Abstract
Purpose
To explore the feasibility of microwave ablation (MWA) of the vertebral growth plate as a minimally invasive treatment for early-onset scoliosis.
Materials and methods
One side of the L1–L3 vertebral growth plates were ablated using different MWA powers. Ablation safety and size were examined. Subsequently, L1–L3 vertebral growth plates were ablated on one side for 40 s at 20 W. At 2, 4, and 6 weeks after the ablation, growth changes of the spine were observed.
Results
No piglets died during and after ablation, and all had modified Tarlov Grade 5. The safe MWA time (time for safely ablating the vertebral growth plate) was 17.0 ± 1.5 s at 50 W, 23.0 ± 2.3 s at 40 W, 31.0 ± 3.1 s at 30 W, 47.0 ± 3.7 s at 20 W, 70.0 ± 4.2 s at 15 W, and 158.0 ± 5.0 s at 10 W. With power <15 W, the vertebral growth plate could not be effectively ablated within the safe ablation time. Within the safe ablation times, the MWA size on hematoxylin and eosin slices on a transverse diameter was between 7 and 10 mm; and that on longitudinal diameter was mainly determined by the ablation needle length. Moreover, the growth plate and annulus fibrosus on the ablated side grew poorly over time, the vertebral body showed significant wedge-shaped changes, and the spine showed significant unbalanced growth.
Conclusion
MWA of the vertebral growth plate can be performed safely when accompanied with appropriate thermometry, and could be a new minimally invasive strategy in regulating spine growth.
Introduction
Scoliosis is a common spinal abnormality in children and adolescents [Citation1]. Although a small curve of the spine in children and adolescents can be tolerated well and does not need further treatment, a large curve may progress and affect an individual’s appearance, seriously interfere with chest wall mechanics, diminish the space available for the lungs and heart, interfere with cardiac and pulmonary functions, and, in extreme cases, lead to cardiopulmonary failure or early death [Citation2,Citation3]. Currently, the surgical treatment for early-onset scoliosis (EOS) has numerous limitations, such as substantial surgical trauma, early spinal fusion, high infection rate, loosening and fracture of internal fixation, multiple operations, and long treatment cycle [Citation4,Citation5]. Thus, developing a minimally invasive technology with fewer complications for the treatment of EOS is vital.
Many studies reported that the curve progression of scoliosis in children and adolescents is due to asymmetric spine growth (Hueter–Volkmann Law) [Citation6–8]. It is commonly assumed that a spine with scoliosis has greater loading on the concave side, and this, together with compression on the concave side causes asymmetric growth, creates vertebral wedging, and leads to the ‘vicious cycle’ of scoliosis progression [Citation9]. Thus, if the growth of bilateral sides of the vertebral body can be selectively regulated, this asymmetric growth could be reversed, the ‘vicious cycle’ interrupted, and the scoliosis curve corrected.
The vertebral growth plate is a cartilage layer between the vertebral body and intervertebral disk tissue. It is similar to the long bone epiphysis and plays an important role in the growth and development of the spine [Citation10]. Regulating proliferation, differentiation, or apoptosis of chondrocytes in the vertebral growth plate may have a vital effect on spine growth in children and adolescents. These processes regulate spinal growth and may be manipulated to correct scoliosis [Citation11]. Epiphyseal block technology is an orthopedic method that completely or partially blocks the process of osteogenesis by changing the symmetrical area and pressure of the epiphyseal plate; it reverses the original growth asymmetry of bone tissues, using the growth imbalance to correct the deformity [Citation12]. Currently, this technology has been widely used in the treatment of limb inequality and scoliosis, using U-shaped nails and tethers [Citation13,Citation14]. However, traditional epiphyseal block techniques involve open surgery, and an internal fixation device is implanted to regulate the growth potential or mechanical stress of the vertebral growth plate. Thus, the surgical trauma is substantial, especially in children and adolescents. Moreover, the epiphyseal block effect is achieved indirectly by adjusting the mechanical stress. However, the mechanism and ability of mechanical stress to regulate spinal growth have not been clarified, and indirect adjustment of mechanical stress to correct scoliosis is limited as it has only been used in patients with mild scoliosis [Citation15,Citation16].
