Abstract
Objective
To investigate the clinical effect of high intensity focused ultrasound (HIFU) ablation combined with gonadotropin-releasing hormone agonist (GnRH-a) and levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of adenomyosis patients who failed to respond to drug therapies.
Study design
A total of 47 patients with adenomyosis who had failed to respond to drug therapies and had no fertility desires were treated with HIFU combined with GnRH-a and LNG-IUS. The score of dysmenorrhea and menstrual volume were measured at pre and 6-, 12-, 18-, 24-month post-HIFU.
Results
All patients completed HIFU ablation without major postoperative complications. Compared with the symptom scores before the HIFU treatment, the score of dysmenorrhea and menstrual volume decreased significantly at 6, 12, 18 and 24 months after HIFU treatment (p < 0.05), but no significant difference was observed between 6, 12, 18 and 24 months after HIFU (p > 0.05). The clinical success rate was 100%, 100%, 95.7% and 93.6% respectively at 6, 12, 18 and 24 months after the combined treatment.
Conclusion
The combined therapeutic regimen of HIFU, GnRH-a and LNS-IUS is safe and effective, which can be an alternative treatment option for patients with adenomyosis who failed to respond to drug therapies to avoid adenomyomectomy or hysterectomy.
Introduction
Adenomyosis, characterized by the invasion of endometrial glands and stroma in the uterine myometrium, is a common benign gynecologic disease. The main symptoms of adenomyosis are hypermenorrhea, dysmenorrhea, and subfertility, which can seriously affect the patient’s quality of life [Citation1,Citation2]. Drug therapies, for example, GnRH-a, dienogest and LNG-IUS can relieve symptoms effectively. However, after the withdrawal of the drugs, the symptoms of adenomyosis and uterine enlargement returned [Citation3,Citation4]. The adverse effects of GnRH-a (such as menopausal symptoms and the risk of osteoporosis) limited its long-term use. Dienogest is generally well tolerated. However, massive metrorrhagia is a major reason for discontinuing treatment with dienogest [Citation5]. LNG-IUS can continuously release intrauterine levonorgestrel and is suitable for long-term treatment. However, spontaneous expulsion of LNG-IUS is the main reason for the failure of LNG-IUS [Citation6,Citation7]. Although GnRH-a combined with LNG-IUS can reduce the LNG-IUS expulsion rate, the expulsion rate at 12 months after insertion is still 14% [Citation8]. Patients with adenomyosis who failed to respond to drug therapies are often recommended for adenomyomectomy or hysterectomy. In fact, the only hysterectomy can radically cure adenomyosis [Citation9]. Due to the unclear boundary of the adenomyotic lesion, recurrence is found as early as within one year after adenomyomectomy [Citation10].
HIFU has emerged as an alternative uterus-sparing option for the treatment of adenomyosis. Compared to surgery, HIFU has fewer complications, quicker recovery, shorter hospital stay and better quality of life [Citation11]. However, symptom relief only lasts for a certain period and the median recurrence time was 12 months after HIFU treatment because of the unclear boundary of the adenomyotic lesion [Citation12]. Conservative treatments such as medicinal, HIFU and surgical methods are all at risk of recurrence. Since there are no specific guidelines to follow for the management of adenomyosis [Citation3], the uterus-sparing treatment of adenomyosis faces a substantial challenge in gynecological practice.
The objective of our study is to specifically evaluate the combined therapeutic effects of HIFU, GnRH-a and LNG-IUS in patients with adenomyosis who failed to respond to drug therapies.
Materials and methods
Patients
From May 2017 to January 2019, 47 patients with adenomyosis who had failed to respond to drug therapies and had no fertility desires were treated with HIFU combined with GnRH-a and LNG-IUS in Shenzhen Maternity and Child Health Hospital Affiliated to Southern Medical University. The inclusion criteria were: (1) confirmation of adenomyosis with depth above 3 cm by magnetic resonance imaging (MRI); (2) patients can communicate with the medical staff during HIFU procedure; (3) patients with no use of steroid hormones or GnRH-a 6 months before treatment and no contraindications to GnRH-a and LNG-IUS; (4) patients were reluctant to have their uterus resected. Exclusion criteria were: (1) the adenomyotic lesion could not be reached by the focused ultrasound beams or could not be visualized; (2) patients with contraindications to MRI; (3) patients with suspected or confirmed uterine malignancy. All patients in the study signed the written informed consent for the HIFU treatment and completed the two-year follow-up after HIFU treatment. This retrospective study was Institutional Review Board approved. The protocol number is SFYLS[2021]017. The requirement for informed consent was waived because the study was retrospective. The identities of the patients were maintained as confidential.
