Abstract
Objectives
To confirm the long-term efficacy and safety of radiofrequency ablation (RFA) for low-risk papillary thyroid microcarcinoma (PTMC).
Methods
We retrospectively reviewed data of 102 primary papillary thyroid carcinoma patients (82 women, 20 men; mean age: 43 [19] years) treated with radiofrequency ablation and thyroid-stimulating hormone (TSH) suppression therapy before December 2018. All patients were at high surgical risk or refused surgery. They were followed up at 1, 3, 6, 9, and 12 months and every 6–12 months thereafter using ultrasound and contrast-enhanced ultrasound. The volume and volume reduction ratio was calculated. Recurrence and lymph node or distant metastasis were evaluated.
Results
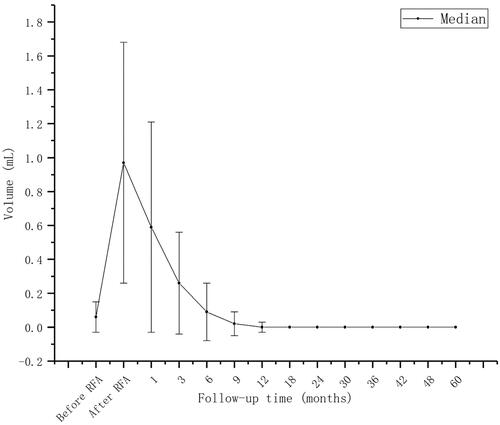
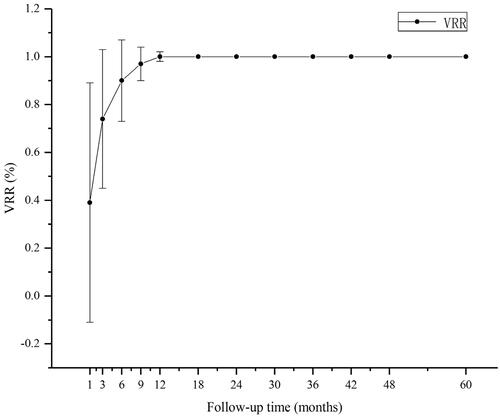
The mean initial tumor diameter was 0.50 (0.29) cm; the mean initial volume was 0.06 (0.09) mL. At 1, 3, 6, 9, 12, 24, 36, 48, and 60 months after RFA, complete resorption rates were 0, 0, 9.8 (10/102), 33.3 (34/102), 91.2 (93/102), 96.1 (98/102), 99 (101/102), 100, and 100%, respectively. Two patients had developed ipsilateral neck lymph node metastasis in regions IV and VI at 30- and 18-month follow-ups, respectively. After RFA, 3/102 patients (2.9%) developed hoarseness—the main side effect. No life-threatening or delayed complications occurred. The TSH value in the initial period was 0.06 (0.02) µIU/mL; the rate of reaching the TSH target was 85.7%. The TSH value at follow-up was 1.47 (0.91) µIU/mL; the compliance rate was 99.3%.
Conclusions
Ultrasound-guided RFA for PTMC is highly effective and safe. RFA can serve as a minimally invasive treatment for PTMC patients who refuse surgery or active surveillance.
