Abstract
Objectives
To evaluate the incidence, risk factors and clinical significance of four types of tumor progression (TP) after microwave ablation (MWA) of single hepatocellular carcinoma (HCC) of <5 cm.
Methods
The data of 340 treatment-naïve, HCC patients with a single HCC of <5 cm underwent MWA between April 2012 and November 2017 were retrospectively reviewed. TPs including local tumor progression (LTP), intrahepatic distant recurrence (IDR), aggressive intrasegmental recurrence (AIR) and extrahepatic distant recurrence (EDR) were reviewed and compared between BCLC stage 0 and A. Univariate and multivariate analysis were performed on clinicopathological variables and different TPs to identify factors affecting long-term overall survival (OS).
Results
In a median follow-up period of 25.6 months (range, 3.1–61.4 months), the rate of LTP, IDR, AIR and EDR was 6.2% (21/340), 29.1% (98/340), 3.2% (11/340) and 7.9% (27/340). The four types of TP occurrence rates in BCLC stage 0 were comparable to those in BCLC stage A (p = 0.492, 0.971, 0.681 and 0.219). Univariate analysis showed that age (p < 0.001, hazard ratio [HR] = 2.783), comorbidities (p = 0.042, HR = 1.864), IDR, AIR and EDR (p = 0.027, HR = 1.719; p = 0.001, HR = 3.628; p = 0.009, HR = 2.638) were independently associated with OS. Multivariate analysis showed older age (p < 0.001, HR = 2.478), the occurrence of AIR (p < 0.001, HR = 2.648) and the occurrence of EDR (p = 0.002, HR = 2.222), were associated with poor OS.
Conclusions
The occurrence rate of IDR is the highest of all TPs following MWA of a single HCC of <5cm. Old age, AIR and EDR had an adverse effect on long-term OS.
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy, ranking sixth among the most common cancers and third among the leading cause of cancer-related mortalities worldwide. Both morbidity and mortality from HCC are continuously increasing [Citation1–3]. Treatment for HCC varies based on the Barcelona Clinic Liver Cancer (BCLC) staging, with treatments ranging from surgical resection, liver transplantation and local radical ablation therapy [Citation4]. As a primary choice for local ablation for patients not suitable for resection, the advantages of microwave ablation (MWA) include a higher intratumoral temperature, short operation time and less dependence on electrical conductivities compared to radiofrequency ablation (RFA) [Citation5–7]. However, post-ablation recurrence remains a relatively frequent occurrence after treatment with MWA, which may result in prognosis, with as high as 52% within 5 years [Citation8–10]. Accumulated evidence demonstrates that untreated micrometastases from the primary tumor, the subsequent transportal spread along intrasegmental branches and vascular invasion contribute to the progression of a tumor in vivo [Citation11], all of which are independent risk factors for survival prognosis.
We recently observed four different types of tumor progression (TP) after MWA treatment in our study. Several studies have reported that local tumor progression (LTP), intrahepatic distant recurrence (IDR) and extrahepatic distant recurrence (EDR) often occur after a period of ablation in HCC cases and early recurrence may lead to a poorer prognosis [Citation9,Citation12,Citation13]. In addition, an interesting pattern of delayed aggressive recurrence, which led to less favorable survival outcomes, was identified. Previous studies suggested that HCC could disseminate via vessels around primary tumor during local-region ablation procedures and cause an aggressive pattern of intrahepatic recurrences [Citation14] and it was defined as aggressive intrasegmental recurrence (AIR), which manifested as either multiple nodular tumors of relatively uniform size or as an infiltrative mass accompanied by a cancer thrombus in the portal vein. This was found to be confined to the peripheral portion of treated segments and HCC cells can propagate through vessels in the proximity of tumors. This propagation can lead to intrahepatic recurrence and metastases [Citation15]. However, the occurrence of AIR remains relatively rare and the range 3.7–15% [Citation16]. However, the incidence, risk factors and clinical significance for the survival of these different TP after MWA treatment have not yet been fully investigated.
