappearance in training cohort.
Prognostic nomogram development
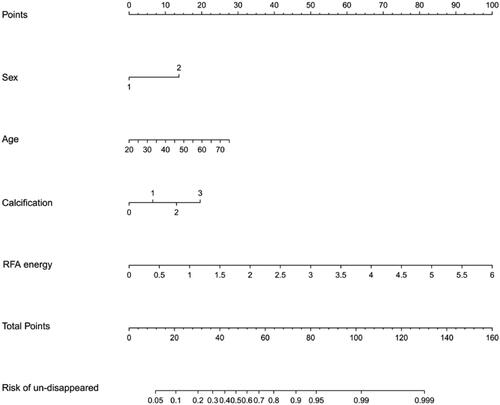
Construction of the prognostic nomogram was based on the multivariate logistic regression analysis. Four variables were selected for model construction: sex, age, calcification type, and RFA energy exposure (). Each variable that contributed to the nomogram model was quantitatively scored in the corresponding column. The total score could be calculated by adding the scores of the four variables, thus, providing the probability of complete ablation zone disappearance for each patient within 12 months after RFA.
Nomogram validation
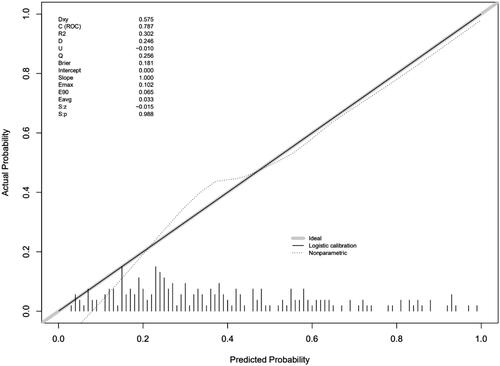
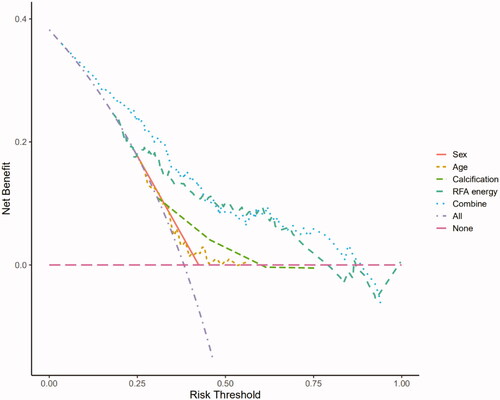
The predictive ability of the nomogram was internally verified with a satisfactory Somers’ d test of statistical significance of 0.575, and a C-index of 0.787 (). The Brier score was 0.181, and there was no significant difference in the Spiegelhalter Z score (p = 0.988). All these results showed good consistency between the calibration curve and the perfect one. In addition, the decision curve analysis showed that patients could obtain satisfactory net benefits, according to the risk assessment of the nomogram ().
Discussion
In this study, we focused on the individual differences that predicted ablation zone disappearance within 12 months. We found that sex, age, calcification type, and RFA energy were independent prognostic factors for ablation zone status within 12 months after RFA. Elderly women with macrocalcification and more energy exposure often had difficulty in completely absorbing the ablated zone in the first year after RFA.
A systematic review conducted by Choi and Jung [Citation12] of the long-term outcomes of thermal ablation in 715 patients with PTMC from 11 studies showed that the pooled estimates of VRR, tumor recurrence rate, and complication rate were 98.1, 0.4, and 3.2%, respectively. Xiao et al. [Citation14] reported the efficacy and safety of thermal ablation in 91 patients with T1bN0M0 PTC, with a good VRR of 99.0%, low tumor recurrence rate of 4.4%, and absence of complications. All these results showed that thermal ablation was an effective and safe technique for the treatment of PTC. However, the pooled proportions of complete disappearance was 57.6 and 56.0% in the studies of Choi [Citation12] and Xiao et al. [Citation14], respectively, with corresponding mean follow-up times of 21.1 months and 20.5 months. This indicated that nearly half the ablation zones remained with a post-ablation imaging that could correspond to a scar or may carry some residual tumor tissue. It is well known that tumor ablation areas seen on follow-up imaging may comprise of complete tumor necrosis [Citation25]. The ablated area including the complete treated tumor and its security margins may be sustained for years following tumor ablation, despite the size of the treated tumors. Thus, it is reasonable to expect that following 12 months since ablation of a microcarcinoma, some will ‘disappear’ on imaging, indicating complete healing. In others cases, they will ‘remain’ as a continuing scar and some of these scars will probably disappear in the future or remain visible in follow-up images [Citation24]. To the best of our knowledge, there are few studies exploring the prognostic factors that could predict complete scar ablation disappearance after thermal ablation treatment.