Introduction
In the past 10 years, the National Cancer Institute’s Surveillance, Epidemiology, and End Results database show that differentiated thyroid cancer (DTC) accounts for 95.7% of thyroid-related cancer cases [Citation1]. Papillary thyroid carcinoma (PTC) has shown a significant increase in the number of cases. The papillary thyroid microcarcinoma (PTMC) is the fastest-growing PTC. It accounts for 50–60% of the PTC cases. However, there is no significant growth in the PTC death rate. Currently, thyroid surgery is the standard treatment for PTMC [Citation2]. The risks of long-term complications, scarring, and lifelong thyroxine replacement resulting from overtreatment with surgery should be minimized because of the good prognosis of low-risk PTMC and the high risks of surgery for patients who cannot tolerate it. In 2015, the American Thyroid Association (ATA) indicated that for low-risk PTMC, active surveillance can replace immediate surgery [Citation2]. The Japan thyroid tumor diagnosis and treatment guidelines, which were published in 2018, classifies patients with T1aN0M0 tumors into the ultra-low risk group. It also recognizes active surveillance as a treatment for these patients [Citation2]. Oda et al. [Citation3] and Ito et al. [Citation4] also found that, compared with the death rate of patients treated with immediate surgeries, the death rate of those under active surveillance without surgeries was not high during the past 10 years. Therefore, active surveillance could be used to replace immediate surgery in cases of low-risk PTMC [Citation4]. Active surveillance has been recognized as a safer and more cost-effective option for low-risk PTMC than immediate surgery [Citation5]. However, many scholars and patients still choose immediate surgery because they have various concerns about active surveillance and anxieties about survival with cancer. There are limited clinical methods to predict the aggressiveness of PTMC. In recent years, an increasing number of scholars have gradually recognized that ultrasound-guided thermal ablation technologies, such as laser ablation (LA), microwave ablation (MWA), or radiofrequency ablation (RFA) have been successfully used to treat benign thyroid nodules as well as PTMC and metastatic lymph nodes in patients at surgical risk [Citation3–7]. Ultrasound-guided RFA technology was first used as a treatment for PTMC in 2016. It proved effective in treating PTMC. Zhang and his colleagues studied 92 patients with PTMC who were followed up for 18 months. The average volume reduction rate (VRR) of the tumor was found to be 0%. In another study, 37 patients with PTMC were followed up for 12 months, and the VRR and recurrence rate was 99.34 ± 3.49 and 0%, respectively [Citation8]. In recent years, several meta-analyses have confirmed that thermal ablation is an excellent method for regional tumor control in patients with low-risk PTMC [Citation9]. The management of PTMC is a growing area of interest. In the present study, we evaluated the effectiveness and safety of RFA in patients with PTMC who were treated with RFA and thyroid-stimulating hormone (TSH) suppression after surgery. TSH suppression therapy is an important part of the post-operative treatment of DTC [Citation10]; it aims to reduce endogenous stimulation of residual DTC cells and thus reduces tumor recurrence and metastasis. Patients were followed up for at least 3 years after the combined treatment. The recurrence rate and metastasis were studied to confirm the effectiveness and safety of RFA with TSH inhibition.
Materials and methods
The First Affiliated Hospital of Dalian Medical University’s institutional review board provided approval for this study. The protocol number is PJ-KS-KY-2020-62. Written consent was obtained from all patients before RFA treatment.
Patients
Between July 2014 and December 2018, 102 patients with PTC with T1aN0M0 lesions were treated at our hospital using ultrasound-guided percutaneous RFA. All patients were at high surgical risk or refused surgery. They were followed up for 3–5 years after their treatment. For all patients, fine-needle aspiration (FNA) was used to confirm PTC. The inclusion criteria were a maximum diameter no larger than 10 mm and absence of capsular infiltration and extrathyroidal invasion on ultrasound (US). Tumors located within 2 mm around the capsule were defined as ‘to the capsule [Citation11].’ Additionally, there was no cervical lymph node metastasis or distant metastasis on imaging. The exclusion criteria were thyroid cancer with severe extra thyroid metastases, lymph node (LN) metastases (imaging or cytological evidence), distant metastases, pregnancy, and severe cardiopulmonary disease. The current retrospective study includes 102 patients with T1N0M0 PTC. The results of the thyroid function test, laryngoscopy, electrocardiography, and lung computed tomography (CT) were normal.
Pre-ablation evaluation
Preoperative ultrasound and contrast-enhanced ultrasound were performed for all patients using the Hitachi Hivision Ultrasound system (Hitachi, Japan). Ultrasound was used to evaluate the orthogonal three-dimensional maximum diameter and size of each tumor nodule. The size of each tumor is calculated based on the formula of V = π ABC/6 (where V is the volume, A is the maximum diameter, and B and C are the other two vertical maximum diameters). After the evaluation of PTMC tumors, a cervical lymph node ultrasound and enhanced cervical CT were performed to assess the presence of LN metastasis. The blood supply of the nodules was assessed on contrast-enhanced ultrasound (CEUS). FNA was performed to confirm the pathology of the lesions. A puncture biopsy was performed to identify suspected LNs. Metastatic LNs were excluded. Meanwhile, routine laboratory tests including thyroid function, serum thyroglobulin, thyroglobulin antibody, blood routine, and coagulation function, and lung CT examination were also performed.
