?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
This study aimed to evaluate the efficacy and safety of ultrasound-guided microwave ablation combined with glucocorticoid therapy for treating idiopathic granulomatous mastitis (IGM).
Methods
From June 2017 to March 2020, 50 consecutive patients diagnosed with IGM using puncture histology were included. All patients received prednisone and ultrasound-guided microwave ablation and were closely monitored for 12–15 months.
Results
A total of 222 lesions in 50 patients were ablated. The results indicated that 78% of cases were cured within 12 months and an additional 20% were cured within 15 months; the recurrence rate was 2%. The clinical and pathological remission rate of the entire group was 98%. The main postoperative complications were local pain, skin ulcerations and sinus formation, skin and areola heat damage, subcutaneous congestion, and fat liquefaction, all of which were conservatively treated.
Conclusion
Microwave ablation combined with glucocorticoid therapy was safe and effective for the treatment of IGM, with a low recurrence rate. In addition, the cosmetic appearance of the affected breast was preserved with little trauma. Therefore, microwave ablation is a viable method that can be successfully applied in clinical practice.
1. Introduction
Idiopathic granulomatous mastitis (IGM) is a chronic inflammatory disease with lesions confined to the lobules of the mammary gland with no caseous necrosis. It was first described by Kessler et al. in 1972 [Citation1] and is characterized by granulomas and abscesses. The disease manifests as breast lumps which often appear suddenly and enlarge over a short period of time. After 1 to 2 weeks, small areas of the skin of the breast overlying the lumps appear reddish and swollen and microabscesses form within; subsequently, local ulceration leads to the formation of sinuses, resulting in a protracted disease course. Other symptoms include galactorrhoea, pain, dented nipples, and secretions. At present, most scholars believe that IGM is an autoimmune disease [Citation2], and its pathogenesis possibly involves milk being deposited into the ducts at the end of the lobules; the milk then exudes into the interlobular connective tissue causing type IV hypersensitivity reactions, giant cell and lymphocyte infiltration, and the formation of granulomas [Citation3].
Based on its clinical manifestations, IGM has the appearance of a condition of ‘benign disease and malignant invasion’ [Citation4]; it is locally invasive and has a recurrence rate as high as 50% [Citation5]. Currently, there is no unified treatment for IGM. Most physicians use glucocorticoids with combined incision and drainage, surgical resection, or extended resection. However, these treatment methods are unsatisfactory in terms of efficacy. In view of this, we explored the efficacy of oral glucocorticoids combined with nonsurgical resection using microwave ablation (MWA) as a possible comprehensive treatment regimen for patients with IGM.
2. Data and methods
2.1. General information
A total of 50 IGM patients treated in our department between June 2017 and March 2020 were retrospectively recruited in this cross-sectional study. This study was approved by the Ethics Committee of the Affiliated Hospital of Putian University (No. 202044). The requirement for informed consent was waived due to the retrospective nature of the study.
The inclusion criteria were 1) Clinical physical examination and ultrasonography indicating the possible presence of clear lesions in the breast; 2) IGM diagnosis confirmed by puncture histopathology (core needle biopsy[CNB]) interpreted based on breast pathology [Citation6] and the typical pathology of IGM (noncaseating necrotic granulomas or microabscesses confined to the breast lobules); 3) patient refusal of surgical resection.
Th exclusion criteria were: 1) severe coagulation dysfunction; 2) acute infectious diseases in other parts of the body; 3) conditions that prohibit the use of glucocorticoids such as diabetes, liver and kidney insufficiency, and viral hepatitis; 4) pregnancy or lactation.
2.2. Equipment
The MWA instrument (KY‐2000, Nanjing Kangyou Medical Technology Cooperation, Nanjing, China) was used at a frequency of 2450 MHz. The outer diameter of the MWA needle was 16 G, and the transmitting tip was 3 mm long. Ultrasound was performed using an American GE Logiq P9 ultrasound instrument (probe: 9–12 L MHz) and the instrument was operated using an ultrasound contrast software platform equipped with an Italian Demetro 16 G semi-automatic biopsy needle.