Here, we evaluate the clinical significance, risk factors and incidence in different TP after MWA treatment in early-stage HCC cases. The relationship between TP and survival prognosis was further analyzed and discussed.
Methods
Study design and patient data
This single-center, the retrospective study protocol was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center (Guangzhou, China) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent to analyze the data was obtained from all patients. The data of 340 consecutive patients (72 females, 268 males; average age 57.6 ± 10.6 years) with a single HCC of ≤5 cm in BCLC stage 0 or A who underwent computed tomography-guided percutaneous microwave ablation (CT-PMWA) from April 2012 to November 2017 were reviewed. HCC was diagnosed as per the guidelines of the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Disease (AASLD) [Citation16]. In 175 patients, the diagnosis of HCC was based on imaging characteristics and tumor marker analysis and another 165 patients, it was based on pathologic findings by biopsy. Inclusion criteria for MWA were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance scores ≤2; (2) Child–Turcotte–Pugh (CTP) grade A or B; (3) single tumor and maximum diameter ≤5 cm; (4) absence of vascular invasion or extrahepatic metastases; (5) MWA was technically successful after the initial treatment; (6) no history of other malignancies. The exclusion criteria were as follows: (1) patients with suboptimal MRI images; (2) those who underwent MWA combined with other treatments; (3) had severe coagulation disorders (i.e., prothrombin time >25 s, prothrombin activity <40%, and platelet count <50 cells × 109/l); (4) lost to follow up within 3 months after MWA; (5) incomplete ablation. MWA was considered to be technically successful when the index tumor was completely covered by the ablation zone on contrast-enhanced MRI within thirty days. As evaluated by the flow chart in , a total of 777 patients were excluded because they could not meet the inclusion criteria. The collected clinical data were as follows: (1) Patient demographics (sex, age) comorbidities (hypertension, diabetes, heart disease, renal disease and esophageal gastric varices), etiology, cirrhosis and CTP grade, α-fetoprotein (AFP) level); (2) tumor features (maximal diameter, shape, capsule, margin and location abutting major vessel - defined as HCC in contact with at least the secondary branches of the portal vein, inferior vena cava, main hepatic veins, or any vessel with a diameter >3 mm); (3) ablation parameters (insertion points, ablation time, ablation power, ablation energy and ablation session); (4) postoperative data (technique effectiveness, complications, and four types of TP). The MWA device and procedure were described previously [Citation17]. All ablative procedures were performed percutaneously by interventional radiologists (J.H.H., and Z.M.H., with 25, and 10 years of experience in performing MWA, respectively).
Definition of survival and TPs
In this study death or recurrence was defined as endpoints. The days between the first session of MWA treatment and death or end of follow-up were used to calculate overall survival (OS). The days between the first session of MWA treatment to tumor progression or the end of follow-up were used to calculate the recurrence-free survival (RFS). In this study, the first occurrence rate of TP was reviewed, including LTP, IDR, AIR, and EDR. The definition of four types of TP were as following:
LTP was defined according to imaging results of abnormal nodular, disseminated, and/or unusual patterns of peripheral enhancement around the ablation site in patients treated with MWA () [Citation18];
Figure 2. The graphical representations of four types of tumor recurrence after MWA. (A) LTP was defined according to imaging results of abnormal nodular, disseminated, and/or unusual patterns of peripheral enhancement around the ablation site in patients treated with MWA; (B) IDR was described as the appearance of an abnormal nodular, disseminated, or unusual patterns of peripheral enhancement of intrahepatic lesions, which were further away from the ablation zone after MWA treatment; (C) AIR was described as the generation of multiple nodular (≥3) distant from the edge of the ablation zone and the infiltrative recurrence in the treated liver segment that showed enhancement in hepatic arterial phase images and washout on portal or delayed venous phase images at follow-up. The AIR was confined to the initial manifestation of tumor recurrence in patients who were previously considered to have a disease-free status at least 6 months after initial MWA to avoid confusion with IDR [Citation18]; (D) EDR was defined as distant metastasis in the extrahepatic, which has the appearance of an abnormal nodular, disseminated, or unusual patterns of peripheral enhancement of intrahepatic lesions.