Ablation procedures
Ablation procedures were performed by a specialist with 10 years of ablation experience using the VIVA RF GENERATOR (STARmed Co., Ltd., Korea) with an 18 G diameter ablation needle (STARmed Co., Ltd., Korea). The 7 F or 10 F working tip was selected based on the volume of the target tumor. The procedure was performed with the patient in the supine position with his/her neck extended. The tumor’s proximity to key areas, such as nerves, the esophagus, and the trachea, was evaluated using ultrasound to prevent damage to the surrounding tissues. Adequate fluid isolation was performed immediately outside the capsule with saline or injected with 5% glucose, which was chosen based on the location of the lesion. Fluid isolation can also be performed simultaneously with regional anesthesia to avoid the nerve area.
A safe puncture route was selected to pass through some normal glands, typically the isthmus. The RFA power was 40–70 W, and the time range was 21–403 s. For a small tumor, the tip of the electrode was fixed to the center of the tumor. Large tumors were treated with unit-by-unit movement. To prevent a marginal recurrence, the ablation was expanded to 3–5 mm away from the surrounding normal tissue or to the capsule. CEUS was used to evaluate the effectiveness of RFA. If there were severe areas in the nodule, complementary ablation was performed immediately to prevent the presence of the residual tumor. Any side effects that occurred during or immediately after ablation were carefully evaluated, and all patients were observed in the hospital for 4–5 h after ablation.
Post-ablation assessment
The assessment of effectiveness mainly included the assessment of ablation range and complications. For the former, attention should be paid to the identification of the expected ablation range related to the treated lesions. The application of CEUS was used to make accurate assessments. Complete ablation was defined as no contrast agent perfusion after the injection of SonoVue into the thyroid nodule. A surgical review was conducted by a physician with more than 3 years of experience. A follow-up was performed 1 month after surgery. Then the follow-ups were completed quarterly for a year and on a semi-year basis thereafter. Follow-ups included ultrasonography to assess tumor size, volume, blood flow, and recurrence of metastasis shown in . Information of delayed surgery (any reason for thyroid surgery during follow-up) was obtained. Thyroid function was also checked during the follow-up visits. The VRR was calculated based on the formula of VRR = [(initial volume − final volume) × 100]/initial volume. If the volume of a nodule decreased gradually or the volume of a nodule was not measurable, the nodule was confirmed to be inactivated. If the volume of a nodule increased, the nodule was considered to have active ingredients; in this case, another option of treatment would be used. All patients were examined using CT 6 months after ablation to detect LN metastasis, and a needle biopsy was required if abnormal lymph nodes were evident on ultrasound and CT. Side effects were the undesired events that occurred during or after a procedure, and recovery time and treatment were recorded. The main immediate (0–24 h) and peri-procedural (1–30 d) side effects are hoarse voice, bleeding, and thyroid abnormalities [Citation12]. Thyroid function (including T3, T4, TSH, FT3, and FT4) was reexamined 1 week after surgery. According to the recommendations of China’s Guidelines for Thyroid Nodules and Differential Thyroid Carcinoma published in 2012 [Citation13], TSH inhibition therapy was performed in compliance with the 2015 ATA dynamic dual risk assessment system [Citation4] and the comprehensive dual risk assessment system. L-T4 was taken before any food. The dose is typically prescribed at 50 μg/d. It can be halved or started with 12.5 μg/d for patients with cardiovascular risk at an interval of 4 weeks at a dose of 12.5–25 μg. In the initial treatment period (in the first year after surgery), TSH was controlled below 0.1 mU/L. In the follow-up period (a year later after surgery), TSH was controlled below 2 mU/L.
Statistical analysis
The SPSS statistical software (version 23.0) was used to perform statistical analysis. Continuous data are presented as medians (interquartile range). The Wilcoxon signed-rank test was used to compare the mean volume of the tumor before RFA with the results of each after RFA at follow-up. A p-value <0.05 was considered to be statistically significant.