In the study conducted by Cho et al. [Citation17] involving the efficacy of RFA for PTMC, the complete disappearance rates at 6, 12, 24, 36, 48, and 60 months were 34.5, 74.1, 98.8, 98.8, 98.8, and 100%, respectively, showing no significant difference in the VRR changes 12 months after RFA. Similarly, a previous study conducted by Zhang et al. [Citation26] showed that the mean tumor volume and the VRR were 0 and 100% at 18 months after RFA, respectively. The data above indicated that the tumor volume and VRR after RFA in patients with PTC had changed significantly during each follow-up period within 12 months, but this change gradually became smaller or even disappeared in subsequent follow-ups.
In our research, we found the RFA energy to maximally contribute to the nomogram model. The study conducted by Trimboli and Deandrea involving the correlation between RFA parameters with VRR showed that only energy exposure was significantly correlated with the VRR of the ablation zones [Citation27]. Deandrea et al. [Citation28] performed univariate and multivariate analyses to explore the correlation of different parameters with 1-year VRR. They found that initial volume and total energy were independently associated with an effective response, defined as VRR above 50% at a 1-year follow-up. Their studies showed that high total RFA energies resulted in larger residual volume. Moreover, another study conducted by Deandrea et al. reported that the smaller the tumor, the higher was the VRR [Citation29]. In our univariate analyses, we observed that the initial volume was significantly correlated with the ablation zone status 12 months after RFA. However, considering that there was a significant relationship between the largest diameter and the initial volume, and between the initial volume and RFA energy [Citation30], we finally included the largest diameter and RFA energy for further analysis. Our results showed that only the RFA energy was closely related to the ablation zone status.
Pan et al. [Citation31] found that age and calcification were influencing factors for PTC prognosis; however, they did not further explore whether these factors influenced complete ablation zone disappearance after RFA. In our study, we found that aged patients and/or patients who had macrocalcification usually had difficulty in achieving complete ablation zone disappearance in the first year after RFA. Furthermore, it is important to note that immunogenic intracellular substrates are released from cells damaged by RFA [Citation25]. According to Weiskopf et al. [Citation32], an age-related decline in immune functions could affect the capacity of the immune system to respond to pathogens and repair damaged tissue. Macrocalcification is defined as calcifications >1 mm with posterior acoustic shadows [Citation33]. Our results showed that the larger the calcification, the less likely is the complete ablation zone disappearance in the first year. The nature of microcalcification is different from that of macrocalcification. The former is closely related to psammoma, while the latter is mainly interstitial calcification and ossification [Citation33,Citation34]. However, whether macrocalcification affected the efficacy of RFA is not clear. In addition, sex also seemed to contribute to the nomogram, in our study. In deeper analysis, we found that the mean age of women in our study was greater than that of men.
The present study had some limitations. First, it had a retrospective design and lacked external verification. Second, some critical prognostic factors such as immune-related laboratory test results could not be retrieved. It is necessary to collect these laboratory data for further investigation.
In conclusion, our study was the first to construct a nomogram for predicting the complete disappearance of PTC within 12 months after RFA, with reasonably good predictive performance. The establishment of this nomogram could help clinicians predict the involution of the ablation zone after RFA treatment more precise and in a personalized way, which would lead to better patient counseling regarding tumor prognosis after RFA.
Ethical approval
This study was conducted with approval from the Ethics Committee of the Chinese People’s Liberation Army General Hospital. The scientific guarantor of this publication is Yukun Luo.
Disclosure statement
No potential conflict of interest was reported by the author(s). All authors declare that they have no relationship with any companies whose products or services may be related to the subject matter of the study.
Data availability statement
The raw data supporting the conclusions of this study are not publicly available but are available from the corresponding author with reasonable request.