![Figure 2. The graphical representations of four types of tumor recurrence after MWA. (A) LTP was defined according to imaging results of abnormal nodular, disseminated, and/or unusual patterns of peripheral enhancement around the ablation site in patients treated with MWA; (B) IDR was described as the appearance of an abnormal nodular, disseminated, or unusual patterns of peripheral enhancement of intrahepatic lesions, which were further away from the ablation zone after MWA treatment; (C) AIR was described as the generation of multiple nodular (≥3) distant from the edge of the ablation zone and the infiltrative recurrence in the treated liver segment that showed enhancement in hepatic arterial phase images and washout on portal or delayed venous phase images at follow-up. The AIR was confined to the initial manifestation of tumor recurrence in patients who were previously considered to have a disease-free status at least 6 months after initial MWA to avoid confusion with IDR [Citation18]; (D) EDR was defined as distant metastasis in the extrahepatic, which has the appearance of an abnormal nodular, disseminated, or unusual patterns of peripheral enhancement of intrahepatic lesions.](/cms/asset/3121f302-da58-4d92-a814-33e8b105ce18/ihyt_a_1962548_f0002_c.jpg)
IDR was described as the appearance of an abnormal nodular, disseminated, or unusual patterns of peripheral enhancement of intrahepatic lesions, which were further away from the ablation zone after MWA treatment ();
AIR was described as the generation of multiple nodular (≥3) distant from the edge of the ablation zone and the infiltrative recurrence in the treated liver segment that showed enhancement in hepatic arterial phase images and washout on the portal or delayed venous phase images at follow-up. The AIR was confined to the initial manifestation of tumor recurrence in patients who were previously considered to have a disease-free status at least 6 months after initial MWA to avoid confusion with IDR () [Citation19];
EDR was defined as distant metastasis in the extrahepatic, which has the appearance of abnormal nodular, disseminated, or unusual patterns of peripheral enhancement of intrahepatic lesions ().
The graphical representations of four types of TP after MWA are shown in Supplementary Figures.
Follow-up protocol
If irregular peripheral enhancement in a scattered, nodular, or eccentric pattern occurred, it represented incomplete ablation and another ablation was to be considered. Otherwise, if complete ablation was achieved, then routine contrast-enhanced images and serum tumor markers were repeated at 1 and 3 months after CT-PMWA and then at every 6 months intervals. For patients with suspected metastasis, chest CT, bone scan or PETCT was performed. Technique success was defined as complete local necrosis 1 month after treatment. If first TP was detected during the follow-up period, an individualized plan for second-line treatment was designed on the basis of characteristics of the recurrent tumor and the patient’s liver function and general condition. Treatment modalities for tumor progression were determined by the multidisciplinary team (H.J.H, H.Z.M, A.C, Z.T.Q with more than 10 years of experience related to CT-PMWA). Complications were classified according to the Society of Interventional Radiology Classification system for Complications by Outcome [Citation20].
Statistical analysis
All patients who met the eligibility criteria at baseline were included, and their data were analyzed. Statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL). The quantitative data were expressed as mean ± standard deviation, and qualitative data were expressed as frequency. OS, RFS, LTP, IDR, AIR and EDR rates were assessed by the Kaplan–Meier method with the log-rank test. A Cox proportional hazards model was used to identify the significant effects of risk factors on survival and recurrence. The parameters for the univariate and multivariate analyses of independent prognostic factors included age, gender, comorbidities, cirrhosis, etiology, AFP, tumor size, CTP grade, BCLC grade, location abutting major vessels, sessions. Variables associated with oncological outcomes were evaluated by means of the forward stepwise Cox regression model. p-Values less than 0.05 were considered to be statistically significant.
