Abstract
Image-guided percutaneous ablation techniques represent an attractive local therapy for the treatment of colorectal liver metastases (CLM) given its low risk of severe complications, which allows for early initiation of adjuvant therapies and spare functional liver parenchyma, allowing repeated treatments at the time of recurrence. However, ablation does not consistently achieve similar oncological outcomes to surgery, with the latter being currently considered the first-line local treatment modality in international guidelines. Recent application of computer-assisted ablation planning, guidance, and intra-procedural response assessment has improved percutaneous ablation outcomes. In addition, the evolving understanding of tumor molecular profiling has brought to light several biological factors associated with oncological outcomes following local therapies. The standardization of ablation procedures, the understanding of previously unknown biological factors affecting ablation outcomes, and the evidence by ongoing prospective clinical trials are poised to change the current perspective and indications on the use of ablation for CLM.
Introduction
Despite notable advances in the clinical application and outcomes of liver ablation for the treatment of colorectal cancer liver metastasis (CLM), substantial gaps in knowledge still exist in respect of its head-to-head comparison to surgical resection. According to international guidelines [Citation1], resection is currently considered the local therapy of choice for patients presenting with resectable CLM. The National Comprehensive Cancer Network guidelines have recommended ablation alone or ablation used in conjunction with resection as long as all visible diseases can be eradicated [Citation2]. Such predilection is predicated by historical data showing that, when compared to ablation, resection is associated with improved overall survival (OS), disease-free survival, and reduced local recurrence rates [Citation3–12]. Notwithstanding, recent understandings on patient selection, tumor biology, ablation technology, and image guidance, along with reported improved outcomes following CLM ablation, have made several investigators revisit the treatment paradigm to the role of surgery and ablation for the management of CLM patients.
In this review, we aim to discuss the current evidence on the management of patients with CLM and to provide up-to-date literature in regards to radiofrequency ablation (RFA) and microwave ablation (MWA), and irreversible electroporation (IRE) use and outcomes on such patient population. We will also expand our discussion on currently known gaps of knowledge on the liver ablation literature. Finally, taking this along with the available literature in respect to surgical resection for CLM, we will debate how such contemporary evidence can be utilized as guidance for appropriate decision-making.
1. Current evidence (the ‘known knowns’)
1.1. Patient selection: a key factor for optimal ablation outcomes
Liver ablation by either percutaneous or intra-operative approach has been traditionally utilized on patients with small CLM [Citation2], with the aim of achieving local curative effect at the ablated lesion and prevent its local recurrence, also known as local tumor progression (LTP) [Citation13]. For patients with extra-hepatic disease, liver ablation can be offered if all intra- and extra-hepatic diseases can be eradicated or if the extent of extra-hepatic metastasis is limited and not thought to be the main driver of prognosis [Citation14–18]. Presently, the role of liver ablation in patients with extensive extra-hepatic disease is of unclear value. It has been proposed that patients with ≤ 5 liver tumors, preferably 1–3, should be considered as inclusion criteria for ablation [Citation16,Citation17,Citation19–21]. Also, tumor size is associated with local tumor control and OS. Several studies have reported that the tumor size < 3 cm is associated with satisfactory local tumor control and with longer OS [Citation15,Citation17,Citation21–26]. In the European Society of Medical Oncology guidelines [Citation1], a maximum tumor diameter of ≤ 3 cm is recommended as it has been demonstrated to be an independent predictor of overall and LTP-free survival (LTPFS) [Citation15,Citation27–29]. To properly estimate tumor burden, patients who are being evaluated for liver ablation should have a recent cross-sectional study with intravenous contrast, such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography-computed tomography (PET-CT), ideally within one month from the ablation procedure day.
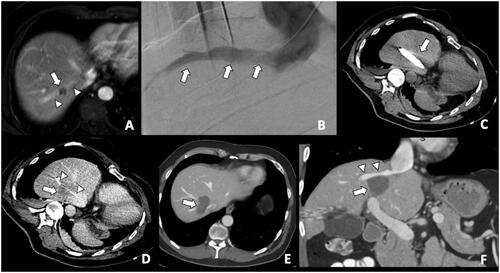
Tumor location can affect both local tumor control and complication rates. Ablation of tumors adjacent to large vessels is associated with the heat-sink phenomenon and with an increased risk of residual tumor or LTP [Citation30–32]. MWA is theoretically less susceptive to heat-sink effects than RFA, although this is still a relevant issue for large vessels with high flow rates [Citation33–35]. The use of thermal ablation modalities to tumors adjacent to critical structures (i.e., major bile ducts, gallbladder, colon, pancreas, among others) can be associated with complications such as bleeding, vessel thrombosis, cholangitis, liver abscess, colon rupture, pancreatitis, among others [Citation36–38]. Such complications can be avoided in a significant number of patients with percutaneous gas/hydrodissection techniques. In such situations, nonthermal ablation techniques such as irreversible electroporation (IRE) can be applied [Citation39,Citation40]. Because of the nonthermal nature of IRE, its efficacy is not impeded by the heat-sink effect by adjacent blood vessels [Citation41]. Additionally, combined ablation and endovascular strategies such as combination with pre-ablation embolization or use of balloon-occlusion of the “adjacent vessel” responsible for heat-sink effect in combination with MWA () should be considered to compensate the heat-sink phenomenon. The influence of subcapsular tumor location on ablation effectiveness has conflicting results in the literature, with most of the authors showing similar LTP rates results when compared to nonsubcapsular-ablated tumors [Citation42–45], whereas some other authors showing higher rates of LTP for subcapsular tumors [Citation22].
Figure 1. A colorectal liver metastasis abutting right hepatic vein treated by combination of percutaneous transhepatic venous temporary balloon occlusion of the right hepatic vein and microwave ablation to compensate the heat-sink phenomenon. (A) Magnetic resonance imaging demonstrating a tumor (arrow) abutting right hepatic vein (arrowheads); (B) Percutaneous transhepatic hepatic venography demonstrating the right hepatic vein (arrows); (C) Intra-procedural CT depicting inflated balloon catheter in the right hepatic vein during ablation; (D) A Clear ablation zone (arrow) covering the right hepatic vein, which is indicated by the catheter (arrowheads); (E) One month follow-up CT revealing satisfactory ablation zone (arrow) without residual tumor; (F) CT coronal multiplanar reconstruction demonstrating the ablation zone (arrow) and patent right hepatic vein (arrowheads).
More recently, the influence of tumor biomarkers on liver ablation outcomes has been reported [Citation45–49]. RAS gene family (KRAS, NRAS, and HRAS) mutations are found in up to 40% of patients with colorectal cancer and portend a worse survival following resection of primary and CLM, more invasive tumor biology, and worse response to preoperative chemotherapy [Citation50–52]. Also, RAS mutation is associated with a higher rate of positive margins after resection of CLM (11.4% mutant-RAS vs 5.4% wild-type RAS, p = 0.007) [Citation53]. On a single-institution retrospective analysis of 92 patients who underwent ablation of 137 CLM, three-year LTPFS rates were significantly worse for mutant RAS patients compared to wild-type RAS patients (35% vs. 71%, p = 0.001) [Citation45]. Similarly, on another single-institution retrospective analysis of 97 patients with CLM treated with RFA, KRAS mutation was a significant predictor for LTP of CLM ablated with margins 1 − 5 mm (LTP rates, 80% vs 41%, mutant vs wild-type RAS, respectively. p = 0.018) [Citation47]. provides a summary of current known patient’s factors associated with CLM ablation outcomes.