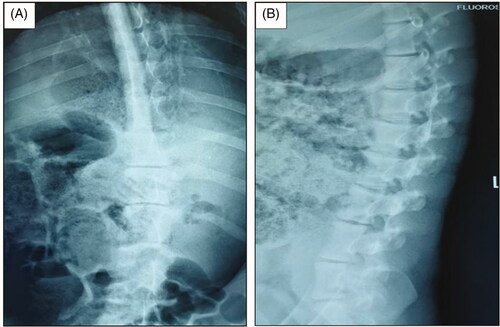
In our previous experiments, we found that EOS in piglets could be induced by directly damaging one side of the vertebral growth plate via percutaneous puncture with thermal injury (high-frequency electrotome) (). However, high-frequency electrocoagulation therapy is not a conventional and mature technology. Among the current thermal therapies, microwave ablation (MWA) is a technique that offers many theoretical advantages over other thermal therapies: high thermal efficiency, i.e., rapid microwave heating and better anti-heat sink effect; sufficiently large ablation volume (single needle puncture can be used); and easy operation (negative plates are not needed, and repeated puncture and recovery of electrodes are not necessary during the operation) [Citation17,Citation18]. Hence, we speculated that MWA may be effective in regulating spine growth.
Figure 1. EOS in piglets induced by high-frequency electrotome at 3 months after the operation. Ablation power, 30 W; ablation time, 30 s; and spine segments ablated, 3. (A) Anteroposterior X-ray image of the spine. (B) Lateral X-ray image of the spine.
MWA has made significant achievements in the ablation of benign and malignant tumors, and its effectiveness has been recognized by most scholars [Citation19,Citation20]. However, the MWA of the vertebral growth plate for EOS treatment has not been investigated, and its feasibility is unknown. The vertebral bodies are surrounded by critical structures, such as the spinal cord and nerves, and excessive or inappropriate ablation energy may put these structures at risk. Thus, this study aimed to investigate the safety and size of MWA on the vertebral growth plate and to explore its feasibility as a minimally invasive intervention for EOS correction.
Materials and methods
The Institutional Ethics Committee of our hospital approved the use of swine in this study, and the protocols were performed by the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23). All experimental piglets received proper care in compliance with the guidelines.
For the MWA in this study, a microwave workstation (ECO-100A13, Yigao Co. Ltd., Nanjing, China) and matched ablation electrode antenna were used. The MWA system can reach a maximum temperature of >100 °C. The adjustable output power of the continuous wave ranges between 0 and 100 W, with an output frequency of 2450 MHz and a maximum ablation diameter of 3 cm when the electrode antenna is operating at full power. A 16 G water-cooled antenna was used in MWA, and the terminal microwave emission shaft was 1.2 cm (the antenna geometry was based on the size of the vertebral body measured in the pre-experiment). There were two cavities in the antenna, which were used for the peristaltic pump to circulate cooled water to the antenna shaft. The temperature-measuring device was an iron-constantan thermocouple wire. The electrocouple wire diameter was 1.0 mm, and the temperature-measuring response time could reach 0.1 s. The electric coupling wire was connected to the data collector, which could collect temperature data accurately to 0.1 °C and read the temperature data at any time.
Grouping
Thirty-five piglets (mean age 45 ± 3 days, weight 15.1 ± 0.3 kg) were provided by the Animal Experiment Center of Kunming Medical University (Kunming, China). Twenty-six piglets were randomly classified into seven groups according to different MWA powers: 50 W (n = 4), 40 W (n = 4), 30 W (n = 4), 20 W (n = 4), 15 W (n = 4), 10 W (n = 4), and sham (n = 2) groups. The experiment groups underwent MWA of the vertebral growth plate (L1–L2 and L2–L3). In the sham group, ablation needles were inserted only for 30 s and no ablation was performed. Subsequently, the other nine piglets were included; L1–L3 vertebral growth plates on one side were ablated using microwave energy (20 W, 40 s). At 2, 4, and 6 weeks after the operation, three of the nine piglets were sacrificed separately to observe the growth changes in the spine, vertebral body, and growth plate.
Preoperative preparation
The experimental pigs were in abrosia for 12 h before the experiment and were deprived of water for 4 h. The body weight, body length, and anal temperature of the experimental piglets were recorded before anesthesia induction. General anesthesia using 3% pentobarbital and atropine was quickly induced, and the anesthesia apparatus connected to the piglet; anesthesia was continued by inhalation (1.2% isoflurane). The depth of anesthesia was maintained at 1 minimum alveolar concentration. Arterial and venous channels were established to monitor blood pressure. After intravenous administration of 1 g cefoperazone in 100 ml of 0.9% sterile physiological saline, the circulation was maintained with balanced salt and colloidal solution alternately.