Results
Baseline characteristics
The characteristics of HCC patients are summarized in . Among the entire group of 340 patients, 134 were found in the very early stage (BCLC stage 0), whereas the remaining 206 patients were in early-stage (BCLC stage A) before MWA according to American National Comprehensive Cancer Network (NCCN) guideline. The complete ablation rate was 100% in all patients’ cohorts. As expected tumor size and ablation parameters differed between the BCLC 0 and A stage (p < 0.001). Clinical parameters were similar between the two groups (p = 0.764–0787). On the basis of follow-up imaging, no significant statistical difference was detected in 100% of technical success rate between the two groups (p = 1.000). The complications included subcapsular tumor implantation (n = 1), pleural effusion in the right side (n = 1) and pleural infection in the right side (n = 1) were found in the BCLC stage A group, and pleural effusion in the right side (n = 1) was found in BCLC stage 0 group, which were comparable to between two groups (p = 0.485).
Table 1. Baseline patient characteristics.
Incidence and risk factors of TP after MWA
Over a 5-years period of follow-up, the occurrence rate of TPs including LTP, IDR, AIR and EDR were observed in 6.2% (21/340), 29.1% (98/340), 3.2% (11/340) and 7.9% (27/340) of the patients with the single HCC who underwent CT-PMWA during the follow-up period, which in BCLC stage 0 were comparable to those in BCLC stage A (p = 0.492–0.219). Baseline characteristics of four types of TP showed in Supplementary Tables 1–4. Univariate analysis revealed that tumor size (p = 0.035) was significant factor in terms of occurrence of LTP, cirrhosis (p = 0.039), CTP grade (p = 0.003) were significant factors in terms of occurrence of IDR and CTP grade (p = 0.018), location abutting major vessels (p = 0.012) were significant factors in terms of occurrence of AIR (). Multivariate analysis results showed that cirrhosis (p = 0.036; hazard ratio [HR] = 0.510; 95% confidence interval [CI]: 0.271–0.958), CTP grade B (p = 0.003; HR = 21.599; 95% CI: 2.861–163.056) were independently associated with high occurrence rate of IDR. Older age (p = 0.036; HR = 2.619; 95% CI: 1.067–6.429), CTP grade B (p = 0.005; HR = 19.863; 95% CI: 2.489–158.454) and location abutting major vessels (p = 0.008; HR = 3.193; 95% CI: 1.356–7.517) were independently associated with high occurrence rate of AIR. Moreover, location abutting major vessels (p = 0.033; HR = 2.284; 95% CI: 1.106–5.236) were independently associated with a high occurrence rate of EDR. (). The initial treatments modalities including RFA, MWA, TACE, surgery, sorafenib therapy and other (i.e., regorafenib, lenvatinib or Chinese medicine). Among these four groups, MWA was used mainly in the treatment of HCC lesions in the IDR group and TACE was used mainly in the treatment of HCC lesions in the AIR group (p < 0.001). The survival outcome of the four groups was a significant statistical difference (p < 0.001). The Kaplan-Meier with the log-rank test was performed to assess the post-MWA recurrence survival for HCC patients, the results suggested that surgical resection for recurrent lesions show superiority to the other treatment (p < 0.001) (Supplementary Figure 2).
Table 2. Univariate analysis for prognostic factors of four types of tumor progressions.
Table 3. Multivariate analysis for prognostic factors of tumor progression.