Results
The follow-up period after RFA lasted for 60 months. All tumor ablation foci disappeared during the follow-up period with the mean tumor diameter at 0.50 (0.29) cm and the mean volume at 0.06 (0.09) mL shown in . Characteristics of the study cohort, tumors, and RFA parameters are shown in . All ablated tumors completely disappeared at the end of the follow-up period. At 1, 3, 6, 9, 12, 24, 36, 48, and 60 months after RFA, the complete resorption rate was 0, 0, 9.8 (10/102), 33.3 (34/102), 91.2 (93/102), 96.1 (98/102), 99 (101/102), 100, and 100%, respectively shown in and . By their 30- and 18-month post-RFA follow-ups, two patients developed ipsilateral neck lymph node metastasis in regions IV and VI, respectively. After RFA, 2.9% (3/102) of patients developed hoarseness—the main side effect. Three patients (2.9%) developed hyperthyroidism 1 week after RFA, but their thyroid functions normalized after a month shown in . One patient was diagnosed with Graves’ disease. Two patients (1.9%) developed subclinical hypothyroidism, and their thyroid functions normalized after a month. The TSH value in the initial treatment period was 0.06 (0.02) μIU/mL, and the rate at which the TSH target was achieved was 85.7%. The TSH value in the follow-up period was 1.47 (0.91) μIU/mL, and the compliance rate was 99.3%.
Table 1. General conditions of patients before radiofrequency ablation.
Table 2. Changes in volume and VRR after radiofrequency ablation (RFA) at follow-up.
Table 3. General conditions of patients after radiofrequency ablation.
Discussion
Most PTMCs are regarded as being low risk because of their limited effect on mortality. Avoiding overtreatment of PTMC is a clinical challenge. In the study by Ito et al., 1235 patients with PTMC underwent 5- and 10-year observations and showed LN metastasis rates at 1.7 and 3.8%, respectively. Of the patients who underwent thyroid surgery for PTMC, 2–6% of them had local recurrence and 1–2% had distant recurrence [Citation14,Citation15]. These outcomes are related to the indolent nature of PTMC. Active surveillance requires patients to have a strong psychological quality because a disease psychologically burdens the patient. Surgery, which radically resects both thyroid glands and regional LNs, remains the cornerstone of treatment for PTC [Citation16,Citation17]. In recent years, new techniques to treat PTMC, such as RFA, LA, and MWA have been attempted with good results [Citation18]. Further, RFA and LA likely represent new options in the treatment of metastatic LNs in the neck that result from PTC [Citation19,Citation20]. RFA treats the tumor only, thus preserving the thyroid function. The results of previous studies confirm that RFA is an effective and safe option to treat patients with low-risk PTMC, with high surgical risk, or those refusing surgery [Citation21]. This paper confirms that RFA can effectively eliminate low-risk PTMC with a low complication rate, and all tumors disappeared after RFA without regional tumor progression or distant metastases. RFA is a promising strategy to treat PTMC because surgical complications can be avoided, and it can reduce the burden of patients who live with the disease.