Surgery
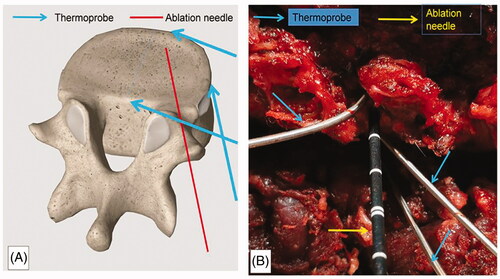
The location of the intervertebral disks was confirmed by C-arm X-ray scanning, and the corresponding body surface was marked. The incision was located ∼1.5 cm from the spinous process, and the skin, subcutaneous tissue, and thoracolumbar dorsal fascia were incised in sequence. The interspace between multifidus and longissimus muscles was bluntly separated to reach the facet joint. The soft tissue was removed by nucleus pulposus forceps; thereafter, the facet joints and intervertebral disks were fully exposed. The paradisiacal soft tissue was separated laterally, and the growth plate punctured at a 45° angle at the junction between the posterior and anterior edges of the intervertebral disk with a Kirschner needle, which was subsequently replaced with an MWA needle (). Three thermometric probes were placed separately at the posterior, lateral, and anterior edges of the puncture site of the intervertebral disk to monitor the temperature variation during ablation (). When inserting thermocouples, make sure the tip of the thermocouple is 5 mm away from the MWA probe to avoid direct heating of the thermocouple.
Figure 2. Placement of the ablation needle and thermoprobe. The ablation needle was placed at a 45° angle at the junction between the posterior and anterior edges of the intervertebral disk. The temperature of the posterior, lateral, and anterior edges of the punctured side of the intervertebral disk was monitored during ablation. (A) Diagrammatic sketch. (B) Intraoperative view.
Ablation procedure
The active tip of the antenna was inserted into the intervertebral disk for at least 2 cm, which corresponded to the antenna length to avoid heating tissues outside the ablation area. The antenna inlet and outlet were connected to a peristaltic pump and cooled with 0.9% sodium chloride solution at 4 °C; thereafter, the cold circulation system was turned on, the actual output power of the microwave generator was adjusted to 50 W, and the ablation mode set to manual. After the thermal ablation was started, the posterior, lateral, and anterior margins of the punctured side of the intervertebral disk were monitored simultaneously; when the temperature of the posterior edge reached 43 °C [Citation25,Citation26] or that of the lateral and anterior edges reached 60 °C during ablation [Citation26], MWA was stopped immediately. Safe ablation time was defined as the elapsed time when the temperature of the posterior wall of the vertebral disk reached 43 °C or that of the lateral wall reached 60 °C; the corresponding effective ablation area was the safe ablation size. Effective ablation was defined as achieving a temperature >60 °C on the vertebral growth plate, with tissue ablation necrosis of pathological sections [Citation27]. Subsequently, the ablation probe was removed, and a thermometric probe was inserted immediately to monitor the ablation center temperature. In accordance with the above steps, the ablation powers used in experimental groups were as follows: 50, 40, 30, 20, 15, and 10 W.
Post-operative management and sampling
The animals were reared in a single cage after waking up, and their vital signs, limb activities, and gait were observed. Neurologic function was evaluated with a modified Tarlov grading system at 1, 3, and 5 days after the operation as follows: Grade 0 – complete paraplegia with no hind extremity motion, Grade 1 – minor joint movements, Grade 2 – major joint movements, Grade 3 – the animal can stand, Grade 4 – the animal can walk, and Grade 5 – the animal can climb a 20° inclined plane [Citation21,Citation22].
A lethal dose of pentobarbital was injected into the neck muscles of pigs for euthanasia when needed. After the experimental animals were sacrificed, the ablated vertebral bodies and disks were isolated, and vertebral growth plate samples were obtained along with the longitudinal and vertical directions of the electrode needles. The specimens were immediately fixed with 4% formaldehyde solution, sectioned, and examined after hematoxylin and eosin (HE), and terminal deoxynucleotidyl nick end labeling (TUNEL) immunofluorescence staining. The morphology and extent of thermal damage were assessed, and the longitudinal and transverse diameters of the section with the greatest damage were measured.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 software (SPSS Inc, Chicago, IL). Quantitative variables were reported as mean (± standard deviation). The size of the ablated intervertebral disks was compared with t-test. The ablation sizes among the groups were compared using analysis of variance, and pairwise comparative analysis was performed using the least significant difference method. Tests were two-sided, and a p-value <0.05 was considered statistically significant.