Long-term survival outcome after MWA
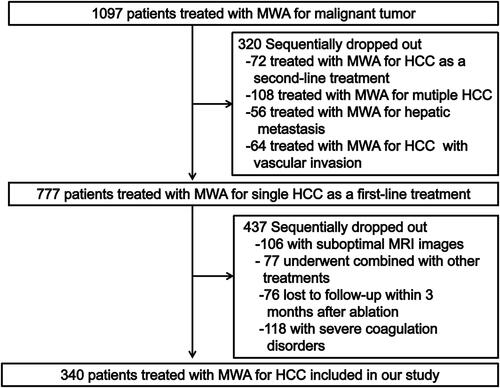
In a median follow-up period of 35.6 months (range, 3.1–61.4 months), The 1-, 3-, and 5-year OS rates of BCLC stage 0 group and BCLC stage A group were 96.2%, 80.8%, 67.7% and 96.4%, 80.5%, 65.5%, respectively, showing no significant statistical difference (p = 0.739). The 1-, 3-, and 5-year RFS rates of BCLC stage 0 group and BCLC stage A group were 80.6%, 64.5%, 59.0% and 80.1%, 67.8%, 58.6%, respectively, showing no significant statistical difference (p = 0.708). 1-, 3-, and 5-year LTP rates of BCLC stage 0 group and BCLC stage A group were 2.4%, 7.2%, 7.2% and 1.0%, 4.1%, 4.1%, respectively, showing no significant statistical difference (p = 0.681). 1-, 3-, and 5-year IDR rates of BCLC stage 0 group and BCLC stage A group were 17.8%, 30.3%, 36.0% and 19.7%, 31.8%, 38.1%, respectively, showing no significant statistical difference (p = 0.971). 1-, 3-, and 5-year AIR rates of BCLC stage 0 group and BCLC stage A group were 1.6%, 5.4%, 8.9% and 3.9%, 9.0%, 11.3%, respectively, showing no significant statistical difference (p = 0.492). 1-, 3-, and 5-year EDR rates of BCLC stage 0 group and BCLC stage A group were 1.5%, 7.4%, 7.4% and 2.4%, 11.5%, 16.7%, respectively, showing no significant statistical difference (p = 0.219). The Kaplan–Meier curves were shown in .
Figure 3. Kaplan–Meier curves comparing OS, RFS and four types of tumor progression outcomes after MWA between BCLC stage 0 and A group. (A) comparing OS between BCLC stage 0 and A group; (B) comparing RFS between BCLC stage 0 and A group; (C) comparing LTP between BCLC stage 0 and A group; (D) comparing IDR between BCLC stage 0 and A group; (E) comparing AIR between BCLC stage 0 and A group; (F) comparing EDR between BCLC stage 0 and A group. OS: overall survival; RFS: recurrence-free survival; MWA: microwave ablation; BCLC: Barcelona clinic liver cancer; LTP: local tumor progression; IDR: intrahepatic distant recurrence; AIR: aggressive intrasegmental recurrence; EDR: extrahepatic distant recurrence.
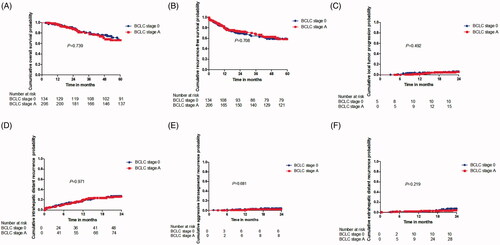
Figure 4. Overall survival curves for patients with and patients without TP of HCC after MWA. Curves were obtained with Kaplan–Meier method. (A) Overall survival curves for patients with and patients without LTP of HCC after MWA; (B) Overall survival curves for patients with and patients without IDR of HCC after MWA; (C) Overall survival curves for patients with and patients without AIR of HCC after MWA; (D) Overall survival curves for patients with and patients without EDR of HCC after MWA. HCC: hepatocellular carcinoma; MWA: microwave ablation; LTP: local tumor progression; IDR: intrahepatic distant recurrence; AIR: aggressive intrasegmental recurrence; EDR: extrahepatic distant recurrence.