Table 1. Patient’s factors affecting oncological outcomes.
1.2. Technical factors: the importance of adequate imaging guidance and ablation margins
Effective ablation treatment relies on the success of four interdependent critical steps: tumor identification; ablation planning; probe placement according to the plan, and assessment of treatment delivery.
1.2.1. Tumor identification
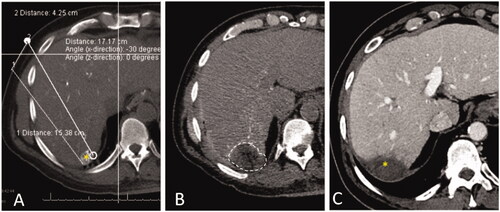
Several studies have demonstrated higher rates of LTP when only unenhanced imaging methods are utilized [Citation14,Citation54,Citation55]. Therefore, the use of intravenous contrast-enhanced cross-sectional (CT or MR) imaging is preferred as it provides optimal tumor margins definition assessment [Citation16]. More recently, the use of transcatheter CT during hepatic arteriography via the hepatic artery has been applied by some investigators for facilitating tumor identification and ablation endpoint assessment () [Citation56,Citation57]. This technique allows tumor identification by injecting smaller amounts of contrast media through the hepatic artery, and its use has been associated with improved local tumor control and superior LTPFS when compared to conventional CT fluoroscopy-guided ablation [Citation56]. Another option to facilitate tumor identification is the use of image fusion, which allows the use of co-registered MRI/CT/PET datasets during ultrasound-guided or CT-guided interventions for ablation planning [Citation58,Citation59]. Also, a whole-body fluorodeoxyglucose (FDG) PET-CT scan can provide additional information for better quantification of liver and extrahepatic metastases and may change the management [Citation60]. MRI is the most accurate imaging for the detection and characterization of hepatic metastases, especially with the hepatocyte-specific MRI contrast agent. It has a high sensitivity for the detection of smaller tumors that may not be easily detected by CT and PET [Citation61].
Figure 2. CT during hepatic arteriography (CTHA) on a patient with colorectal liver metastasis undergoing liver ablation. (A) CTHA prior to MWA antenna placement to localize target tumor (*); (B) postablation CTHA demonstrating large ablation zone (dotted line) around the treated tumor, which is no longer visualized; (C) 32-months CT follow-up demonstrating involution of the ablation zone without recurrence (*).
1.2.2. Ablation planning
To achieve sufficient ablation margins (>5 mm) around the tumor, careful planning of the procedure is the key. Recent studies indicated that ablation may provide acceptable oncologic outcomes for patients with small CLMs that can be ablated with sufficient margins [Citation15,Citation22,Citation62]. One study has reported that margins 10 mm or larger are associated with no local tumor progression within a 24-month follow-up period [Citation44]. The maximum size of the lesion that may be treated with one probe position depends on the short diameter of the expected ablation zone (which is related to the type of the ablation device and the liver perfusion at the tumor location). The in vivo short diameters of the ablation zone after using standard RFA and MWA probes range from 1.6 cm to a maximum of 3.6 cm [Citation63,Citation64]. Therefore, if the probe is perfectly placed in the lesion center the maximum tumor size that can be treated with one probe position ranges from 0.6 to 2.6 cm (depending on the type of probe/ablation system used). For larger lesions, multiple overlapping ablation zones have to be obtained, which can be facilitated by the stereotactic navigation system and 3 D-planning of probe trajectories which enable highly accurate probe positioning [Citation65].
1.2.3. Probe placement
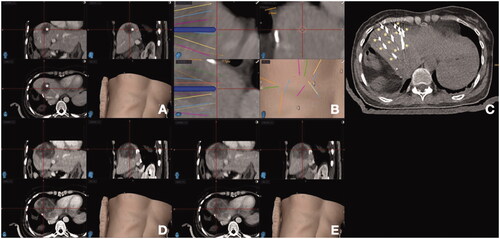
Precise probe placement at the target tumor can be facilitated by stereotactic navigation systems that take into consideration tumor location, shape, size, as well distance between the ablation needles (). Stereotactic radiofrequency ablation (SRFA) via 3 D imaging has been advocated by some authors as a reproducible method that allows improved local tumor control [Citation65]. This is a commercially available, optical-based, frameless stereotactic navigation system combined with 3 D-planning software allowing for accurate planning of elaborate trajectories for single or multiple probes to cover an ablation zone with a clear margin [Citation65]. On a retrospective analysis performed by Bale et al. [Citation66], 63 consecutive patients submitted to 98 SRFA sessions for 189 CLM showed similar LTP rates between CLM <3 cm, 3–5 cm or >5 cm in diameter (17.7% vs 11.1% vs 17.4%, respectively. p = 0.6). 1-, 3- and 5-year survival rates were 87%, 44%, and 27%. Resectable patients had improved median OS rates when compared to unresectable ones (58 vs 27 months, respectively. p = 0.002). Based on such results, the authors concluded that SRFA can be considered as an alternative to surgical resection as a first-line treatment for CLM on an individual patient basis [Citation66].
Figure 3. Stereotactic navigation systems on a patient with colorectal liver metastasis (CLM) undergoing liver ablation. (A) Posthepatectomy recurrent CLM measuring 5 cm in diameter (*); (B) Planning of 13 percutaneous guide needles trajectories, represented by different lines; (C) CT evaluation of guide needles placement (*); (D) Postablation CT for treatment verification. (E) Fusion of pre- and post-ablation CT for verification of ablation margin, showing complete tumor coverage with ablation with sufficient ablation margin.
The percutaneous approach of ablation is usually favored over the surgical approach due to less post-treatment morbidity and shorter hospital stay. The reported major complications rates range from 3.1 to 4.4% for laparoscopic ablation, 9.6 to 32% for open ablation, and from 0 to 4.7% for percutaneous ablation [Citation7,Citation15,Citation17,Citation22,Citation23,Citation54,Citation67–72]. A study of 233 patients with malignant hepatic tumors found shorter hospital stays and fewer complications of percutaneous RFA comparing to the surgical approach, although there was no significant difference in oncologic outcomes [Citation73]. A meta-analysis has reported that there was no significant difference in 5-year overall and disease-free survival rates between percutaneous and open/laparoscopic approaches for ablation of CLM [Citation74].