Results
General information
Successful operations were achieved in all 35 pigs in the experiments (16 males and 19 females, 26 pigs in experiment one, and nine pigs in experiment two). In experiment one, the transverse diameter of the ablated L1–L2 and L2–L3 intervertebral disks was 28.0 ± 1.4 and 28.5 ± 1.9 mm, respectively; the longitudinal diameter was ∼17.8 ± 1.6 and 18.8 ± 2.3 mm, respectively. No significant difference was found between the size of the L1–L2 and L2–L3 intervertebral disks (p > 0.05); thus, we deduced that there was no difference in the size of the corresponding vertebral growth plate ().
Table 1. Comparison of the sizes of the ablated intervertebral disks (L1–L2 and L2–L3).
Safe ablation time and ablation size
In this study, there was no obvious resistance during the antenna pullout process, no carbonized tissue adhesion on the outside shaft, and no burning changes in the tissue at the antenna puncture site.
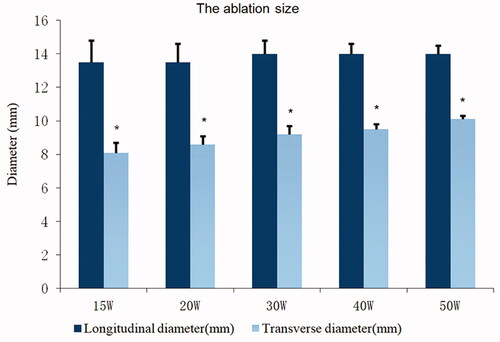
At the start of ablation, the posterior wall of the vertebral disk first reached the safe ablation temperature threshold; hence, the safe ablation time of the vertebral growth plate is mainly determined by the temperature of the posterior wall of the vertebral disk. In this study, the safe ablation time of the vertebral growth plate was 17.0 ± 1.5 s at 50 W, 23.0 ± 2.3 s at 40 W, 31.0 ± 3.1 s at 30 W, 47.0 ± 3.7 s at 20 W, 70.0 ± 4.2 s at 15 W, and 158.0 ± 5.0 s at 10 W, which shows that as the output power increases, the safe ablation time shortens (). When the power was <15 W, the vertebral growth plate could not be effectively ablated within the safe ablation time; the HE sections only showed scattered tissue dehydration and no tissue necrosis (). Within the safe ablation time and in effective ablation, the median energy dose received at different ablation powers was 938 ± 50 J. Moreover, within the safe ablation time, the energy tolerated by the vertebral growth plate gradually decreased and as the ablation power increased and changed significantly at 15 W ().
Figure 3. Safe ablation time under different powers. The safe ablation time changed significantly at 15 W.
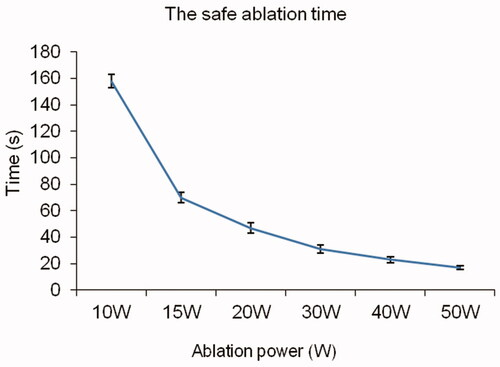
Figure 4. Safe ablation energy under different powers. The energy tolerated by the vertebral growth plate changed significantly at 15 W.
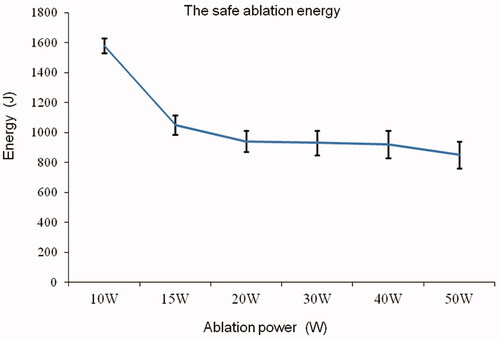
After MWA, ablated tissues showed marked differences on visual inspection. The ablation area of the vertebral growth plate was ellipsoidal in shape and had no clear boundary with normal tissues. The ablation area was mainly divided into four: normal vertebral growth plate (white area), transitional area (white-yellow area), damaged necrosis area (yellow area), and needle track carbonization area (slightly black area) (). After HE staining, the vertebral growth plate around the needle track showed coagulative necrosis of varying degrees, the tissue structure disappeared, cell morphology disappeared, and the nucleus showed pyknosis (). On the HE section, there was an obvious boundary between the non-ablated and ablated tissue (). The reserve and proliferation layers were absent and only the hypertrophic layer remained in the ablated cartilage (); in the non-ablated cartilage, cell layers were clearly seen (). After TUNEL immunofluorescence staining, chondrocyte apoptosis in the ablation area was confirmed (), whereas no apoptosis occurred in the non-ablated area. The size of necrosis was 1–2 mm larger than the ablation size shown on the HE section, and no clear boundary was noted. Given the obvious boundary in the HE slices, the ablation size was defined as that seen on the HE section. Based on the HE slices, the ablation size at different powers on a transverse diameter and within the safe time was between 7 and 10 mm (), and that on a longitudinal diameter was mainly determined by the ablation needle penetration length. Under the maximum safe ablation time, the ablation size was significantly different between groups with a power >15 W (*p < 0.05) ().