Influence on long-term overall survival
Of the 340 patients enrolled, 68 (20%) died of HCCs progression. The death occurred in 4 of the 21 patients (19.0%) in the group with LTP and in 62 of the 319 (19.5%) without LTP. The 1-, 3-, and 5-year OS rates in the LTP group and non-LTP group were 95.9%, 66.3%, and 50.2% and 96.2%, 81.2%, and 68.1%, respectively, (, showing no significant statistical difference (p = 0.674). The death occurred in 32 of the 98 patients (32.7%) in the group with IDR and in 36 of the 242 (14.9%) without IDR. The 1-, 3-, and 5-year OS rates in the IDR group and non-IDR group were 97.9%, 75.8%, and 52.8% and 95.6%, 83.0%, and 77.0%, respectively, (), showing significant statistical difference (p = 0.025). The death occurred in eight of the 11 patients (71.4%) in the group with AIR and in 55 of the 329 (16.6%) without AIR. The 1-, 3-, and 5-year OS rates in the AIR group and non-AIR group were 78.2%, 55.0%, and 55.0% and 96.7%, 83.5%, and 74.3%, respectively, ((C)), showing significant statistical difference (p = 0.018). The death occurred in four of the 27 patients (14.8%) in the group with EDR and in 64 of the 313 (20.3%) without EDR. The 1-, 3-, and 5-year OS rates in the EDR group and the non-EDR group were 92.1%, 63.7%, and 45.1% and 96.7%, 82.4%, and 70.1%, respectively, (), showing significant statistical difference (p = 0.001). The univariate analysis showed statistically significant differences in terms of OS rates, depending on the age (p < 0.001; HR:2.783; 95% CI: 1.703–4.547), comorbidities (p = 0.042; HR: 1.864; 95% CI: 1.023–3.397), occurrence of IDR (p = 0.027; HR:1.719; 95% CI: 1.065, 2.774), AIR (p = 0.001; HR: 3.628; 95% CI: 2.041–6.449) and EDR (p = 0.009; HR:2.638; 95% CI: 1.462–4.763). The multivariate analysis showed that risk factors that significantly affected the OS rate were older age (p < 0.001; HR:2.478; 95% CI: 1.503–4.084), the occurrence of AIR (p < 0.001; HR = 2.648; 95% CI: 1.457–4.815), and occurrence of EDR (p = 0.002; HR = 2.222; 95% CI: 1.218–4.054) ().
Table 4. Univariate and multivariable analysis of prognostic factors for overall survival after MWA.
Discussion
The probability of various TP after MWA for HCC has been previously reported [Citation21,Citation22]. Most opinions suggest that TP after MWA is closely related to incomplete ablation and intravascular spread. The current study provides new knowledge related to the incidence, risk factors and clinical significance of four types of TP after MWA in a large cohort of HCC cases with long-term follow-ups. After MWA as a first-line of treatment for patients with the single, early-stage HCC, we found that the occurrence rate of IDR was the highest (29.1% in all patients) and the occurrence rate of AIR was the lowest (3.1% in all patients) for these TP. High-power MWA was applied in this study, which was regarded as less affected by the ‘heat-sink’ effect, producing a larger ablation volume compared to RFA [Citation23]. Previous studies reported an enlarged thermal field closely related to lower LTP rates as well as the observation that the perivascular tumor did not affect LTP after high-power MWA [Citation24]. With a 100% technique success rate, LTP with a low occurrence rate is a reasonable explanation. However, IDR seemed to have little relationship with the same size of the ablation margin. A vital risk factor is invisible micrometastases around the tumor. Toshimori et al. reported that the risk for IDR depends mainly on the carcinogenic potential of noncancerous tissue [Citation13]. According to our study, it is difficult to prevent recurrence outside the ablation zone by local control of MWA.
It is interesting that most AIR were found in the liver parenchyma peripheral to MWA zones and present an aggressive growth pattern. Kim et al. reported [Citation25] occurrence rates of AIR to be 3.7% after RFA for a single HCC, which was similar to the 3.1% occurrence rate identified in our study. AIR of HCC manifested as multiple nodules appearing simultaneously or combined with tumor thrombus within the portal vein after MWA. EDR of HCC manifested as lesions occurring metastasis after MWA. In view of the aggressive characteristics of AIR and EDR, these two types of outcomes were regarded as more severe TP, which need to be alerted by radiologists. Previous studies showed that increased intratumoral pressures caused by heating could lead to tumor cell spread and dislodgement through the vessel system in the ablation zone [Citation26–29]. Although the detailed mechanisms of intravascular spread of tumors are not fully known, rapid temperature increases in the tumor resulting from MWA have been suggested as a possible cause of cancer spread to the liver via nearby vessels.