The role of anesthetic techniques in ablation is critical since it reduces the pain, anxiety, and patient’s movements during the procedure, therefore facilitating tumor targeting and potentially improving ablation outcomes [Citation75]. Several anesthetic methods are used, such as general anesthesia, and sedation using fentanyl, midazolam, or propofol. General anesthesia is preferred because of controllable respiration during the procedure. Adjunctive methods such as intermittent breath-hold during probe placement, low tidal volume settings, and high-frequency jet ventilation can also be applied with that intent [Citation16,Citation27,Citation76]. Precise respiratory triggering is mandatory if stereotactic techniques are used [Citation66]. On a recent retrospective analysis by Puijk et al of 90 patients submitted to 114 ablation procedures under general anesthesia (n = 22), midazolam (n = 32), or propofol (n = 60), authors reported that sedation with propofol and general anesthesia was associated with better local tumor control than sedation with midazolam, providing local tumor progression rates of 4.3%, 5.7%, and 45.2%, respectively (p < 0.001) [Citation77].
1.2.4 Ablation assessment
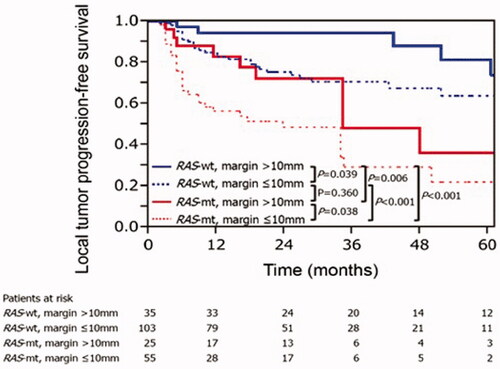
Sufficient minimal ablation margins are considered an essential technical factor for reducing rates of local tumor progression [Citation15,Citation22,Citation24,Citation44,Citation62]. A minimal ablation margin of > 5 mm is considered essential for achieving acceptable local tumor control rates, which have been reported to be around 15%. It has been reported in two studies with long-term follow-up periods that ablation margin smaller than 5 mm was an independent risk factor for LTP [Citation15,Citation22]. Moreover, if minimal ablation margins are > 10 mm, LTP rates fall significantly to 0–5%, therefore making it the desired treatment endpoint for CLM ablation as it is on par with marginal recurrence rates after resection of CLM [Citation11,Citation15,Citation16,Citation44,Citation62,Citation78,Citation79]. Similar to the surgical literature, the interplay between minimal ablation margins and tumor biology has also been recently evaluated for thermal ablation. Calandri et al. [Citation46] have demonstrated on a two-institution retrospective study of 136 patients with 218 ablated CLM that three-year LTPFS were significantly worse in mutant than in wild-type RAS in both CLM subgroups with minimal ablation margin ≤ 10 mm (29% vs. 70%, p < 0.001) and >10 mm (48% vs. 94%, p = 0.006), respectively. Such results demonstrate that even when minimal ablation margins > 10 mm are achieved, LTP among mutant and wild-type RAS patients were still significantly different (). Therefore, the authors concluded that ablation margins > 10 mm are crucial for mutant RAS CLM [Citation46].
Figure 4. Kaplan–Meier curves for local tumor progression-free survival according to RAS and minimal ablation margin. RAS-wt: RAS wild-type; RAS-mt: RAS mutant. Adapted from Calandri M, Yamashita S, Gazzera C, Fonio P, Veltri A, Bustreo S, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumor progression-free survival. Eur Radiol. 2018;28(7):2727–2734. Used with permission.
Despite the widely recognized need for sufficient minimal ablation margins on a three-dimensional plane, fundamental limitations still exist on how to properly assess such ablation margins intra- and post-procedurally. This is due to a series of factors: the tumor is obscured by the ablation zone, precluding its mapping within the ablation zone; the complex changes in the patient’s position, post-ablation tissue retraction and inflammation, and imaging resolution [Citation80,Citation81]. Ongoing efforts such as the use of deformable imaging registration methods aim to provide accurate modeling of ablation zone assessment () [Citation80,Citation82–86]. Furthermore, some intraoperative techniques are used to facilitate the targeting of tumors and ablation assessment. A split-dose technique for FDG PET/CT guidance has been developed, which allows prompt tumor targeting and immediate postablation assessment [Citation87–89]. The main concept of this technique is that a smaller first dose of FDG before the ablation will be significantly decayed by the time the second larger dose is administered, allowing for the detection of FDG activity within any residual viable tumor. Another technique using intraprocedural nitrogen 13 ammonia perfusion PET has been developed to assess the ablation margins [Citation86]. For ultrasound guidance, using intravenous contrast can improve tumor detection sensitivity [Citation85,Citation90]. A post-ablation contrast-enhanced ultrasound can provide an immediate evaluation of residual tumors and guidance to supplementary ablation [Citation90].
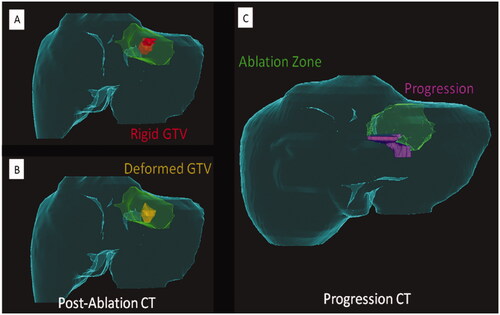
Figure 5. Importance of a deformable registration model use for assessing ablation margins. (A) 3 D CT reconstructionu using a rigid registration model. Gross tumor volume (GTV, red) is depicted onto the postablation CT maps within the ablation zone (green area); (B) 3 D CT reconstruction using a deformable registration model depicting GTV (yellow) in close contact with the ablation zone margin (green area) caudally, reflecting insuficient ablation margins (2 mm) at that region; (C) 6-month postablation 3 D CT reconstruction using a deformable registration depicting local tumor progression (purple area) and the caudal aspect of the ablation zone (green area), which was deemed to have insufficient ablation margins per biomechanical deformable registration model. Courtesy: Brian Anderson, PhD and Kristy Brock, PhD.
Besides imaging assessment, intraprocedural pathology assessment can provide clinical information that has prognostic significance for LTP. The identification of viable Ki-67-positive tumor cells from the tissue fragment adherent to electrodes or tissue obtained from the biopsy of the tumor center and the suspected minimal margin of the ablation zone has been reported to be associated with LTP and OS [Citation91–93]. A prospective study performed a biopsy of the ablation zone showing minimal margins <5 mm were likely to have biopsies with viable tumor cells (p = 0.019) [Citation91]. In addition, immediate fluorescent assessment of ablation zone [Citation94] or in vivo diffuse reflectance spectroscopy [Citation95] has shown to be feasible for real-time identification of residual viable tumor cells.
1.3. Longitudinal sequential local therapies: a must from the get-go
In recent years, a shift in the oncological liver surgery approach of patients with CLM has occurred. The previous approach of performing major hepatectomies for wider tumor clearance and resultant ‘less at-risk liver’ has been superseded by the parenchymal-sparing hepatectomy (PSH) [Citation96–99]. Such change has been driven by several reports showing that major hepatectomy is more frequently associated with postoperative liver insufficiency, while it does not prevent intrahepatic recurrence or improve survival [Citation100]. Moreover, growth factors and cytokines, which might get elevated depending on the extent of liver resection, may also act as pro-oncogenic factors, ultimately promoting tumor progression [Citation101–103]. Finally, it is known that up to 75% of the patients with CLM submitted to hepatectomy as the initial local curative modality will unfortunately present with recurrence during their disease course, with the liver representing a significant proportion of such recurrences [Citation78,Citation104,Citation105].