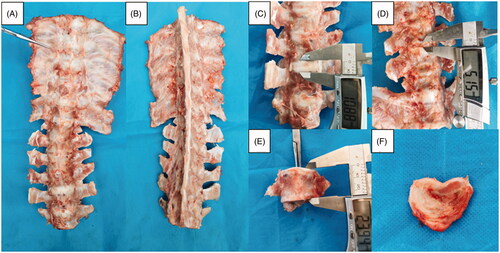
Figure 5. Ablated area of the vertebral growth plate. (A) Visual inspection. (B) Hematoxylin and eosin (HE) section overview. (C) Normal vertebral growth plate on HE staining clearly showing cell layers. (D,E). Ablated vertebral growth plate on HE staining showing that the reserve and proliferation layers were absent and only the hypertrophic layer remained in the ablated area. (F) TUNEL immunofluorescence staining showing chondrocyte apoptosis in the ablation area (The red circles show necrosis of chondrocytes, and the blue circles show no necrosis of chondrocytes).
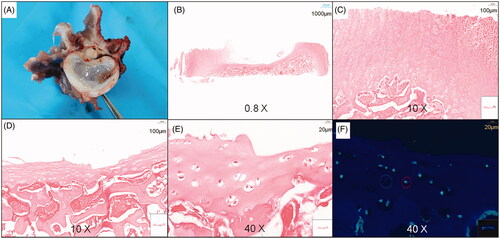
Figure 6. Ablation size on hematoxylin and eosin (HE) slices under different powers. (A–C) The ablation size of different powers on a transverse diameter with a power >15 W was between 7 and 10 mm within the safe MWA time. (D) No cell necrosis in the vertebral growth plate was observed for a power <15 W within the safe WMA time.
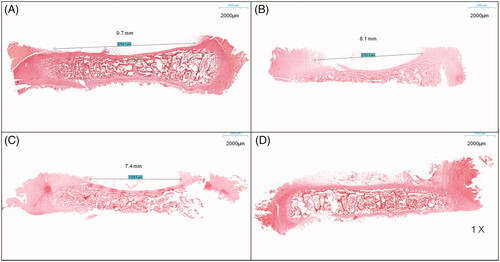
Effect of MWA on spine growth
At 2, 4, and 6 weeks after MWA, the ablated segmental spine grew asymmetrically and developed structural scoliosis over time (). Unilateral growth plates of the vertebral bodies of the ablated segments showed a marked lack of growth, significant vertebral height effect, significant wedge-shaped vertebral body changes (), and dysplastic disks and annulus fibrosus on the ablated side (). The contralateral side showed normal growth (). No spinal canal stenosis was found in all experimental pigs, and the neurological outcome was rated as modified Tarlov grade 5.
Figure 8. Asymmetric segmental spine growth and structural scoliosis after the operation. (A–C) Spine in the neutral position with progressive scoliosis within 6 weeks. (D,E) Flexion and extension positions showing a structural scoliosis curve at 6 weeks after the operation.
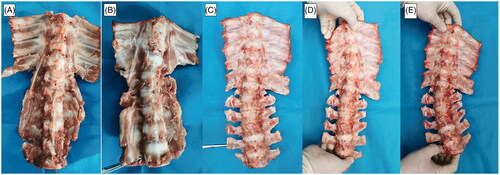
Figure 9. Asymmetric segmental spine growth and structural scoliosis at 6 weeks after the operation in piglets with MWA. (A,B) Spine in the neutral position showing mild scoliosis. (C,D) Dysplastic disk annulus fibrosus on the ablated side, and normal contralateral side. (E) Significant wedge-shaped changes in the vertebral bodies. (F) Intervertebral disk dysplasia on the ablated side and normal contralateral side.