Procedure of HIFU
All patients were given instructions about bowel preparation three days before HIFU treatment. Enema was performed in the morning of the treatment day following 8-h fasting. The skin was degreased and degassed right before HIFU treatment. The urinary catheter was inserted to control the bladder volume to optimize the therapeutic acoustic pathway.
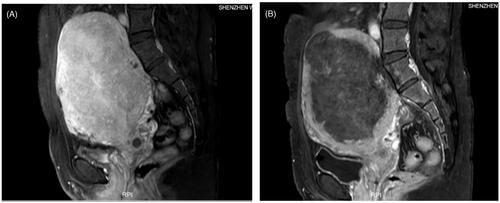
The adenomyotic lesion was treated by ultrasound-guided high-intensity Focused Ultrasound Tumor Therapeutic System JC200D (Chongqing Haifu Medical Co. Ltd). Fentanyl and Midazolam were administered preoperatively for sedation and analgesia. A degassed water balloon was used to compress and push away bowels from the acoustic pathway during treatment to avoid intestinal injury. Sonication started from the middle section and the posterior part of the lesion, with the focal point at least 1.5 cm away from the margin of the uterus in every patient to ablate the adenomyotic lesion and the endometrium near the lesion. The power and sonication energy were adjusted according to patient feedback and real-time gray-scale changes on ultrasonographic imaging. The treatment was repeated on a section-by-section basis until the entire target lesion was covered by hyperechoic changes. Close attention was paid to monitor adverse effects during treatment. At the end of treatment, contrast-enhanced ultrasound was performed to visualize the non-perfusion range of the lesion and thereby evaluate the ablation effect. Contrast-enhanced MRI was performed 1–2 days after HIFU to evaluate the non-perfused volume (NPV) of the lesions (). The NPV ratio was calculated according to the following equation: NPV ratio=the volume of the necrotic tissue/the volume of the targeted lesions × 100%.
Figure 1. Contrast MRI obtained from a 41-year-old patient with adenomyosis who had failed to respond to drug therapies. (A) Contrast-enhanced MRI obtained before HIFU showed the enhancement of the adenomyosis. (B) MRI obtained one day after HIFU showed the non-perfused area of the adenomyotic lesion and endometrium.
GnRH-a administration and LNG-IUS insertion
After HIFU treatment all patients were administered with 3 doses of GnRH-a (Goserelin, AstraZeneca, Cambridge, UK) treatment and LNG-IUS (Mirena, Bayer Ag, Turku, Finland). The first GnRH-a was given within 1 week after the completion of HIFU ablation. The interval between the rest two injections was 28 days. LNG-IUS was inserted when the third dose of GnRH-a was given. Transvaginal sonography ascertained the proper position of the device immediately after LNG-IUS insertion.
Observation and measurement
Dysmenorrhea score and menstrual volume score were measured at the 6, 12, 18 and 24 months after HIFU ablation by telephone follow-up. The perception of pain was clinically evaluated using a visual analogue scale (VAS) and menorrhagia was defined by a pictorial blood loss assessment chart (PBAC) score of greater than 100, as described by Higham et al. [Citation13].
The efficacy of treatment was evaluated at 6 months after treatment using the following method: (1) inefficient: with a VAS score reduction of ≤20%; (2) partial relief: with a VAS score reduction of 20% to 50%; (3) significant relief: with a VAS score reduction of 50% to 80%; (4) complete relief: with a VAS score reduction of ≥80%. A result of either (2), (3) or (4) was defined as clinical success. Dysmenorrhea recurrence was defined as VAS scores at 12 months after HIFU rose back to more than 80% that of pre-HIFU.