For patients with DTC, the metastasis rate of cervical LN is 20–50% [Citation22–25]. The micrometastasis incidence rate is approaching 90% [Citation26]. The sensitivity and specificity of ultrasound in predicting PTC metastasis in the central neck were 30.0 and 86.8%, respectively [Citation27]. In a study by Myung et al. [Citation28], the recurrent rate was 1.4% for patients followed up to 5–6 years after surgery. In this study, 1.9% (2/102) patients developed ipsilateral neck LN metastasis in regions IV and VI at their follow-ups of 30 and 18 months after RFA, respectively. All patients with metastatic LNs underwent surgery, and no recurrence was identified in those on limited follow-up; delayed surgery did not affect the outcome. The two tumors were located in the middle of the thyroid gland and not close to the capsule, and the TSH value after RFA was in the range of the target. Micrometastases were 100% confined to the ipsilateral LNs regardless of the site or size of the tumor [Citation26]. This may be related to the macrometastasis before RFA, which cannot be found on ultrasound because of its low sensitivity. However, this does not influence the outcomes of patients who had a macrometastasis before RFA. For low-risk PTMC, surgery causes far more harm to the patient than the disease. A meta-analysis has shown that the occurrence rate of permanent RLN palsy is 1.8 and 1.1% for thyroidectomy and total thyroidectomy, respectively, and the occurrence rate of temporary RLN palsy is 10.1 and 8.1% for thyroidectomy and total thyroidectomy, respectively. The occurrence rate of permanent hypocalcemia is 2.8% for thyroidectomy and 3.3% for total thyroidectomy [Citation29]. These patients were also unable to accept the permanent presence of scarring in the neck and the impact of lifelong medication on the quality of their lives. The main post-RFA complication in this study was hoarseness with an occurrence rate of 2.9% (3/102). Hoarseness was resolved 30 min to 2 weeks after surgery. In previous studies, several thermal ablation methods have shown good regional control of low-risk PTMCs with a VRR of 0% and no regional tumor recurrence or distant metastases [Citation19]. Thermal ablation can be considered an alternative approach for patients with primary thyroid cancer who refuse surgery or are unable to undergo surgery. Though long-term follow-up of thermal ablation for PTMC has been reported in the past, the number of cases was small. There are limited studies on the long-term follow-up of RFA for PTMC. PTC develops gradually, and lymph node metastasis usually occurs 5 years after surgery. Therefore, long-term follow-up is necessary to assess the effectiveness of RFA and to detect lymph node metastasis. RFA for PTMC is not recommended as first-line treatment in the guidelines but is recommended as a palliative treatment for recurrent thyroid cancer. It is a treatment for regional recurrent metastatic cancer in the 2015 ATA guidelines [Citation2]. The 2017 Korean Society of Thyroid Radiology (KSThR) [Citation30] guidelines suggest that thermal ablation therapy can be used as an alternative treatment for PTMC if patients refuse surgery or cannot undergo surgery. Therefore, long-term follow-up is important for the assessment of the effectiveness and safety of RFA for PTMC.
In the past few years, studies from multiple centers have shown that RFA is an effective and safe alternative treatment for low-risk PTMC patients who are concerned about having surgery or post-operative complications [Citation8,Citation31–36]. RFA is also considered as an alternative for PTMC patients who refuse surgery or are ineligible for surgery. Most of the ablation foci are absorbed and disappear 12 months after the treatment. In this study, the whole absorption rate at 1, 3, 6, 9, 12, 24, 36, 48, and 60 months was 0, 0, 9.8 (10/102), 33.3 (34/102), 91.2 (93/102), 96.1 (98/102), 99 (101/102), 100, and 100%, respectively. Three months after the RFA, the VRR value increased significantly by 0.74 (0.29), which is consistent with the results of previous studies. The ablation foci exhibit the fastest volume reduction in the first 3 months after RFA; the absorption rate is the highest according to ultrasonography [Citation30,Citation37]. During the follow-up period, one (0.9%) patient chose to delay surgery owing to anxiety, and he had post-operative pathology of fibrotic thyroid nodules with calcification and no lymph node metastasis. Three patients (2.9%) had hyperthyroidism 1 week after RFA. Their hyperthyroidism returned to normal after a month. One patient was diagnosed with Graves’ disease, and two (1.9%) patients had subclinical hypothyroidism with normalization of thyroid function after 1 month of follow-up. Temporary thyroid function abnormalities (including hyperthyroidism and hypothyroidism) occurred at a rate of 4.9% (5/102). They are the most common complications after RFA because thyroid follicular cells are destroyed during the treatment; thus, the thyroid hormones released into the blood cause hyperthyroidism or subclinical hyperthyroidism.