In this scenario, allowing sufficient liver remnant following first local liver curative therapy might allow further application of local therapies such as re-resection or liver ablation at the time of recurrence, which has been shown to positively impact patient outcomes [Citation71,Citation98,Citation106,Citation107]. On a retrospective analysis of 300 solitary CLM patients, Mise et al. [Citation98] showed that patients undergoing PSH, when compared to non-PSH patients, did not have worse OS, recurrence-free, and liver-only recurrence-free survival. More importantly, repeat hepatectomy was more frequently performed in the PSH group (68% vs 24%, p < 0.01) and, among patients with liver-only recurrence, better 5-year OS from initial hepatectomy and liver recurrence was shown on the PSH group versus the non-PSH. Finally, no difference in OS was noted after liver recurrence between patients who received repeated resection versus ablation at the time of recurrence (p = 0.143) [Citation98].
Also, subsequent ablation to LTP after ablation could improve the survival compared to LTP who did not undergo re-treatment. Solbiati et al. reported that the median OS rates of patients undergoing repeated RFA for LTP and who did not re-treated were 45.5 months and 31.0 months, respectively (p < 0.001) [Citation108]. Another study from Sofocleous et al. reported that patients undergoing repeated RFA for LTP had a significantly prolonged 3-year survival than those who didn’t (89% vs 23%, p = 0.03) [Citation71]. These results support percutaneous thermal ablation as a longitudinal sequential local therapy for salvage treatment after disease recurrences.
Recent publications on the use of liver ablation for recurrent CLM after hepatectomy have shown LTP rates ranging from 5.1% to 16.7% [Citation106,Citation109,Citation110]. On a retrospective analysis, Schullian et al. [Citation109] demonstrated their experience with multi-probe stereotactic RFA in 64 patients with a total of 217 ablated CLMs. Despite the inclusion of patients with up to 25 metastases (median, n = 2) with diameters of up to 7.5 cm (median, 2.7 cm), LTP rate of 11.5%, median OS of 33.1 months, with 1-, 3- and 5-year OS rates of 90.1%, 46.2% and 34.8% after the first SRFA were achieved. Of the 25 tumors with local tumor progression, there were 13 (52%) tumors with size ≥ 3 cm and 3 (12%) with size ≥ 5 cm. Notably, 31 patients (48.4%) developed distant tumor recurrence away from the prior ablation of whom 15 patients received repeated SRFA [Citation109]. Such results highlight the need for planning for sequential therapies on the longitudinal care of such patients and the ability to achieve OS rates with ablation similar to re-resection.
1.4. Ablation: standing against (and side-to-side) with resection
With its minimally invasive approach, faster recovery, superior safety profile, and reduced costs when compared to surgical resection, percutaneous ablation is an appealing treatment alternative for patients with small CLM. Ablation combined with systemic chemotherapy has been shown on a prospective phase II study to significantly prolong the OS in patients with unresectable CLM when compared to the use of chemotherapy alone [Citation111]. Despite such potential advantages, to date, there are no available prospective randomized studies providing a definitive answer to this matter [Citation4–8]. Retrospective non-randomized studies have reported that RFA has similar outcomes to resection with 5-year survival rates up to 55% [Citation112–116]. A comparison of the CLOCC and EPOC trials showed that ablation had similar outcomes to resection for small tumors, which made authors support the use of RFA for local control in patients with limited metastases [Citation29]. Contrarily, several meta-analyses have reported that liver resection was significantly superior to RFA in respect of overall and disease-free survival, although RFA showed a significantly lower rate of complications [Citation8,Citation74,Citation117,Citation118]. Recently, a case-matched study of 271 patients reported no difference in 3-year OS between patients submitted to MWA and hepatectomy as the first intervention for CLM (76 vs. 76%; p = 0.253) [Citation119]. An ongoing noninferiority phase-III single-blind prospective randomized controlled trial comparing thermal ablation versus liver resection for CLM (the COLLISION trial) will add much needed evidence on the role of ablation as a first local therapy option [Citation120]. In this trial, patients with at least one resectable and ablatable CLM (≤ 3 cm) and up to ten lesions are considered eligible ( and ). Additional unresectable tumors should be ≤ 3 cm and ablatable and additional unablatable tumors should be resectable. Patients undergoing any surgical resection or focal ablative liver therapy, systemic treatment ≤ 6 weeks before the procedure, or extrahepatic disease are excluded. The primary endpoint is OS. The main secondary endpoints are disease-free survival, time-to-progression, primary and assisted technique efficacy, mortality, length of hospital stay, assessment of the quality of life, and cost-effectiveness [Citation120].
Figure 6. Assessment flowchart per-tumor of COLLISION trial. Adapted from Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and Ablatability Criteria for the Treatment of Liver Only Colorectal Metastases: Multidisciplinary Consensus Document from the COLLISION Trial Group. Cancers (Basel). 2020;12(7):1779. Published 2020 Jul 3.
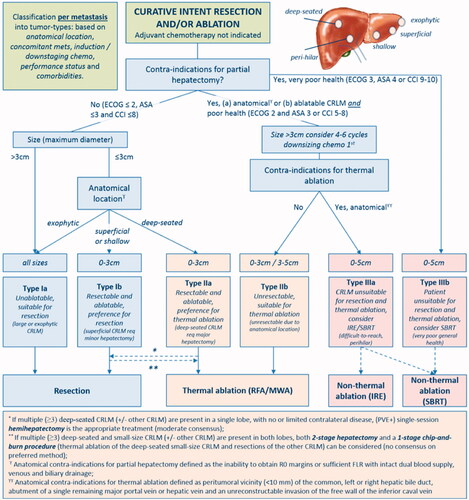
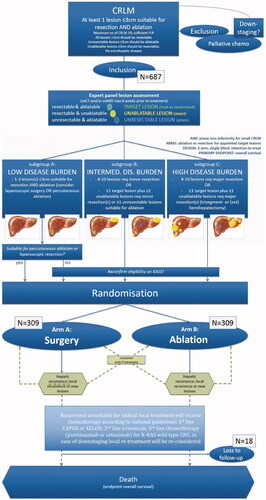
Figure 7. Flow diagram of study procedure from COLLISION trial. Adapted from Puijk RS, Ruarus AH, Vroomen LGPH, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18(1):821. Published 2018 Aug 15.