2.3. Research methods
2.3.1. Preparation before microwave treatment
Patient data for all recorded IGM cases are summarized in . Under ultrasound guidance, the 16 G core needle was used for needle biopsy of the intramammary lesions. After examination by two professional pathologists, the diagnosis of IGM was confirmed. After the confirmation of IGM, treatment with oral prednisone acetate tablets (20 mg qd) was initiated and continued for 6–8 days; the largest diameter of the main lesion was measured and recorded daily. When the maximum diameter of the lesion was reduced by <5%, the patient was admitted to the hospital and participated in a detailed preoperative discussion.
Table 1. General data of IGM patients undergoing microwave ablation.
2.3.2. Methods
Ultrasound was used to locate the lesions; the locations, sizes, amounts, blood flow signals, and echo characteristics of the lesions were recorded. Regarding anesthesia, local anesthesia was the first-line option though intravenous general anesthesia was used in cases of large lesions. Local infiltration anesthesia was administered under the skin and the posterior space of the breast. Lesions closer to the skin, areola, and muscles were injected with normal saline to create a barrier to avoid heat damage. An individualized ablation treatment plan was formulated based on the lesion ultrasound results. Components of the treatment plan included: selection of the needle insertion point, depth of needle insertion, number of needle insertions, fluid aspiration from the lesion, and ablation duration. The microwave power was set to 25–30 W. The active tip of the MWA needle was placed in the lesion under ultrasound guidance, and the entire process was continued under multisectional dynamic ultrasound observation. The expanded breast duct was gradually ablated along the tube wall from deep to shallow depth. Fixed-point ablation was used for micro-abscesses, whereas multi-point, multi-level mobile ablation was required for multiple lesions and larger lesions (maximum diameter >2 cm). Contrast-enhanced ultrasound (CEUS) was used to determine whether ablation was complete [intraoperative ultrasound monitoring was completed by bolus injection of SonoVue (2.4 ml, through the cubital vein)]. When no contrast agent perfusion was observed in the ablation area, the ablation was considered to be complete. If ablation was not complete, the procedure had to be repeated immediately. The patient was discharged 4–6 h after treatment with local dressing and an ice pack.
2.4. Observation and postoperative follow-up
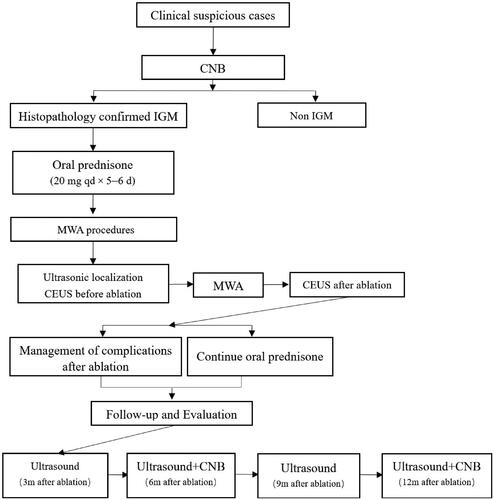
After microwave treatment, the patient was continued on oral prednisone acetate tablets at 20 mg once daily for 3 days followed by 5 mg once daily until the drug was discontinued. The entire course of glucocorticoid treatment did not exceed 2 weeks. Outpatient follow-up visits were conducted at 3 months, in June, in September, and at 12 months following treatment. The following indicators were reviewed using ultrasound and recorded during follow-ups: lesion size, presence or absence of local effusion and sinus formation, blood flow signal, and the time to complete regression. All patients underwent two pathological reexaminations (needle biopsies) of the original breast lesions at 6 and 12 months. shows a flowchart of patient enrollment and the MWA treatment procedures used in this study.
Recovery was judged based on the following criteria: 1) an ultrasound with no lesion detected—thereby indicating that the original lesion was completely absorbed and that there were no new lesions (on the ipsilateral side); 2) no requirement of additional MWA treatment, oral hormone administration, or surgical resection; 3) the return of normal breast appearance and softness. The follow-up period was extended to 15 months for patients who did not recover within 12 months. Additionally, we surveyed patients' post-procedural satisfaction with their breast appearance and softness.
2.5. Statistical methods
SPSS 22.0 statistical software was used for data analyses and image plotting. The measurement data are expressed as mean ± standard deviation (±s).
3. Results
A total of 222 lesions were ablated in 50 patients. Information regarding MWA is presented in .
Table 2. General information for microwave ablation.