The response and recurrence of menorrhagia were defined similarly with the indicator of PBAC [Citation14]. All patients were closely observed for complications of HIFU ablation, GnRH-a, and LNG-IUS.
Statistical analysis
Statistical analysis was completed with SPSS 20.0 (IBM, Armonk, NY), and p < 0.05 was defined as statistically significant. All continuous variables were presented as mean ± standard deviation (SD). The continuous variables were compared using ANOVA for the repeated measures.
Results
Baseline characteristics
A total of 47 patients with adenomyosis who received HIFU treatment combined with GnRH-a and LNG-IUS for conservative management were investigated. The baseline characteristics of the patients were listed in . All patients had failed to respond to drug therapies including GnRHa, LNG-IUS and dienogest. Their uterine volumes were all larger than 150 ml. LNG-IUS was inserted in 41 patients, which were spontaneously expulsed 47 times. The overall median time of spontaneous expulsion after insertion of the LNG-IUS was 6 months (range: 10 days − 24 months).
Table 1. Baseline characteristics of the patients.
Treatment
All the patients completed HIFU ablation in one session. The mean acoustic sonication power was 395.64 ± 15.70 (range: 299–400) W. The treatment time was 70.26 (range: 21–162) min. The mean sonication time was 699.51 ± 345.34 (range: 181–2178) s. The mean NPV ratio was 82.81 ± 13.79 (range: 50–98) %. The mean intrauterine depth at the insertion of LNG-IUS was 8.81 ± 0.99 (range: 7–11) cm (). The intrauterine depth at the insertion of LNG-IUS was larger than 9 cm in 10 of 47 patients (range: 9.5–11 cm).
Symptom improvement
Compared with the symptom scores before treatment, scores of dysmenorrhea and menstrual volume decreased significantly at 6, 12, 18 and 24 months after HIFU treatment (p < 0.05) (). Mean dysmenorrhea scores were all less than 3 and no significant difference was observed among 6, 12, 18 and 24 months after HIFU (p > 0.05). Mean menstrual volume scores were all less than 40, and no significant difference was observed among 6, 12, 18 and 24 months after HIFU (p > 0.05).
Table 2. Evaluation of treatment efficacy on dysmenorrhea and menstrual volume before and 6, 12, 18, 24 months after HIFU treatment.
Success rate and recurrence rate
The clinical success rate of the combined treatment of adenomyosis patients who had failed to respond to drug therapies was 100%, 100%, 95.7% and 93.6% respectively at 6, 12, 18 and 24 months. During the 24-month follow-up, the long-term recurrence rates were low, with 4.3% (2/47) at 18 months and 6.4% (3/47) at 24 months. Three patients experienced spontaneous expulsion of the LNG-IUS after HIFU treatment combined with GnRH-a and LNG-IUS. In the same month, symptom scores rose back to the level of pre-HIFU ablation. The three patients all had a history of spontaneous expulsion of LNG-IUS within 6 months before HIFU treatment. One of the three patients completed a second HIFU ablation combined with GnRH-a and LNG-IUS after she failed the first combination treatment. The symptom relief after the second combination treatment was significant and so far lasted for 8 months.
Adverse effects
After HIFU treatment, no major post-HIFU complications such as infection, skin burn, leg pain, hematuresis and intestinal injury were observed in any of these patients. Scanty vaginal bleeding occurred after HIFU treatment in all patients and such symptoms disappeared within one month. None of the patients had experienced night sweats, hot flashes, and insomnia during three cycles of GnRH-a administration. Irregular vaginal bleeding was documented in 30 (63.83%) patients and relieved within 3 months after LNG-IUS implantation. All patients continued the combined therapy and completed 2 years of follow-up.
Discussion
Up to now, a satisfactory conservative treatment strategy for adenomyosis is lacking. Whereas for patients with adenomyosis who failed to respond to drug therapies, resection can be performed. Excision of adenomyosis is difficult and common complications in this surgery are blood loss, postoperative high fever and uterine hematomas [Citation15]. Meanwhile, conservative surgical treatment usually cannot completely remove the lesion, which may lead to a high recurrence rate [Citation16]. We sought to examine whether adenomyomectomy or hysterectomy can be avoided after HIFU combined with GnRH-a and LNG-IUS administration. To our knowledge, this is the first study to describe the long-term management of HIFU combined with GnRH-a and LNG-IUS administration for patients with adenomyosis who failed to respond to drug therapies.