Complications and safety
All piglets were able to move freely and eat normally after recovery from anesthesia and had modified Tarlov Grade 5 at 1, 3, and 5 days after the operation. No symptoms of nerve root palsy or spinal cord injury were observed, and no other serious complications were found. Cell necrosis was not observed in the HE staining of the spinal cord. No piglet died during and immediately after the ablation procedure, and all piglets were sacrificed as planned.
Discussion
The application of MWA in benign and malignant tumors has been extensively studied, and the relevant ablation parameters have been widely reported. Noventa et al., reported MWA using charts of time and power in human renal tumors and compared them to the charts by HS AMICA [Citation23]. Khan et al., performed an experiment on 69 patients with 102 painful spinal metastases undergoing MWA and cementoplasty, to determine the efficacy and safety of MWA in the treatment of spinal metastases [Citation24]. In their experiment, they used the following parameters: median ablation time, 4 min 30 s ± 7 s, and energy dose, 4.1 ± 1.6 kJ. Blood supply to the vertebral growth plate is insufficient to significantly cool the treated area; hence, heat could not be dissipated in time. Moreover, the spinal cord and nerves are located behind the vertebral body, and intervertebral disk and heat accumulation may lead to nerve injury; thus, MWA parameters that are common in other applications cannot be directly used in the ablation of the vertebral growth plate.
In this study, we first explored the feasibility of applying MWA to the vertebral growth plate. We found that after a period of ablation, the temperature of the posterior edge of the vertebral disk reached a safe threshold before the anterior and lateral edges. Moreover, the safe ablation time of the vertebral growth plate was mainly limited by the temperature of the posterior wall of the vertebral disk () and changed significantly at 15 W, which may be attributed to the local heat balance (). In addition, with an increase in output power, the ablation size slightly increased; a statistically significant difference in the increase in ablation size was observed in the groups with >15 W of power (statistically significant differences in ablation on transverse diameter, but not on longitudinal diameter, were found; the significance level of mean difference was 0.05) (). With power <15 W, the temperature of the vertebral growth plate did not reach 60 °C within the safe ablation time, only scattered tissue dehydration was found in the pathological section, with no definite tissue necrosis (). In the sham group, no cell necrosis was observed in the growth plate. The safe MWA time of the vertebral growth plate was significantly shorter than that of other tissues [Citation19,Citation20].
The spinal cord is surrounded by cerebrospinal fluid and a dural sac. In actual experiments, stripping the dural sac to measure the spinal cord temperature is not possible as such action would inevitably affect the local thermal circulation. Thus, the temperature probe in our experiment was placed between the posterior edge of the vertebral body and the dural sac. Animal studies show that the maximum heat dose without obvious complications after localized hyperthermia in regions of the central nervous system (CNS) lies within the range of 40–60 min at 42–42.5 C or 10–30 min at 43 °C [Citation25,Citation26]. Hence, in our experiment, the safe temperature of the posterior edge of the vertebral body was set at 43 °C, and ablation was stopped when the temperature of the posterior edge of the vertebral body reached 43 °C. In addition, spinal cord blood flow and cerebrospinal fluid circulation can dissipate a certain amount of heat and play a protective role in the spinal cord. Previous studies have demonstrated that a temperature >60 °C results in irreversible damage to the tissues outside the nerve [Citation27]. Thus, we set the safe temperature of the anterior and lateral edges of the vertebral growth plate at 60 °C in our experiment, which allowed the experimental animals to move freely and eat normally after vertebral growth plate ablation; all pigs had modified Tarlov Grade 5 after the operation, and cell necrosis was not observed in the HE staining of the spinal cord. These results prove that a transient temperature of 43 °C at the posterior edge and 60 °C at the anterior and lateral edges of the vertebral growth plate are safe during microwave ablation of the vertebral growth plate.
In our experiment, the safe energy that can be tolerated at different power levels varied. Effective ablations performed within the safe ablation time had median energy levels of 938 ± 50 J at a power >15 W (). The higher the ablation power, the lower the safe energy that can be tolerated, which may be because the accumulated energy cannot be emitted in time. If the accumulated energy and local heat dissipation are balanced, the local temperature would not increase and effective tissue ablation would not be achieved. Moreover, given the different sizes of the vertebral body and disk across different ages, the acceptable safe energy should likely be varied accordingly. We suggest that an appropriate power and ablation time be used to avoid extremely high ablation energy during MWA of the vertebral growth plate, and local energy accumulation should be considered to avoid substantial deviation of ablation energy, which affects ablation effectiveness and safety. In addition, magnetic resonance thermometry may be a better, less invasive approach to monitoring the ablation size and safety during operation.