Patient complications are shown in according to the Society of Interventional Radiology classification [Citation7]. The most common complication after MWA was local pain, with an incidence of 76% (38/50); in most patients, the pain resolved on its own within 3 days. During ablation, the lesions and surrounding tissues were isolated using ice-cold normal saline, and an ice pack was applied after ablation to effectively prevent the incidence of scalds caused by thermal ablation. Excluding the skin sinuses that were present before ablation, new-onset skin ulceration/sinus formation was found in 20 patients within 2 to 12 weeks following ablation and most patients had one sinus with an opening of 3–4 mm. A small amount of intermittent turbid yellow liquid or a small amount of white, bean dreg-like necrotic tissue discharged from the sinus and the sinus opening healed on its own after drainage. After routine dressing changes (typically 2–5 times), no obvious scars were observed. In summary, most complications were mild, short-lived, easily managed, and without serious consequences.
Table 3. Complications of SIR classification system and treaments.
Postoperative observation indicators are listed in . One patient refused to continue follow-up observation due to recurrence of redness, swelling, and pain on the affected side at 4 months post-MWA and required a total mastectomy (nipple-sparing mastectomy [NSM] +IMP). Additionally, one patient acquired IGM on the contralateral side 17 months after the original ablation and received follow-up MWA on the newly affected side; IGM was cured on both sides using this treatment. Postoperative follow-up revealed that 78% of the patients were cured at 12 months, and an additional 20% were cured at 15 months. No additional glandectomy was required in the 50 patients. illustrate the changes in a typical case of IGM before and after ablation.
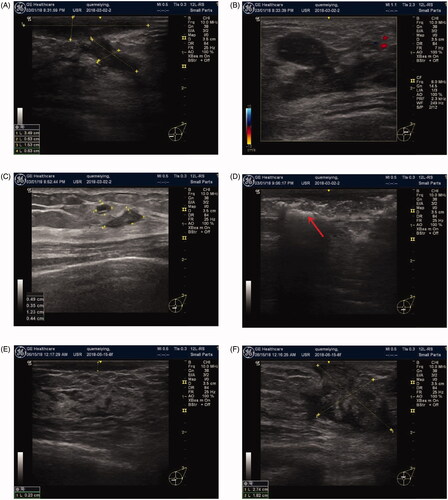
Figure 2. Changes in IGM on color Doppler ultrasound before and after ablation. Before ablation: (A) subcutaneous sinus, (B) primary IGM lesion, (C) microabscess and satellite lesions. During ablation: (D) The arrow indicates the lesion covered by hyperechoic signals after ablation. Postoperative follow-up: (E, F) 3rd month; (G, H) 6th month; (I, J) 12 months after ablation. IGM lesions were gradually reduced after microwave ablation and disappeared on color Doppler ultrasound at 12 months after ablation. Imaging shows echoes of normal tissue, suggesting complete absorption of the lesions.
Figure 3. Contrast-enhanced ultrasound images before and after MVA. (A) Before ablation: ultrasound CEUS blood perfusion showed high enhancement. (B) After ablation: ultrasound CEUS blood perfusion showed no enhancement.
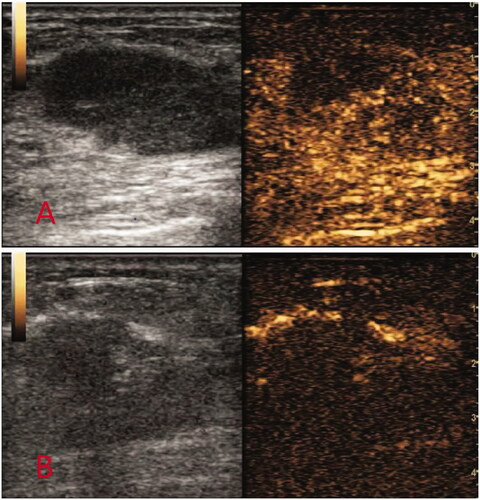
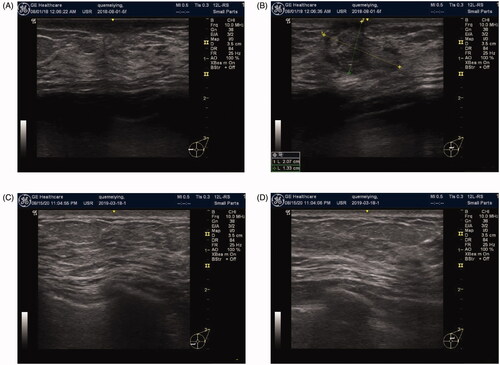
Figure 4. Changes in IGM before and after ablation. (A) Before ablation: the marker indicates palpable lesions and the expected ablation range under ultrasound. (B) At 9 months after ablation: the appearance and texture of the breasts are completely back to normal, with no scars.