The combination of hepatic resection with ablation has been proposed to achieve cure and preserve future liver remnants for patients with an extensive distribution of CLM [Citation121–123]. Studies have reported that combined therapy of hepatic resection and ablation had comparable survival compared to hepatic resection alone [Citation118,Citation121,Citation124]. Nevertheless, a study compared two-stage hepatectomy to one-stage hepatectomy combined with RFA for bilobar CLM and found an improved 5-year OS rate (35 vs. 24%; p = 0.01) and a lower incidence of postoperative hepatic insufficiency (6% vs 28%, p < 0.0001) in patients undergoing two-stage hepatectomy [Citation125]. The authors inferred those surgeons may inadvertently over-ablate the lesions in an attempt to reduce the local recurrence rate but caused more unplanned damage to the liver remnant. Lastly, a new developed sequential treatment strategy, planned incomplete resection followed by postoperative percutaneous CT/MR-guided completion ablation for intentionally-untreated lesions, may provide better local tumor control when compared with intraoperative US-guided ablation (5-year local tumor recurrence: 31.7 vs. 62.4%; p = 0.03), while offering significantly lower rates of major postoperative complication [Citation122]. provides an overview of the most relevant ablation studies recently published.
Table 2. Selected recently published relevant ablation studies.
2 Current gaps in knowledge (the ‘known unknowns’)
2.1. The relevance of co-mutations in liver resection
Although mutant RAS status of CLM has been already linked to worse outcomes after thermal ablation [Citation45,Citation46], it is currently unknown what is the interplay between co-mutations and ablation outcomes. RAS mutation in patients with wild-type TP53 and SMAD4 was not associated with a worse prognosis than wild-type RAS after CLM resection [Citation126]. Additionally, the double mutations of TP53 with either KRAS, NRAS, or BRAF were associated with significantly worse survival compared with mutations in both gene groups alone [Citation127]. Coexisting mutations in RAS, TP53, and SMAD4 were associated with significantly worse recurrence and survival than coexisting mutations in any 2 or 1 of these genes [Citation126]. It is unknown that these genes with distinct signaling pathways can ‘cross-talk’ between each other or accumulate the hazard for survival.
2.2. The impact of prior hepatectomy
It has been reported that the history of prior liver resection is associated with improved local tumor control and survival of CLM thermal ablation. Odisio et al. reported that 3-year LTPFS (73 vs. 34%; p < 0.001), recurrence-free survival (23 vs. 9.1%; p = 0.026), and OS (78 vs. 48%; p = 0.003) were improved in patients with previous hepatic resection submitted to percutaneous ablation than on patients without previous hepatic resection submitted to percutaneous ablation [Citation106]. Survival rates for patients submitted to liver ablation after hepatectomy in this series are in keeping with current survival rates reported after the first and second hepatectomy for CLM [Citation128–130]. Although the patients with prior hepatic resection were younger, had ablation earlier on smaller CLM, and received less chemotherapy, the multivariable analysis did not disclose any of those factors associated with LTPFS. The authors suggested that the process of selecting patients for hepatic resection might have translated into factors positively affecting ablation outcomes of CLM who developed following resection. This might indicate the existence of currently unknown biological factors influencing ablation outcomes associated with patient selection for surgical resection. An ablation clinical risk score adapted from surgical clinical risk score [Citation131], including the nodal status of the primary tumor, the time interval from primary resection to CLM diagnosis, number of tumors, and size of the largest tumor, is associated with local tumor control and OS [Citation15,Citation71,Citation132]. This also has similar impacts on outcomes of ablation to the history of prior hepatectomy. Such observations further rebuke retrospective comparison of local recurrence rates between surgically resected and ablated patients, since such patient populations might be fundamentally different.
2.3. Influence of micrometastatic disease
The detection of circulating free tumor DNA, plasma microRNA, and circulating tumor cells, which are encompassed by the term ‘liquid biopsy’, is currently used clinically for therapeutic guidance, especially for the tumors with intratumor heterogeneity and clonal heterogeneity such as colorectal cancers. The technique could supplement existing clinical tools by improving screening, early detection, staging, recurrence identification, and prediction of clinical outcomes after treatment. Serial monitoring of the levels of circulating free tumor DNA during treatments can provide early detection of early disease progression in patients undergoing anti-EGFR therapies and immune-checkpoint inhibitors [Citation133–136]. Similarly, the circulating tumor cells might represent a predictive biomarker of early response to chemotherapy and targeted agents [Citation137,Citation138], which might guide escalation or de-escalation of systemic treatment. Also, a challenge in CLM treatment is the development of resistance to systemic treatment, and ablation has been linked in some animal studies with aggressive tumor biological changes and tumor growth promotion [Citation139,Citation140]. It has been reported that liquid biopsy can detect oncogenic mutations in plasma before radiologic progression [Citation141,Citation142]. The liquid biopsy may provide serial monitoring of tumor heterogeneity and evolution over time and guiding clinical decisions [Citation143]. Despite its intriguing potential, the clinical practice of liquid biopsy on patients undergoing ablation is limited due to the lack of standardization of the test and clear demonstration of its clinical benefits to date.
2.4. Salvage local therapy after the first resection of colorectal liver metastases: the impact of histopathological growth patterns
The histopathological growth patterns (HGP) of hepatic tumors appear at the interface between the tumor border and surrounding liver parenchyma. This includes three distinct growth patterns: a desmoplastic, a pushing, and a replacement type. They have been suggested to have the potential to predict the oncological outcomes of CLM and validated as prognostic biomarkers in patients undergoing resection of CLM with a replicable scoring method in international consensus guidelines [Citation144–148]. Studies of CLM patients undergoing hepatic resection have reported more recurrence, microscopic residual tumor, and worse OS in non-desmoplastic HGP than in desmoplastic HGP [Citation146,Citation149]. Additionally, a higher rate of intrahepatic only recurrence and local treatments with curative intent for recurrence after hepatic resection was reported in desmoplastic HGP, inferring patients with better prognosis can still take salvageable local treatments [Citation149]. Although the tumors with nondesmoplastic HGP exhibit features associated with aggressive cancer biology and worse outcomes after hepatic resection [Citation150], its impact on oncological outcomes of ablation is unknown, warranting further investigation.
Conclusions
The fast-pacing evolving understanding of ablation therapies technology, imaging guidance, tumor biology, and artificial intelligence has the potential to clarify the current gaps in knowledge and to bring to light variables that were completely overlooked in the management of patients with CLM. When taken together, such observations can fundamentally change our perspective on the use of liver ablation. Moreover, by standardizing ablation planning, monitoring, and immediate response assessment, complete ablation (A0) with sufficient margins (ideally > 10 mm) can be achieved, which translates into lower rates of LTP that are on par with current surgical literature.
Based on the contemporary literature, aggressive local therapy with ablation (and, once possible, in combination with surgical resection) is associated with prolonged OS in patients with unresectable CLM. In addition, ablation for salvage after CLM resection recurrence can be considered in selected patients as the first-line modality of choice given similar OS and local recurrence rates to re-resection, and faster patient recovery. Finally, the role of ablation as first-line local therapy in comparison to surgical resection will have to be examined once the results of the COLLISION trial are available. Nevertheless, it is expected that both ablation and resection will play a synergistic role in the management of such patients, further highlighting the need for multi-disciplinary care for this patient population.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- National Comprehensive Cancer Network. Colon Cancer (Version 2.2021) 2021. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428.