Additional information
Funding
References
- Kitahara CS, Brenner AV. Thyroid cancer. New York: Oxford University Press; 2018.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Gabrichidze T, McHedlishvili I, Zhizhilashvili A, et al. Temporal trends of cervical cancer mortality in Georgia, 2011–2018. Georgian Med News. 2020;309:17–21.
- Alobuia W, Gillis A, Kebebew E. Contemporary management of anaplastic thyroid cancer. Curr Treat Options Oncol. 2020; 21(10):78.
- Haddad RI, Nasr C, Bischoff L, et al. NCCN guidelines insights: thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw. 2018;16(12):1429–1440.
- Roman BR, Brito JP, Saucke MC, et al. National survey of endocrinologists and surgeons regarding active surveillance for low-risk papillary thyroid cancer. Endocr Pract. 2021;27(1):1–7.
- Solis-Pazmino P, Salazar-Vega J, Lincango-Naranjo E, et al. Thyroid cancer overdiagnosis and overtreatment: a cross-sectional study at a thyroid cancer referral center in Ecuador. BMC Cancer. 2021;21(1):42.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653.
- Sanabria A, Kowalski LP, Shah JP, et al. Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck. 2018;40(4):855–866.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Yan L, Lan Y, Xiao J, et al. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol. 2021;31(2):685–694.
- Xiao J, Zhang Y, Zhang M, et al. Ultrasonography-guided radiofrequency ablation vs. surgery for the treatment of solitary T1bN0M0 papillary thyroid carcinoma: a comparative study. Clin Endocrinol. 2021;94(4):684–691.
- Sanabria A. Experience with active surveillance of thyroid low-risk carcinoma in a developing country. Thyroid. 2020;30(7):985–991.
- Hegedus L, Frasoldati A, Negro R, et al. European thyroid association survey on use of minimally invasive techniques for thyroid nodules. Eur Thyroid J. 2020;9(4):194–204.
- Cho SJ, Baek SM, Lim HK, et al. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid. 2020;30(12):1745–1751.
- Lin W-C, Kan N-N, Chen H-L, et al. Efficacy and safety of single-session radiofrequency ablation for benign thyroid nodules of different sizes: a retrospective study. Int J Hyperthermia. 2020;37(1):1082–1089.
- Chung SR, Baek JH, Choi YJ, et al. Efficacy of radiofrequency ablation for recurrent thyroid cancer invading the airways. Eur Radiol. 2021;31(4):2153–2160.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897–4903.
- Kong QF, Lv B, Wang B, et al. Association of von Willebrand factor (vWF) expression with lymph node metastasis and hemodynamics in papillary thyroid carcinoma. Eur Rev Med Pharmacol Sci. 2020;24(5):2564–2571.
- Yan L, Luo Y, Zhang Y, et al. The clinical application of Core-Needle biopsy after radiofrequency ablation for low-risk papillary thyroid microcarcinoma: a large cohort of 202 patients study. J Cancer. 2020;11(18):5257–5263.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Trimboli P, Deandrea M. Treating thyroid nodules by radiofrequency: is the delivered energy correlated with the volume reduction rate? A pilot study. Endocrine. 2020;69(3):682–687.
- Deandrea M, Trimboli P, Mormile A, et al. Determining an energy threshold for optimal volume reduction of benign thyroid nodules treated by radiofrequency ablation. Eur Radiol. 2021;31(7):5189–5197.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Heerink WJ, Solouki AM, Vliegenthart R, et al. The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol. 2018;28(8):3228–3236.
- Pan Q, Yuan T, Ding Q. Clinical value of matrix metalloproteinase-2 and -9 in ultrasound-guided radiofrequency ablation treatment for papillary thyroid carcinoma. J Int Med Res. 2020;48(8):300060520917581.
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22(11):1041–1050.
- Ferreira LB, Gimba E, Vinagre J, et al. Molecular aspects of thyroid calcification. Int J Mol Sci. 2020;21(20):7718.
- Ha J, Lee J, Jo K, et al. Calcification patterns in papillary thyroid carcinoma are associated with changes in thyroid hormones and coronary artery calcification. J Clin Med. 2018;7(8):183.