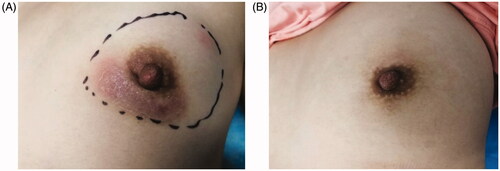
Figure 5. Pathological changes in IGM after ablation. Histopathological manifestations of IGM: (A) destruction of the normal lobular structure. (B) non-caseous granuloma, which is composed of lymphocytes, epithelioid tissue cells, and multinucleated giant cells surrounded by clustered neutrophils and confined to the lobules of the mammary gland. Microabscesses and lipid vacuoles are located at the center of the lobules. (C, D) IGM treated by MWA. The lobular structure damage is reduced. The number of inflammatory cells has decreased significantly, and the interfibrillar substance is mucinous.
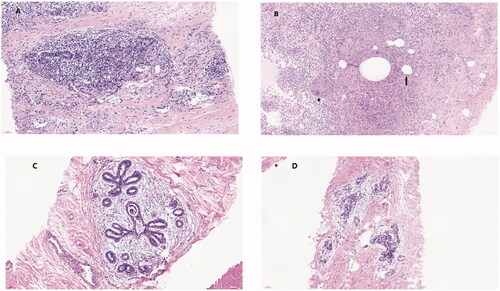
Table 4. Observation indicators after microwave ablation.
4. Discussion
IGM is a benign breast disease that occurs in young women (average age: 34 years [Citation8]) with a history of childbearing and lactation within 5 years of developing IGM [Citation5]. It accounts for 1.8% of all benign breast diseases [Citation9]. In recent years, the incidence of IGM in China has shown a clear upward trend [Citation10]. In the early stages of IGM, there is almost no clear etiological evidence; furthermore, no pathogenic bacteria are cultured in most reported cases [Citation11] and the effectiveness rate of antibiotic treatment of IGM is only 5% [Citation12]. The clinical manifestations of IGM are nonspecific, and the early symptoms involve the appearance of masses in most cases (70%). Ultrasound often reveals irregular hypoechoic clumps in a crab-claw shape that are heterogeneous, have blurred borders, have no envelope, and they are often rich in blood flow signals. Therefore, IGM must be differentiated from breast cancer [Citation13,Citation14]. IGM imaging has poor specificity, lacks typical imaging features, and easily leads to misdiagnosis. Clinical studies have shown that histopathological diagnosis is the only way to confirm IGM [Citation15].
Although it has been reported that IGM is a self-limiting disease [Citation16], during the disease’s active period, the patient may suffer from breast pain, abscess formation, secondary sinuses and ulcers, repeated attacks, and a protracted disease course. Furthermore, self-healing is usually accompanied by breast appearance deformity and hardening breast texture, which can lead to a serious decline in quality of life. Therefore, IGM patients often have an urgent desire to seek medical attention.
Donn et al. [Citation17] proposed in 1994 that glucocorticoid treatment could reduce IGM masses based on the relationship between IGM and autoimmunity. When IGM is diagnosed, hormone therapy is usually attempted first and oral administration of prednisone is the common choice. However, there is no agreed-upon standard for the dosage and course of oral prednisone administration. Some studies have reported that the initial dosage should be 30–60 mg/d for at least 2 weeks with a gradual decrease in dosage upon relief of the clinical symptoms; this process could span anywhere from 6 weeks to 1 year [Citation18–20]. Prednisone treatment of IGM usually results in a high remission rate in the acute phase, but because the disease develops progressively, it is not truly ‘cured’ [Citation21]. We advocate the use of small- and medium-dose, short-term, oral prednisone regimens to minimize side effects (i.e., obesity) caused by glucocorticoids.