Many studies have demonstrated that HIFU is a safe and effective non-invasive treatment for adenomyosis [Citation17,Citation18]. However, it is also difficult to ablate the adenomyotic lesion completely by means of HIFU because of its unclear boundary which will cause a high relapse rate [Citation19]. The previous studies have shown that combining HIFU with LNG-IUS or GnRH-a yields a superior clinical effect compared to HIFU treatment alone [Citation20]. Another report by Yang X et al. [Citation21] revealed that a combined therapeutic regimen of HIFU, GnRH-a and LNS-IUS was effective for curing severe adenomyosis (greater than at 12 weeks of gestation). A 5-year follow-up study on HIFU ablation in combination with GnRH-a and LNG-LUS treatment for adenomyosis found low long-term recurrence rates, with 5.68% and 7.91% in dysmenorrhea and menorrhagia, respectively [Citation14]. These results suggested that HIFU ablation was effective for adenomyosis when synergized with other agents to improve efficacy. In this study, compared with the symptom scores before HIFU treatment, the average dysmenorrhea score and menstrual volume were significantly lower at 6, 12, 18 and 24 months after HIFU treatment. At 6 months and 12 months after HIFU treatment, the clinical success rates were 100% and remained 95.7% at 18 months and remained 93.6% at 24 months. Therefore, HIFU treatment combined with GnRH-a and LNG-IUS was effective for patients with adenomyosis who had failed to respond to drug therapies.
LNG-IUS is suitable for the long-term treatment of adenomyosis. The uterine volume of more than 150 ml is the only independent factor for the failure of LNG-IUS [Citation22]. LNG-IUS is not optimal for women with a large lesion of adenomyosis [Citation23]. Previous expulsions can be considered a risk factor for re-expulsion for the LNG-IUS users [Citation22]. In this study, a total of 47 patients failed to respond to drug therapies and their uterine volumes were all larger than 150 ml. Among them, 41 patients had a history of spontaneous expulsion of LNG-IUS before HIFU treatment and the overall median time of spontaneous expulsion was 6 months. LNG-IUS retention was achieved within 24 months of the combination therapy with the exception of 3 patients. The three patients all had a history of spontaneous expulsion of LNG-IUS within 6 months before HIFU treatment. After the combination therapy, two patients experienced LNG-IUS expulsion at 18 months and one patient experienced LNG-IUS expulsion at 24 months. In adenomyosis with large uterine volume, HIFU treatment combined with GnRH-a and LNG-IUS have sustained long-term symptom relief and may reduce the expulsion rate of LNG-IUS. One of the three patients had a second session of combination treatment and enjoyed significant symptoms relief. This observation suggested that the combination regimen can be repeated to achieve the desired therapeutic effects. Although both adenomyomectomy and HIFU can treat adenomyosis, fibrotic scars resulted from adenomyomectomy may cause difficulty in re-operation when adenomyosis recurs. In contrast, as a noninvasive treatment, HIFU is easy to re-perform.
The greater the NPV ratio, the longer the long-term symptom relief sustained [Citation24]. Because of the unclear boundary of adenomyotic lesion, the focal point was at least 1.5 cm away from the margin of the uterus in order to ablate more lesions near the uterine serosa and to prevent intestinal injury at the same time. As the 47 patients had no fertility desires, the endometrium near adenomyotic lesions was also ablated during HIFU treatment in order to ablate more lesions and to improve the NPV ratio. In this study, the mean NPV ratio was 82.81% after HIFU. Meanwhile, endometrial ablation may help reduce menstrual volume. Scanty vaginal bleeding occurred within one month after HIFU treatment in all patients because of the endometrial ablation near adenomyosis lesions. No major post-HIFU complications were observed in this cohort. Previous studies showed a sufficient superior effect of endometrial ablation combined with LNG-IUS in women with adenomyosis [Citation25]. In this study, the ablation of the endometrium near adenomyotic lesions may help to increase the effects of HIFU treatment combined with GnRH-a and LNG-IUS and reduce the expulsion rate of LNG-IUS, which need further research to confirm.