- Di Martino M, Rompianesi G, Mora-Guzmán I, et al. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur J Surg Oncol. 2020;46(5):772–781.
- Otto G, Düber C, Hoppe-Lotichius M, et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251(5):796–803.
- Nishiwada S, Ko S, Mukogawa T, et al. Comparison between percutaneous radiofrequency ablation and surgical hepatectomy focusing on local disease control rate for colorectal liver metastases. Hepatogastroenterology. 2014;61(130):436–441.
- Berber E, Tsinberg M, Tellioglu G, et al. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointest Surg. 2008;12(11):1967–1972.
- McKay A, Fradette K, Lipschitz J. Long-term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surg. 2009; 2009:346863.
- Kron P, Linecker M, Jones RP, et al. Ablation or resection for colorectal liver metastases? A systematic review of the literature. Front Oncol. 2019;9:1052.
- Lee WS, Yun SH, Chun HK, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008;42(8):945–949.
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759–766.
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722.
- Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, et al. Impact of surgical margin width on recurrence and overall survival following R0 hepatic resection of colorectal metastases: a systematic review and meta-analysis. Ann Surg. 2018;267(6):1047–1055.
- Ahmed M, Solbiati L, Brace CL, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–1705.e4.
- Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30(7):567–574.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19(5):1206–1213.
- Petre EN, Jia X, Thornton RH, et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer. 2013;12(1):37–44.
- Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION trial group. Cancers (Basel). 2020;12(7):1779.
- Siperstein AE, Berber E, Ballem N, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246(4):559–565.
- Wang CZ, Yan GX, Xin H, Liu ZY. Oncological outcomes and predictors of radiofrequency ablation of colorectal cancer liver metastases. World J Gastrointest Oncol. 2020;12(9):1044–1055.
- Han K, Kim JH, Yang SG, et al. A single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology. 2021;298(1):212–218.
- Shi Y, Wang Z, Chi J, et al. Long-term results of percutaneous microwave ablation for colorectal liver metastases. HPB (Oxford)). 2021;23(1):37–45.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of Colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Qin S, Liu GJ, Huang M, et al. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: a review of a 4-year experience at a single center. Int J Hyperthermia. 2019;36(1):36–43.
- Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23(7):1358–1364.
- Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate Meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171.
- Ayav A, Germain A, Marchal F, et al. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg. 2010;200(4):435–439.
- Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European organisation for research and treatment of cancer #40004 and #40983. Eur J Cancer. 2014;50(5):912–919.
- Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19(7):1087–1092.
- Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore)). 2015;94(9):e580.
- Rhaiem R, Kianmanesh R, Minon M, et al. Microwave thermoablation of colorectal liver metastases close to large hepatic vessels under pringle maneuver minimizes the "Heat Sink Effect”. World J Surg. 2020;44(5):1595–1603.
- Bhardwaj N, Dormer J, Ahmad F, et al. Microwave ablation of the liver: a description of lesion evolution over time and an investigation of the heat sink effect. Pathology. 2011;43(7):725–731.
- Ringe KI, Lutat C, Rieder C, et al. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS One. 2015;10(7):e0134301.
- van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-Based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39(10):1438–1446.
- Marchal F, Elias D, Rauch P, et al. Biliary lesions during radiofrequency ablation in liver. Study on the pig. Eur Surg Res. 2004;36(2):88–94.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.
- Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–118.
- Dollinger M, Zeman F, Niessen C, et al. Bile duct injury after irreversible electroporation of hepatic malignancies: evaluation of MR imaging findings and laboratory values. J Vasc Interv Radiol. 2016;27(1):96–103.
- Lee EW, Wong D, Prikhodko SV, et al. Electron microscopic demonstration and evaluation of irreversible electroporation-induced nanopores on hepatocyte membranes. J Vasc Interv Radiol. 2012;23(1):107–113.
- Liu C, He J, Li T, et al. Evaluation of the efficacy and postoperative outcomes of hydrodissection-assisted microwave ablation for subcapsular hepatocellular carcinoma and colorectal liver metastases. Abdom Radiol (NY). 2021;46(5):2161–2172.
- Sartori S, Tombesi P, Macario F, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248(2):670–679.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: Ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275.e1.
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–768.
- Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28(7):2727–2734.
- Shady W, Petre EN, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017;8(39):66117–66127.
- Jiang BB, Yan K, Zhang ZY, et al. The value of KRAS gene status in predicting local tumor progression of colorectal liver metastases following radiofrequency ablation. Int J Hyperthermia. 2019;36(1):211–219.
- Lin YM, Taiji R, Calandri M, et al. Tumor biomarkers and interventional oncology: Impact on local outcomes for liver and lung malignancy. Curr Oncol Rep. 2021;23(6):67.
- Van Cutsem E, Lenz HJ, Kohne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700.
- Zimmitti G, Shindoh J, Mise Y, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(3):834–842.
- Schramm K, Krause K, Bittroff-Leben A, et al. D. Activated K-ras is involved in regulation of integrin expression in human Colon carcinoma cells. Int J Cancer. 2000;87(2):155–164.
- Brudvik KW, Mise Y, Chung MH, et al. RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23(8):2635–2643.
- Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31(5):948–956.
- van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13(5):651–658.
- Puijk RS, Nieuwenhuizen S, van den Bemd BAT, et al. Transcatheter CT hepatic arteriography compared with conventional CT fluoroscopy guidance in percutaneous thermal ablation to treat colorectal liver metastases: a single-center comparative analysis of 2 historical cohorts. J Vasc Interv Radiol. 2020;31(11):1772–1783.
- Lin Gc EY, Brock KB, Avritscher R, et al. Intra-arterial CT hepatic angiography for tumor detection and ablation endpoint assessment: a proof-of-concept analysis. Cardiovasc Intervent Radiol. 2019;42(3):65–549.
- Mauri G, Cova L, De Beni S, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38(1):143–151.
- Schullian P, Johnston E, Laimer G, et al. Thermal ablation of CT 'invisible' liver tumors using MRI fusion: a case control study. Int J Hyperthermia. 2020;37(1):564–572.
- Moulton CA, Gu CS, Law CH, et al. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. Jama. 2014;311(18):1863–1869.
- Oba A, Mise Y, Ito H, et al. Clinical implications of disappearing colorectal liver metastases have changed in the era of hepatocyte-specific MRI and contrast-enhanced intraoperative ultrasonography. HPB (Oxford)). 2018;20(8):708–714.
- Kurilova I, Bendet A, Petre EN, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer. 2021;20(2):e82–e95.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79(1):124–130.
- Berber E. The first clinical application of planning software for laparoscopic microwave thermosphere ablation of malignant liver tumours. HPB (Oxford)). 2015;17(7):632–636.
- Bale R, Widmann G, Stoffner DI. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol. 2010;75(1):32–6.
- Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol. 2012;22(4):930–937.
- Agcaoglu O, Aliyev S, Karabulut K, et al. Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg. 2013;37(6):1333–1339.
- Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol. 2011;18(7):1947–1954.
- Reuter NP, Woodall CE, Scoggins CR, et al. Ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13(3):486–491.
- Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg. 2013;37(6):1340–1347.
- Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–761.
- Jakobs TF, Hoffmann RT, Trumm C, et al. Radiofrequency ablation of colorectal liver metastases: mid-term results in 68 patients. Anticancer Res. 2006;26(1b):671–680.
- Wong J, Lee KF, Yu SC, et al. Percutaneous radiofrequency ablation versus surgical radiofrequency ablation for malignant liver tumours: the long-term results. HPB (Oxford)). 2013;15(8):595–601.
- van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Fütterer JJ, et al. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a Meta-analysis. HPB (Oxford)). 2017;19(9):749–756.
- Piccioni F, Poli A, Templeton LC, et al. Anesthesia for percutaneous radiofrequency tumor ablation (PRFA): a review of current practice and techniques. Local Reg Anesth. 2019;12:127–137.
- Denys A, Lachenal Y, Duran R, et al. Use of high-frequency jet ventilation for percutaneous tumor ablation. Cardiovasc Intervent Radiol. 2014;37(1):140–146.
- Puijk RS, Ziedses Des Plantes V, Nieuwenhuizen S, et al. Propofol compared to midazolam sedation and to general anesthesia for percutaneous microwave ablation in patients with hepatic malignancies: a Single-Center comparative analysis of three historical cohorts. Cardiovasc Intervent Radiol. 2019;42(11):1597–1608.
- Muratore A, Ribero D, Zimmitti G, et al. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol. 2010;17(5):1324–1329.
- Izaaryene J, Drai M, Deniel C, et al. Computed tomography-guided microwave ablation of perivascular liver metastases from colorectal cancer: a study of the ablation zone, feasibility, and safety. Int J Hyperthermia. 2021;38(1):887–899.
- Laimer G, Schullian P, Putzer D, et al. Can accurate evaluation of the treatment success after radiofrequency ablation of liver tumors be achieved by visual inspection alone? Results of a blinded assessment with 38 interventional oncologists. Int J Hyperthermia. 2020;37(1):1362–1367.
- Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30(5):2463–2472.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29(5):2698–2705.
- Kim YS, Lee WJ, Rhim H, et al. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195(3):758–765.
- Sibinga Mulder BG, Hendriks P, Baetens TR, et al. Quantitative margin assessment of radiofrequency ablation of a solitary colorectal hepatic metastasis using MIRADA RTx on CT scans: a feasibility study. BMC Med Imaging. 2019;19(1):71.
- Bo XW, Xu HX, Guo LH, et al. Ablative safety margin depicted by fusion imaging with post-treatment contrast-enhanced ultrasound and pre-treatment CECT/CEMRI after radiofrequency ablation for liver cancers. BJR. 2017;90(1078):20170063.
- Shyn PB, Casadaban LC, Sainani NI, et al. Intraprocedural ablation margin assessment by using ammonia perfusion PET during FDG PET/CT-guided liver tumor ablation: a pilot study. Radiology. 2018;288(1):138–145.
- Ryan ER, Sofocleous CT, Schöder H, et al. Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology. 2013;268(1):288–295.
- Cornelis FH, Petre EN, Vakiani E, et al. Immediate postablation 18F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med. 2018;59(9):1360–1365.
- Cornelis F, Silk M, Schoder H, et al. Performance of intra-procedural 18-fluorodeoxyglucose PET/CT-guided biopsies for lesions suspected of malignancy but poorly visualized with other modalities. Eur J Nucl Med Mol Imaging. 2014;41(12):2265–2272.
- Francica G, Meloni MF, Riccardi L, et al. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol. 2018;105:96–101.
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: Biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19(13):4262–4269.
- Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249(1):364–374.
- Sotirchos VS, Fujisawa S, Vakiani E, et al. Fluorescent tissue assessment of colorectal cancer liver metastases ablation zone: a potential real-time biomarker of complete tumor ablation. Ann Surg Oncol. 2019;26(6):1833–1840.
- Tanis E, Spliethoff JW, Evers DJ, et al. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur J Surg Oncol. 2016;42(2):251–259.
- Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100(2):278–284.
- DeMatteo RP, Palese C, Jarnagin WR, et al. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4(2):178–184.
- Mise Y, Aloia TA, Brudvik KW, et al. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Annal Surg. 2016;263(1):146–152.
- Moris D, Dimitroulis D, Vernadakis S, et al. Parenchymal-sparing hepatectomy as the new doctrine in the treatment of liver-metastatic colorectal disease: beyond oncological outcomes. Anticancer Res. 2017;37(1):9–14.
- Kokudo N, Tada K, Seki M, et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001;181(2):153–159.
- Tanaka K, Shimada H, Matsumoto C, et al. Impact of the degree of liver resection on survival for patients with multiple liver metastases from colorectal cancer. World J Surg. 2008;32(9):2057–2069.
- Mizutani J, Hiraoka T, Yamashita R, et al. Promotion of hepatic metastases by liver resection in the rat. Br J Cancer. 1992;65(6):794–797.
- Lim C, Cauchy F, Azoulay D, et al. Tumour progression and liver regeneration-insights from animal models. Nat Rev Gastroenterol Hepatol. 2013;10(8):452–462.
- Chan KM, Wu TH, Cheng CH, et al. Prognostic significance of the number of tumors and aggressive surgical approach in colorectal cancer hepatic metastasis. World J Surg Oncol. 2014;12:155.
- Park MS, Yi NJ, Son SY, You T, et al. Histopathologic factors affecting tumor recurrence after hepatic resection in colorectal liver metastases. Ann Surg Treat Res. 2014;87(1):14–21.
- Odisio BC, Yamashita S, Huang SY, et al. Impact of prior hepatectomy history on local tumor progression after percutaneous ablation of colorectal liver metastases. J Vasc Interv Radiol. 2018;29(3):395–403 e1.
- Brouquet A, Vauthey JN, Badgwell BD, et al. Hepatectomy for recurrent colorectal liver metastases after radiofrequency ablation. Br J Surg. 2011;98(7):1003–1009.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968.
- Schullian P, Johnston EW, Putzer D, et al. Stereotactic radiofrequency ablation (SRFA) for recurrent colorectal liver metastases after hepatic resection. Eur J Surg Oncol. 2021;47(4):866–873.
- Fan XX, Lv SY, Zhang MW, et al. Clinical analysis of ultrasound-guided radiofrequency ablation for recurrent colorectal liver metastases after hepatectomy. World J Surg Oncol. 2020;18(1):76.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015.
- Karanicolas PJ, Jarnagin WR, Gonen M, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg. 2013;148(7):597–601.
- Hof J, Joosten HJ, Havenga K, et al. Radiofrequency ablation is beneficial in simultaneous treatment of synchronous liver metastases and primary colorectal cancer. PLoS One. 2018;13(3):e0193385.
- Lee H, Heo JS, Cho YB, et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: a propensity score analysis. WJG. 2015;21(11):3300–3307.
- Hof J, Wertenbroek MW, Peeters PM, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103(8):1055–1062.