Currently, most physicians use oral glucocorticoids combined with surgical resection to treat IGM. The expert consensus on the diagnosis and treatment of non-lactation mastitis in China [Citation22] is that surgery should be performed during the non-acute stage when the lesion is stable and limited. The underlying principle is that all visible lesion tissues should be completely and fully excised during surgery, otherwise, recurrence is likely. Nonetheless, the recurrence rate is still 5.5% after treatment [Citation23–27]. Most patients with IGM are women of childbearing age who are uncomfortable with the side effects of simple mastectomy, such as a large surgical resection range, long postoperative scars, deformity of the affected breast, and reduced breast volume. Moreover, when mastectomy is performed for benign diseases, overtreatment is suspected.
In this study, we treated IGM by first applying a short course of oral glucocorticoids to reduce the size of the lesions, then we performed ultrasound-guided MWA therapy to ablate the lesions. With precise ultrasound-guided puncture of the lesions, MWA regulates the effective thermal field of microwave emissions and uses the high temperature resulting from water molecule oscillations in the tissue to directly coagulate and denature the proteins of the lesion cells and this further results in total necrosis of the lesion tissues. Advantages of the procedure include a continuous high temperature, large tumor ablation volume, short operation time, and low pain score. With ultrasound guidance, precise positioning can be achieved without missing lesions. When the proteins of the lesion tissue are denatured at high temperatures, the immunogenicity of the lesion tissue is lost. Consequently, the factors that initiate IGM recurrence can be fundamentally eliminated. This study revealed that in addition to lesions that could be clearly detected, color Doppler ultrasound could also detect suspicious microabscess lesions around the primary lesions in most patients; the maximum number of microabscess lesions detected reached 14. Residual microabscesses are a common cause of failure when using traditional surgical resection for IGM. Color Doppler ultrasound-guided MWA can more intuitively identify potential lesions and reduce the risk of recurrence. The overall recurrence rate of the patients in this study was only 2%, which was significantly lower than that of traditional treatments. Some studies () report a higher recurrence rate than our study.
Table 5. Summary of articles in classifcation of IGM.
Through the application of MWA, we quickly inactivated the antigenicity of the lesions, effectively controlled the aforementioned clinical symptoms, significantly shortened the duration of symptoms, and enabled the ‘quiet’ state of self-repair (without the need for frequent medical treatment, long-term oral corticosteroid-use, or dressing changes). Finally, MWA does not cause breast deformities or psychological burden.
In this study, histological specimens were observed under an optical microscope during the postoperative follow-up period. The results showed that, at the beginning of the follow-up period, the ablation zone gradually transformed into a large red-stained, nonstructural necrotic area. As the follow-up period progressed, proliferative fibrous tissue could be seen again in these areas—which is consistent with the process of tissue trauma repair. The clinical manifestations of this treatment process were as follows: first, the texture of the ablation area hardened after ablation— usually within 1 to 3 months; then, with the degradation of the necrotic tissue and the formation of new fibrous tissue, the texture of the ablation area gradually softened and finally achieved the same feel and shape of healthy breasts in patients whose lesions did not involve the adipose layer.
We believe that a 1-year postoperative ultrasound reexamination and histopathological reexamination (biopsy) are important for patients. These examinations were performed to fulfill the following objectives: 1) timely extraction of liquid secretions from the affected breast; 2) timely evaluation of breast tissue repair and possible new or missed lesions; and 3) reliable assessments of IGM to eliminate the risk of co-occurring breast malignancies.
In summary, oral administration of glucocorticoids combined with MWA provides a new option for the treatment of IGM. We believe that tissue inactivation of IGM lesions is a better choice than extensive resection of breast tissue.
4.1. Limitation of study
The development of the technology in our study requires the operator to possess considerable knowledge and judgment regarding breast ultrasound The operator should try not to omit possible tiny lesions and microabscesses, and must be skilled at breast tissue puncture techniques. In the present study, we evaluated the adequacy of ablation using CEUS; however, if more lesions are present, the ablation of secondary lesions should be directly evaluated using ultrasound. This requires the operator to possess experience in predicting the duration of ablation for lesions of different sizes to avoid over-ablation or under-ablation. Further studies with larger cohorts and long-term follow-up are required to confirm our findings.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Medical Ethics Committee of the Affiliated Hospital of Putian University(202044).