In our cohort of patients, several risk factors appeared to induce IDR, EDR and AIR of HCC after MWA, including cirrhosis, CTP grade B and lesions adjacent to major vessels. Previous studies reported biology, microvascular invasion and tumor grade were risk factors for early recurrence (within 12 months) after RFA in the treatment of HCC [Citation30–33]. However, we did not find any known tumor-related factors, including tumor size, tumor number, and AFP levels, instead, liver-related factors were found to be related to the occurrence of IDR and AIR. Especially lesions adjacent to major vessels were predictive of AIR and EDR. This result was similar to report from the Kim group [Citation15]. Based on our findings, we hypothesized that cirrhosis and poor liver function may result in the emergence of a crisis of potential tumor micrometastases and vascular microinvasion, which was closely related to IDR. The spread of tumor was partly caused by perivascular tumors from the ablative zone spreading to the major vessel zone through irregular arterioportal communications in tumor microenvironments [Citation9,Citation34]. However, this speculation needs further investigation to validate the detailed mechanisms regarding AIR and IDR in HCC after MWA treatment.
Observing HCC lesions in BCLC early stage, different oncological and survival outcomes including OS, RFS, LTP, IDR, EDR and AIR between 0 stage and A stage, were not significantly different, This result demonstrated that tumor size did not affect survival and recurrence when single tumors in early-stage received MWA, and was further confirmed by univariate and multivariate analyses. In all patients with four types of TP, the OS can be affected by the occurrence of AIR during follow-up. Although intrahepatic and extrahepatic distant recurrence was also a significant adverse factor for OS, the relatively low occurrence rate in the four types of TP seemed to be a more serious problem compared to other outcomes, which usually arises from multistep or de novo carcinogenesis in the setting of a preneoplastic cirrhotic liver. In order to decrease the risk of AIR, TACE for small and multiple tumors (to avoid increasing intratumoral pressure and several repositioning) can also be used [Citation35]. In summary, we believe that further studies comparing these different strategies would be useful to decrease AIR.
This study had numerous limitations including selection bias in a retrospective study. In addition, potential risk factors were not investigated in this study, thus there was not sufficient evidence proving the exact cause of TP. Additionally, the selection of treatment for recurring tumors was based on the specific experience, affecting long-term OS rates. Finally, because there were not enough biopsies of HCCs in this study, the degree of histologic differentiation was not provided in the multivariable analysis.
This retrospective study investigated the incidence, risk factors and clinical significance in different TP after MWA treatment of early-stage HCC. Liver-related factors were closely related to IDR and AIR. AIR occurs rarely but is the most severe TP after MWA. Indeed, AIR and EDR had an adverse effect on the OS rate. Further prospective randomized studies are needed to confirm these conclusions.
Supplemental Material
Download PDF (260.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30(6):571–579.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66(6):1172–1173.
- Liu Y, Zheng Y, Li S, et al. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68(1):21–26.
- Bhardwaj N, Strickland AD, Ahmad F, et al. Microwave ablation for unresectable hepatic tumours: clinical results using a novel microwave probe and generator. Eur J Surg Oncol. 2010;36(3):264–268.
- Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2019;23:114–118.
- Kim R, Jeong WK, Kang TW, et al. Intrahepatic distant recurrence after radiofrequency ablation of hepatocellular carcinoma: relationship with portal hypertension. Acta Radiol. 2019;60(12):1609–1618.
- Sparchez Z, Mocan T, Radu P, et al. Prognostic factors after percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma. Impact of incomplete ablation on recurrence and overall survival rates. J Gastrointestin Liver Dis. 2018;27(4):399–407.
- Wilkinson NW, Shapiro AJ, Harvey SB, et al. Port-site recurrence reproduced in the VX-2 rabbit carcinoma model: an in vivo model comparing laparoscopic port sites and open incisions. JSLS. 2001;5:221–226.
- Zhai H, Liang P, Yu XL, et al. Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: an analysis of 11 cases. Int J Hyperthermia. 2015;31(8):863–868.