- Vietti Violi N, Duran R, Demartines N, et al. Local recurrence rate in patients with colorectal cancer liver metastasis after wedge resection or percutaneous radiofrequency ablation. Int J Hyperthermia. 2018;34(7):1020–1028.
- Weng M, Zhang Y, Zhou D, et al. Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS One. 2012;7(9):e45493.
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204.
- Tinguely P, Dal G, Bottai M, et al. Microwave ablation versus resection for colorectal cancer liver metastases - A propensity score analysis from a population-based nationwide registry. Eur J Surg Oncol. 2020;46(3):476–485.
- Puijk RS, Ruarus AH, Vroomen L, COLLISION Trial Group, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18(1):821.
- Philips P, Groeschl RT, Hanna EM, et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg. 2016;103(8):1048–1054.
- Okuno M, Kawaguchi Y, De Bellis M, et al. A new sequential treatment strategy for multiple colorectal liver metastases: Planned incomplete resection and postoperative completion ablation for intentionally-untreated tumors under guidance of cross-sectional imaging. Eur J Surg Oncol. 2021;47(2):311–316.
- Faitot F, Faron M, Adam R, et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg. 2014;260(5):822–827.
- Tanaka K, Shimada H, Nagano Y, et al. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139(2):263–273.
- Mizuno T, Cloyd JM, Omichi K, et al. Two-stage hepatectomy vs one-stage major hepatectomy with contralateral resection or ablation for advanced bilobar colorectal liver metastases. J Am Coll Surg. 2018;226(5):825–834.
- Kawaguchi Y, Kopetz S, Newhook TE, et al. Mutation status of RAS, TP53, and SMAD4 is superior to mutation status of RAS alone for predicting prognosis after resection of colorectal liver metastases. Clin Cancer Res. 2019;25(19):5843–5851.
- Datta J, Smith JJ, Chatila WK, McAuliffe JC, et al. Coaltered ras/B-raf and TP53 is associated with extremes of survivorship and distinct patterns of metastasis in patients with metastatic colorectal cancer. Clin Cancer Res. 2020;26(5):1077–1085.
- Denbo JW, Yamashita S, Passot G, et al. RAS mutation is associated with decreased survival in patients undergoing repeat hepatectomy for colorectal liver metastases. J Gastrointest Surg. 2017;21(1):68–77.
- Ali MA, Di Sandro S, Lauterio A, et al. Repeat hepatectomy for recurrent colorectal liver metastases: is it worth the challenge? J Gastrointest Surg. 2015;19(12):2192–2198.
- Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg. 2013;100(6):808–818.
- Creasy JM, Sadot E, Koerkamp BG, et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163(6):1238–1244.
- Urbonas T, Anderson EM, Gordon-Weeks AN, et al. Factors predicting ablation site recurrence following percutaneous microwave ablation of colorectal hepatic metastases. HPB (Oxford). 2019;21(9):1175–1184.
- Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795–801.
- Germano G, Mauri G, Siravegna G, et al. Parallel evaluation of circulating tumor DNA and circulating tumor cells in metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17(1):80–83.
- Khan KH, Cunningham D, Werner B, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov. 2018;8(10):1270–1285.
- Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779.
- Tol J, Koopman M, Miller MC, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21(5):1006–1012.
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–3221.
- Rozenblum N, Zeira E, Scaiewicz V, et al. Oncogenesis: an "off-Target" effect of radiofrequency ablation. Radiology. 2015;276(2):426–432.
- Ahmed M, Kumar G, Moussa M, et al. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-Met inhibition. Radiology. 2016;279(1):103–117.
- Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532–536.
- Diaz LA, Jr., Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537–540.
- Underwood JJ, Quadri RS, Kalva SP, et al. Liquid biopsy for cancer: review and implications for the radiologist. Radiology. 2020;294(1):5–17.
- Falcão D, Alexandrino H, Caetano Oliveira R, et al. Histopathologic patterns as markers of prognosis in patients undergoing hepatectomy for colorectal cancer liver metastases - pushing growth as an independent risk factor for decreased survival. Eur J Surg Oncol. 2018;44(8):1212–1219.
- Galjart B, Nierop PMH, van der Stok EP, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22(2):355–368.
- Nierop PMH, Höppener DJ, van der Stok EP, et al. Histopathological growth patterns and positive margins after resection of colorectal liver metastases. HPB (Oxford). 2020;22(6):911–919.
- Buisman FE, van der Stok EP, Galjart B, et al. Histopathological growth patterns as biomarker for adjuvant systemic chemotherapy in patients with resected colorectal liver metastases. Clin Exp Metastasis. 2020;37(5):593–605.
- van Dam PJ, van der Stok EP, Teuwen LA, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117(10):1427–1441.
- Nierop PMH, Galjart B, Höppener DJ, et al. Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis. 2019;36(2):109–118.
- Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294–1302.
- Meijerink MR, Ruarus AH, Vroomen L, et al. Irreversible electroporation to treat unresectable colorectal liver metastases (COLDFIRE-2): a phase II, two-center, single-arm clinical trial. Radiology. 2021:203089.
- Fan XX, Lv SY, Zhang MW, et al. Clinical analysis of ultrasound-guided radiofrequency ablation for recurrent colorectal liver metastases after hepatectomy. World J Surg Oncol. 2020;18(1):76.
- Zimmermann M, Pedersoli F, Schulze-Hagen M, et al. Salvage RFA in patients with intrahepatic recurrence after major hepatic surgery for colorectal cancer liver metastases: mid-term outcome. Eur Radiol. 2020;30(2):1221–1227.
- Cornelis FH, Petre EN, Vakiani E, et al. Immediate postablation (18)F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med. 2018;59(9):1360–1365.
- Schicho A, Niessen C, Haimerl M, et al. Long-term survival after percutaneous irreversible electroporation of inoperable colorectal liver metastases. Cancer Manag Res. 2019;11:317–322.
- Mao R, Zhao JJ, Bi XY, et al. Resectable recurrent colorectal liver metastasis: can radiofrequency ablation replace repeated metastasectomy? ANZ J Surg. 2019;89(7–8):908–913.
- van Amerongen MJ, van der Stok EP, Fütterer JJ, et al. Results after simultaneous surgery and RFA liver ablation for patients with colorectal carcinoma and synchronous liver metastases. Eur J Surg Oncol. 2019;45(12):2334–2339.
- Dupré A, Jones RP, Diaz-Nieto R, et al. Curative-intent treatment of recurrent colorectal liver metastases: a comparison between ablation and resection. Eur J Surg Oncol. 2017;43(10):1901–1907.
- Imai K, Allard MA, Castro Benitez C, et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg. 2017;104(5):570–579.
- Sasaki K, Margonis GA, Andreatos N, et al. Combined resection and RFA in colorectal liver metastases: stratification of long-term outcomes. J Surg Res. 2016;206(1):182–189.
- Valls C, Ramos E, Leiva D, et al. Safety and efficacy of ultrasound-guided radiofrequency ablation of recurrent colorectal cancer liver metastases after hepatectomy. Scand J Surg. 2015;104(3):169–175.