Consent for publication
Due to the retrospective nature of this study the usual requirement for signed written informed consent forms was waived.
Author contributions
Lisheng Lin and Hongling Wang conceived the study, and drafted the manuscript; Zifang Zheng analyzed the data; Jinfan Zhang Xiaoli Liu and Daren Chen designed the study and collected the data. All authors have read and approved the final manuscript.
Acknowledgments
The authors thank the Follow-up Office established by the Department of Breast Surgery, The Affiliated Hospital of Putian University, Putian, Fujian Province, China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kessler E, Wolloch Y. Wolloch y: granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58(6):642–646.
- Fletcher A, Magrath IM, Riddell RH, et al. Granulomatous mastitis: a report of seven cases. J Clin Pathol. 1982;35(9):941–945.
- D'Alfonso TM, Ginter PS, Shin SJ. A review of inflammatory processes of the breast with a focus on diagnosis in core biopsy samples. World J Clin Cases. 2015;49(4):279–287.
- Yabanoğlu H, Çolakoğlu T, Belli S, et al. A comparative study of conservative versus surgical treatment protocols for 77 Patients with Idiopathic Granulomatous Mastitis. Breast J. 2015;21(4):363–369.
- Going JJ, Anderson TJ, Wilkinson S, et al. Granulomatous lobular mastitis. J Clin Pathol. 1987;40(5):535–540.
- Epstein JI. Biopsy interpretation of the breast. 2nd ed. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 47.
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28(10):1432–1437.e3.
- Taylor GB, Paviour SD, Musaad S, et al. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35(2):109–119.
- Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation]. World J Surg. 2007;31(8):1677–1681.
- Xu B, Wang L, Liu XY, et al. Investigation on present status of diagnosis and treatment of granulomatous lobular mastitis in China. J N Chin Med. 2019;51(2):279–283.
- Bashir MU, Ramcharan A, Alothman S, et al. The enigma of granulomatous mastitis: a series. Breast Dis. 2017;37(1):17–20.
- Hovanessian Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment]. AJR Am J Roentgenol. 2009;193(2):574–581.
- Aghajanzadeh M, Hassanzadeh R, Alizadeh S, et al. Granulomatous mastitis: presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast. 2015; 24(4):456–460.
- Sheybani F, Sarvghad M, Naderi H, et al. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015;125(4):801–807.
- Kiyak G, Dumlu EC, Kilinc I, et al. Management of idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. BMC Surg. 2014;14:66.
- Wang Q, Yang JM, Yu HJ. Granulomatous mastitis: diagnonsis and treatment. Chin J Prac Surg. 2016;36(7):734–738.
- Donn W, Rebbeck P, Wilson C, et al. Idiopathic granulomatous mastitis. A report of three cases and review of the literature. Arch Pathol Lab Med. 1994;118(8):822–825.
- Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17(6):661–668.
- Mizrakli T, Velidedeoglu M, Yemisen M, et al. Corticosteroid treatment in the management of idiopathic granulomatous mastitis to avoid unnecessary surgery. Surg Today. 2015;45(4):457–465.
- Sakurai K, Fujisaki S, Enomoto K, et al. Evaluation of follow-up strategies for corticosteroid therapy of idiopathic granulomatous mastitis. Surg Today. 2011;41(3):333–337.
- Pandey TS, MacKinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis-a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20(3):258–266.
- Women's health care branch of chinese preventive medicine association. Expert consensus on diagnosis and treatment of non-puerperal mastitis. Chin J Prac Surg. 2016;36(7):755–758.
- Shin YD, Park SS, Song YJ, et al. Is surgical excision necessary for the treatment of granulomatous lobular mastitis?. BMC Womens Health. 2017;24(1):49.
- Kuba S, Yamaguchi J, Ohtani H, et al. Vacuum-assisted biopsy and steroid therapy for granulomatous lobular mastitis: report of three cases. Surg Today. 2009;39(8):695–699.
- Gurleyik G, Aktekin A, Aker F, et al. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012;15(1):119–123.
- Li JQ. Diagnosis and treatment of 75 patients with idiopathic lobular granulomatous mastitis. J Invest Surg. 2019;32(5):414–420.
- Prasad S, Jaiprakash P, Dave A, et al. Idiopathic granulomatous mastitis: an institutional experience. Turk J Surg. 2017;33(2):100–103.