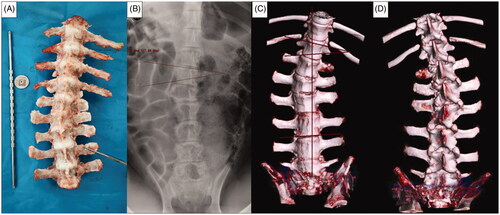
Numerous studies have reported that limb growth plate injury can lead to limb shortening and deformity [Citation28,Citation29]. In our previous experiments, we found that thermal injury on one side of the vertebral growth plate can induce EOS in an animal model. Thus, the ability of the thermal intervention to adjust spine growth may be used for spontaneous correction of EOS. However, the mechanism involved in EOS due to thermal injury of the vertebral growth plate is unclear. The vertebral growth plate area and the number of segments that need to be ablated during MWA have not yet been established. Hence, we assumed that in the early experimental stage, striving for the maximum ablation area within the safe ablation time may be a better approach to achieving spinal growth regulation. In our subsequent experiment, we successfully induced EOS in piglets using 20 W (for 40 s) to ablate different segments of the vertebral growth plates adjacent to L1–L4; single or double needles were employed (0.5 cm apart). The Cobb angle of single-segment ablation at 3 months after the operation ranged from 10 to 15° (mean, 13°) and that of three-segment ablations ranged from 12 to 20° (mean, 17°); no statistically significant difference between single and double-needle groups was found (). Some ablated spine segments showed bony fusion at 3 months after the ablation, and the bony fusion ratio was 30%. However, in our preliminary experiment, no fusion was found in the ablated segment at 6 weeks.
Figure 10. EOS in piglets induced by MWA at 3 months after the operation. Ablation power, 20 W; ablation time, 40 s; spine segments ablated, 3; needle ablation, single. (A) Gross spine image. (B) Anteroposterior X-ray image of the spine. (C,D) Three-dimensional computed tomography (CT) image of the spine.
MWA of the vertebral growth plate can be performed by a percutaneous puncture; thus, the trauma is less than that in traditional surgical techniques for EOS [Citation4,Citation5]. As internal fixation is not needed in MWA, thus, complications, such as infection and loosening and fracture of internal fixation, can be effectively avoided. Compared with the traditional indirect epiphyseal block technique [Citation15,Citation16], MWA of the vertebral growth plate can directly interfere with the growth of the vertebral growth plate and may have a better blocking effect. Thus, MWA of the vertebral growth plate could be a novel minimally invasive method for the treatment of EOS. Nonetheless, this new strategy has numerous challenges that need to be addressed. In this preliminary study, we strived for the largest ablation under safe conditions to achieve spine growth regulation. However, a large ablation volume may put nearby critical structures, such as the spinal cord and spinal nerves, at risk. Currently, the ablation volume with correction ability has not been clearly identified. In future experiments, the relationship between ablation volume and spinal growth should be studied in detail to achieve precise growth control and to reduce the risks. In addition, thermal injury and degenerative changes of the intervertebral disk may limit the clinical applicability of this technique.
This study has several limitations. First, the samples in this study were small, and the size of the animal possibly influenced the thermal and ablation effects on the intervertebral disks. Second, the ablation time was measured manually; electronic and automatic measurement is more accurate. Lastly, this study tested only the lumbar vertebrae of piglets; thus, the parameters in this study may not be safe in the thoracic vertebrae or lumbar vertebrae of other animals of different sizes.
In conclusion, our pre-clinical study in a piglet model demonstrates that MWA of the vertebral growth plate can be performed safely when accompanied with appropriate thermometry. The technique requires further characterization and testing, but it is a promising minimally invasive strategy for regulating spine growth and correcting scoliosis in children and adolescents.
Acknowledgments
We would like to acknowledge ‘Taylor & Francis Editing Services’ for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wessell NM, Martus JE, Halanski MA, et al. What's new in pediatric spine growth modulation and implant technology for early-onset scoliosis? J Pediatr Orthop. 2018;38(1):e3–e13.
- Harris LR, Andras LM, Sponseller PD, et al. Comparison of percentile weight gain of growth-friendly constructs in early-onset scoliosis. Spine Deform. 2018;6(1):43–47.
- Redding GJ, Hurn H, White KK, et al. Persistence and progression of airway obstruction in children with early onset scoliosis. J. Pediatr. Orthop. 2020;40(4):190–195.
- Thakar C, Kieser DC, Mardare M, et al. Systematic review of the complications associated with magnetically controlled growing rods for the treatment of early onset scoliosis. Eur Spine J. 2018;27(9):2062–2071.