The unique feature of this paper is that TSH suppressive therapy was used with RFA. The concept of TSH suppression therapy has changed in recent years because DTC patients generally have an excellent survival rate because of the increasing prevalence of PTMC [Citation38,Citation39]. It is not recommended to administer TSH suppressive therapy during the period of active surveillance because the side effects of the drugs are more harmful than the disease itself. Adverse effects of TSH suppression include cardiovascular and bone loss risks [Citation40]. TSH suppressive therapy was also not performed after RFA to not affect the quality of life of low-risk patients [Citation41]. There is little evidence to guide TSH targets or the use of thyroid hormone in low-risk patients who have undergone lobectomy or RFA [Citation2]. In this paper, we combined treatments for the following reasons: first, PTMC is defined not based on the presence of high-risk features, such as lymph node metastases and/or distant metastases but as PTC with a maximum diameter of 1 cm, no clinical lymph node metastases or distant metastases, and FNA findings not indicating high-risk pathological subtypes. The 10-year recurrence rate is 30%, and the specific survival rate is 74.1% [Citation42]. Some patients still have metastases after surgery, and post-operative recurrence is the main cause of death [Citation31]. Second, the biological characteristics of tumors can never be evaluated by size only. So far, it remains impossible to reliably identify inert occult and high-risk cancers [Citation43]. Related studies have confirmed that recurrence and metastasis of PTC are closely related to age, venereal disease, and tumor diameter size [Citation40]. Recently, specific molecular profiles, such as the coexistence of BRAF with other oncogenic mutations like the TERT promoter or TP53 mutations, may serve as more specific markers of a less favorable outcome of PTC. Only active surveillance can identify if a tumor is progressive PTMC or harmless PTMC. Third, related studies and clinical data have shown that thyroid cell proliferation is dependent on TSH; a key point of DTC treatment is to reduce endogenous TSH values of low-risk patients or suppress TSH values of high-risk patients by obtaining exogenous thyroid hormones to reduce the release of TSH [Citation44]. RFA is an in-situ treatment of tumors, and the thyroid gland and LNs are not removed. The two patients whose TSH values met the standards during TSH suppression therapy (TSH <0.1 μIU/mL at primary treatment and TSH <2 μIU/mL at follow-up) developed lymph metastases. To prevent post-operative recurrence and metastasis, it is recommended that TSH suppression therapy be used after surgery for micropapillary thyroid cancer.
There are some limitations to this study. First, it is a single-center retrospective study, and we need to conduct further prospective multicenter studies. Second, the detection of metastatic lymph nodes using ultrasound at an earlier stage cannot completely exclude the possibility of negative metastases. Third, the standards of TSH suppression we used were based on the surgical treatment of thyroid gland removal. Collaboration among different study fields and long-term follow-ups will provide high-quality evidence for TSH suppressive therapy.
Conclusion
Ultrasound-guided RFA for PTMC is highly effective and safe. RFA can serve as a minimally invasive treatment for PTMC patients who refuse surgery or active surveillance.
Acknowledgments
I wish to thank clinical investigators who took care of study patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
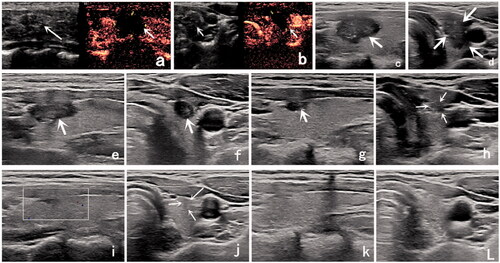
Figure 1. Ultrasound (US) image of a 33-year-old woman with a low-risk papillary thyroid microcarcinoma (PTMC). (a) Before radiofrequency ablation (RFA), US image showing a tumor (arrow) located in the right thyroid lobe; it had an initial volume of 307.13 mm3. (b) Immediately after RFA, the volume of the ablation area (arrow) was 852.82 mm3 on contrast-enhanced ultrasound. (c,d) One month after RFA, the volume of the ablation area (arrow) was 556.09 mm3. (e,f) Three months after RFA, the volume of the ablation area (arrow) was 164.12 mm3. (g,h) Six months after RFA, the volume of the ablation area (arrow) was 26.90 mm3. (i,j) Nine months after RFA, the ablation area could not be identified on the longitudinal US image. There was only a focal concavity in the capsule caused by shrinkage of the scar (arrow) on the transverse US image. (k,l) Twelve months after RFA, the ablation area completely disappeared.
Additional information
Funding
References
- Howlader N, Krapcho M, Miller D, et al. SEER cancer statistics review, 1975–2016 [November 2018 SEER data submission posted to the SEER web site, April 9, 2020]. Bethesda, MD: National Cancer Institute; 2020. Available from: https://seer.cancer.gov/csr/1975_2016/
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Oda H, Miyauchi A, Ito Y, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26(1):150–155.
- Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34(1):28–35.
- Wang TS, Goffredo P, Sosa JA, et al. Papillary thyroid microcarcinoma: an over-treated malignancy? World J Surg. 2014;38(9):2297–2303.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol. 2021;11:575152.
- Guang Y, Luo Y, Zhang Y, et al. Efficacy and safety of percutaneous ultrasound guided radiofrequency ablation for treating cervical metastatic lymph nodes from papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(8):1555–1562.
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717.
- Cho SJ, Baek SM, Na DG, et al. Five-year follow-up results of thermal ablation for low-risk papillary thyroid microcarcinomas: systematic review and meta-analysis. Eur Radiol. 2021. DOI:https://doi.org/10.1007/s00330-021-07808-x
- Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg. 2016;40(3):516–522.
- Anatomical location classification of benign thyroid nodule and expert consensus on risk prevention and control of thermal ablation. Chinese J Med Ultrasonogr. 2020;17(01):6–10.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Endocrinology Society of Chinese Medical Association, Endocrinology Group of Chinese Medical Association Surgery Society, Head and Neck Tumor Professional Committee of China Anti-Cancer Association, Chinese Medical Association Nuclear Medicine Society. Guidelines for the diagnosis and treatment of thyroid nodules and differentiated thyroid cancer. Int J Clin Endocrinol Metab. 2012;4(10):779–797.
- Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007;13(5):498–512.
- Li YJ, Wang YZ, Yi ZB, et al. Comparison of completion thyroidectomy and primary total surgery for differentiated thyroid cancer: a meta-analysis. Oncol Res Treat. 2015;38(10):528–531.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid NODULES-2016 UPDATE. Endocr Pract. 2016;22(5):622–639.
- Haugen BR. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017;123(3):372–381.
- Min Y, Wang X, Chen H, et al. Thermal ablation for papillary thyroid microcarcinoma: how far we have come? Cancer Manag Res. 2020;12:13369–13379.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Kim J, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Lim HK, Cho SJ, Baek JH, et al. Erratum: US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2020;21(4):510.
- Nam-Goong IS, Kim HY, Gong G, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol. 2004;60(1):21–28.
- Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):43–63.
- Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18(4):559–568.
- Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98(1):31–40.
- Qubain SW, Nakano S, Baba M, et al. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131(3):249–256.
- Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121(3):487–491.
- Lee MC, Kim MJ, Choi HS, et al. Postoperative thyroid-stimulating hormone levels did not affect recurrence after thyroid lobectomy in patients with papillary thyroid cancer. Endocrinol Metab. 2019;34(2):150–157.
- Li YJ, Wang YZ, Yi ZB, et al. Comparison of completion thyroidectomy and primary total surgery for differentiated thyroid cancer: a meta-analysis. Oncol Res Treat. 2015;38(10):528–531.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Robenshtok E, Fish S, Bach A, et al. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 97(8):2706–2713.
- Kim JH, Baek JH, Sung JY, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33(2):212–219.
- Lim HK, Cho SJ, Baek JH, et al. US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2019;20(12):1653–1661.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Xiao J, Zhang M, Zhang Y, et al. Efficacy and safety of ultrasonography-guided radiofrequency ablation for the treatment of T1bN0M0 papillary thyroid carcinoma: a retrospective study. Int J Hyperthermia. 2020;37(1):392–398.
- Wu R, Luo Y, Tang J, et al. Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: a retrospective analysis of 198 patients. Int J Hyperthermia. 2020;37(1):168–174.
- Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25(1):112–117.
- Mazzaferri EL, Massoll L. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002;9(4):227–247.
- Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German thyroid cancer study group. An American College of Surgeons Commission on cancer patient care evaluation study. Cancer. 2000;89(1):202–217.
- Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010;20(2):135–146.
- Zhang M, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years' follow-up. Thyroid. 2020;30(3):408–417.
- Sugitani I, Toda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222–1231.
- Miyauchi A, Ito Y, Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid. 2018;28(1):23–31.
- Recognition and challenge of thyrotropin inhibition therapy after thyroid microcarcinoma. Chinese J Pract Surg. 2016;36(05):524–527.