In conclusion, patients with adenomyosis and without fertility desires can adopt a life-long management plan through a combination of HIFU, GnRH-a and LNG-IUS while minimizing side effects. The combined therapeutic regimen is safe and effective, which can be an alternative treatment option in patients with adenomyosis who failed to respond to drug therapies to avoid adenomyomectomy or hysterectomy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dueholm M. Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):569–582.
- Kwon YS, Roh HJ, Ahn JW, et al. Conservative adenomyomectomy with transient occlusion of uterine arteries for diffuse uterine adenomyosis. J Obstet Gynaecol Res. 2015;41(6):938–945.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109(3):398–405.
- Neriishi K, Hirata T, Fukuda S, et al. Long-term dienogest administration in patients with symptomatic adenomyosis. J Obstet Gynaecol Res. 2018;44(8):1439–1444.
- Nagata C, Yanagida S, Okamoto A, et al. Risk factors of treatment discontinuation due to uterine bleeding in adenomyosis patients treated with dienogest. J Obstet Gynaecol Res. 2012;38(4):639–644.
- Yoo HJ, Lee MA, Ko YB, et al. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet. 2012;285(1):161–166.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2):193–215.
- Zhang P, Song K, Li L, et al. Efficacy of combined Levonorgestrel-releasing intrauterine system with gonadotropin-releasing hormone analog for the treatment of adenomyosis. Med Princ Pract. 2013;22(5):480–483.
- Tsui KH, Lee WL, Chen CY, et al. Medical treatment for adenomyosis and/or adenomyoma. Taiwan J Obstet Gynecol. 2014;53(4):459–465.
- Younes G, Tulandi T. Conservative surgery for adenomyosis and results: a systematic review. J Minim Invasive Gynecol. 2018;25(2):265–276.
- Elhelf IAS, Albahar H, Shah U, et al. High intensity focused ultrasound: the fundamentals, clinical applications and research trends. Diagn Interv Imaging. 2018; 99(6):349–359.
- Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study . Medicine. 2016;95(3):e2443.
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97(8):734–739.
- Haiyan S, Lin W, Shuhua H, et al. High-intensity focused ultrasound (HIFU) combined with gonadotropin-releasing hormone analogs (GnRHa) and levonorgestrel-releasing intrauterine system (LNG-IUS) for adenomyosis: a case series with long-term follow up. Int J Hyperthermia. 2019;36(1):1179–1185.
- Mikos T, Lioupis M, Anthoulakis C, et al. The outcome of fertility-sparing and nonfertility-sparing surgery for the treatment of adenomyosis. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2020;27(2):309–331.e3.
- Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 1998;4(4):323–336.
- Dong X, Yang Z. High-intensity focused ultrasound ablation of uterine localized adenomyosis. Curr Opin Obstet Gynecol. 2010;22(4):326–330.
- Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2018; 51:119–137.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96(6):707–714.
- Guo Q, Xu F, Ding Z, et al. High intensity focused ultrasound treatment of adenomyosis: a comparative study. Int J Hyperthermia. 2018;35(1):505–509.
- Ki Hwan L, Jang Kew K, Min AL, et al. Relationship between uterine volume and discontinuation of treatment with levonorgestrel-releasing intrauterine devices in patients with adenomyosis. Arch Gynecol Obstet. 2016;294(3):561–566.
- Park DS, Kim ML, Song T, et al. Clinical experiences of the levonorgestrel-releasing intrauterine system in patients with large symptomatic adenomyosis. Taiwan J Obstet Gynecol. 2015;54(4):412–415.
- Yang X, Zhang X, Lin B, et al. Combined therapeutic effects of HIFU, GnRH-a and LNG-IUS for the treatment of severe adenomyosis. Int J Hyperthermia. 2019;36(1):486–492.
- Zhou M, Chen JY, Tang LD, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril. 2011;95(3):900–905.
- Zheng J, Xia E, Li TC, et al. Comparison of combined transcervical resection of the endometrium and levonorgestrel-containing intrauterine system treatment versus levonorgestrel-containing intrauterine system treatment alone in women with adenomyosis: a prospective clinical trial. J Reprod Med. 2013;58(7–8):285–290.