- Fedorak GT, Garg S, Heflin JA, et al. Management of early-onset scoliosis-from Mehta casts to magnetically expandable rods: state of the art in 2019. Instr Course Lect. 2020;69:641–650.
- Huynh AM, Aubin CE, Rajwani T, et al. Pedicle growth asymmetry as a cause of adolescent idiopathic scoliosis: a biomechanical study. Eur Spine J. 2007;16(4):523–529.
- Li QY, Zhong GB, Liu ZD, et al. Effect of asymmetric tension on biomechanics and metabolism of vertebral epiphyseal plate in a rodent model of scoliosis. Orthop Surg. 2017;9(3):311–318.
- Labrom FR, Izatt MT, Contractor P, et al. Sequential MRI reveals vertebral body wedging significantly contributes to coronal plane deformity progression in adolescent idiopathic scoliosis during growth. Spine Deform. 2020;8(5):901–910.
- Stokes IA, Gardner-Morse M. Muscle activation strategies and symmetry of spinal loading in the lumbar spine with scoliosis. Spine. 2004;29(19):2103–2107.
- Bylski-Austrow DI, Glos DL, Wall EJ, et al. Scoliosis vertebral growth plate histomorphometry: comparisons to controls, growth rates, and compressive stresses. J Orthop Res. 2018;36(9):2450–2459.
- Driscoll M, Aubin CE, Moreau A, et al. Spinal growth modulation using a novel intravertebral epiphyseal device in an immature porcine model. Eur Spine J. 2012;21(1):138–144.
- Stevens PM, Klatt JB. Guided growth for pathological physes: radiographic improvement during realignment. J Pediatr Orthop. 2008;28(6):632–639.
- Sunni N, Askin GN, Labrom RD, et al. The effect of vertebral body stapling on spine biomechanics and structure using a bovine model. Clin Biomech. 2020;74:73–78.
- Bogie R, Roth AK, Willems PC, et al. The development of a representative porcine early-onset scoliosis model with a standalone posterior spinal tether. Spine Deform. 2017;5(1):2–10.
- Newton PO. Spinal growth tethering: indications and limits. Ann Transl Med. 2020;8(2):27.
- Cheung ZB, Selverian S, Cho BH, et al. Idiopathic scoliosis in children and adolescents: emerging techniques in surgical treatment. World Neurosurg. 2019;130:e737–e742.
- Di Martino M, Rompianesi G, Mora-Guzmán I, et al. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur J Surg Oncol. 2020;46(5):772–781.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Facciorusso AG, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: an updated review. World J Gastrointest Pharmacol Ther. 2016;7(4):477–489.
- Aufranc V, Farouil G, Abdel-Rehim M, et al. Percutaneous thermal ablation of primary and secondary lung tumors: comparison between microwave and radiofrequency ablation. Diagn Interv Imaging. 2019;100(12):781–791.
- Bohlman HH, Bahniuk E, Raskulinecz G, et al. Mechanical factors affecting recovery from incomplete cervical spinal cord injury: a preliminary report. Johns Hopkins Med J. 1979;145(3):115–125.
- Tarlov IM, Klinger H. Spinal cord compression studies: II. Time limits for recovery after acute compression in dogs. AMA Arch Neurol Psychiatry. 1954;71(3):271–290.
- Novanta A, Herpe G, Vesselle G, et al. Chart for renal tumor microwave ablation from human study. Diagn Interv Imaging. 2018;99(10):609–614.
- Khan MA, Deib G, Deldar B, et al. Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma. Am J Neuroradiol. 2018;39(7):1376–1383.
- Carlander J, Koch C, Brudin L, et al. Heat production, nerve function, and morphology following nerve close dissection with surgical instruments. World J Surg. 2012;36(6):1361–1367.
- Haveman J, Sminia P, Wondergem J, et al. Effects of hyperthermia on the central nervous system: what was learnt from animal studies? Int J Hyperthermia. 2005;21(5):473–487.
- Zhou P, Liang P, Yu X, et al. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg. 2009;13(2):318–324.
- Hell AK, von Laer L. [Growth behaviour after fractures of the proximal radius: differences to the rest of the skeleton]. Unfallchirurg. 2014;117(12):1085–1091.
- Wang KK, Novacheck TF, Rozumalski A, et al. Anterior guided growth of the distal femur for knee flexion contracture: clinical, radiographic, and motion analysis results. J. Pediatr Orthop. 2019;39(5):e360–e365.