- Toshimori J, Nouso K, Nakamura S, et al. Local recurrence and complications after percutaneous radiofrequency ablation of hepatocellular carcinoma: a retrospective cohort study focused on tumor location. Acta Med Okayama. 2015;69(4):219–226.
- Kim SM, Shin SS, Lee BC, et al. Imaging evaluation of ablative margin and index tumor immediately after radiofrequency ablation for hepatocellular carcinoma: comparison between multidetector-row CT and MR imaging. Abdom Radiol. 2017;42(10):2128–2527.
- Hocquelet A, Balageas P, Frulio N, et al. Aggressive intrasegmental recurrence of periportal hepatocellular carcinoma after radiofrequency ablation: role of ablative technique and heat-sink effect. Radiology. 2015;276(3):932–933.
- Sherman M, Bruix J, Porayko M, et al. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology. 2012;56(3):793–796.
- Ni JY, An C, Zhang TQ, et al. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int J Hyperthermia. 2019;36(1):328–336.
- Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Song KD, Lee MW, Rhim H, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after combined transarterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2016;207(5):1122–1127.
- Duszak R, Mabry MR. Clinical services in interventional radiology: results from the national medicare database and a society of interventional radiology membership survey. J Vasc Interv Radiol. 2003;14(1):75–81.
- Brunello F, Carucci P, Gaia S, et al. Local tumor progression of hepatocellular carcinoma after microwave percutaneous ablation: a preliminary report. Gastroenterology Res. 2012;5(1):28–32.
- Yu J, Liang P, Yu XL, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol. 2015;25(4):1119–1126.
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and Meta-analysis. Int J Hyperthermia. 2019;36(1):264–272.
- Dou JP, Yu J, Yang XH, et al. Outcomes of microwave ablation for hepatocellular carcinoma adjacent to large vessels: a propensity score analysis. Oncotarget. 2017;8(17):28758–28768.
- Kang TW, Lim HK, Lee MW, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276(1):274–285.
- Nitta H, Allard MA, Sebagh M, et al. Prognostic value and prediction of extratumoral microvascular invasion for hepatocellular carcinoma. Ann Surg Oncol. 2019;26(8):2205–2568.
- Xu G, Yang HY, Xu HF. Prediction of microvascular invasion in hepatocellular carcinoma with preoperative imaging radiomic analysis: is it ready for prime time. Hepatobiliary Pancreat Dis Int. 2019;18(3):289–290.
- Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29(7):3595–3605.
- Kotoh K, Nakamuta M, Morizono S, et al. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int. 2005;25(3):542–547.
- Yuan ZG, Wang ZY, Xia MY, et al. Comparison of diffusion kurtosis imaging versus diffusion weighted imaging in predicting the recurrence of early stage single nodules of hepatocellular carcinoma treated by radiofrequency ablation. Cancer Imaging. 2019;19(1):30.
- Cho JY, Choi MS, Lee GS, et al. Clinical significance and predictive factors of early massive recurrence after radiofrequency ablation in patients with a single small hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(4):477–486.
- Ishii T, Numata K, Hao Y, et al. Evaluation of hepatocellular carcinoma tumor vascularity using contrast-enhanced ultrasonography as a predictor for local recurrence following radiofrequency ablation. Eur J Radiol. 2017;89:234–241.
- Takahashi H, Akyuz M, Aksoy E, et al. Local recurrence after laparoscopic radiofrequency ablation of malignant liver tumors: results of a contemporary series. J Surg Oncol. 2017;115(7):830–834.
- Xie X, Jiang C, Peng Z, et al. Local recurrence after radiofrequency ablation of hepatocellular carcinoma: treatment choice and outcome. J Gastrointest Surg. 2015;19(8):1466–1475.
- Kumar A, Bal C, Srivastava DN, et al. Management of multiple intrahepatic recurrences after radiofrequency ablation of hepatocellular carcinoma with rhenium-188-HDD-lipiodol. Eur J Gastroenterol Hepatol. 2006;18(2):219–223.