Abstract
Objective
A systematic review of clinical trials on thermal ablation of T1b RCC was conducted to assess oncologic outcomes of those procedures. The primary endpoint was the rate of local recurrence. Secondary endpoints included technical efficacy, progression to metastatic disease, cancer-specific mortality, complications and renal function decrease.
Methods
PubMed (MEDLINE) and Embase databases were searched in June 2020 for eligible trials following the PRISMA selection process. Prevalence of local recurrence and per procedural major adverse effects were calculated using double arcsine transformation and a random-effects model.
Results
Nine clinical trials (all retrospective) involving 288 patients with T1b renal clear cell carcinoma treated with either percutaneous microwave ablation, cryoablation or radiofrequency ablation were analyzed. Using a random-effects model, the overall prevalence of local recurrence following percutaneous ablation was 0.08 (0.04–0.14; p = 0.05). Primary technical efficacy was 226/263 (86%) patients and secondary technical efficacy was 247/263 (94%). Overall, 10/176 (6%) patients presented metastatic locations following the ablation. Major adverse effects prevalence was 0.09 (0.06–0.14; p = 0.05).
Conclusions
Thermal ablations are feasible, safe, and effective to treat T1b renal clear cell carcinoma. More trials are necessary to determine the rate of the evidence of the benefit.
Thermal ablations are feasible and safe to treat T1b renal clear cell carcinoma.
Oncologic outcomes appear to be very good on both local control and distant progression.
Due to small number and heterogeneity of studies more trials are necessary to determine the rate of the evidence of the benefit.
Highlights
Introduction
Worldwide, renal cell carcinoma (RCC) represents the sixth most frequently diagnosed cancer in men and the 10th in women, accounting for 5% and 3% of all oncological diagnoses, respectively [Citation1]. Incidence rates are increasing, likely to be partially due to an increase in the incidental detection of renal masses when abdominal imaging is performed in higher-income settings. Percutaneous ablations have proved to be effective treatments for localized renal cancer [Citation2] and guidelines generally recommend considering percutaneous thermal ablation as an option for lesions <3cm [Citation3–5]. The well-described advantages of thermal ablation techniques compared to surgical resection are their minimally invasive nature, nephron-sparing and greater safety [Citation6,Citation7].
Contrary to percutaneous RFA limited to a smaller lesion, cryoablation (CA) and microwave ablation (MWA) may attain a higher rate of tumor coverage by the ablative zone resulting in lower rates of retreatment [Citation8].
International guidelines recommended surgery (radical nephrectomy (RN) or partial nephrectomy (PN)) as the best treatment for T1b RCC (4≤ tumor ≤7 cm) [Citation9]. However, thermal ablation of T1b RCC has been published with good results recently [Citation8,Citation10–18].
The aim of this review is to assess the current evidence for long-term oncologic efficacy after percutaneous RFA, cryoablation (CA) or microwave ablation (MWA) of stage T1b RCC.
Evidence acquisition
A systematic review and meta-analysis of published literature on T1b RCC ablation therapies was performed in adherence to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [Citation19]. This study only included peer-reviewed articles written in English. PubMed (MEDLINE) and Embase databases were searched. The literature search was last updated in June 2020. No date limitation was set in the constraints but all articles identified were published between 2011 and 2020.
Eligibility criteria
We conducted a 3-step selection process. Literature database search was performed according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) statements. First, the search was performed using varying combinations of the following keywords on PubMed, Cochrane library and Embase: renal, carcinoma, RCC, ablation, thermal ablation (TA), T1b, percutaneous, microwave (MWA), radiofrequency (RFA), cryoablation (CA). To be included, articles had to describe local recurrence and other oncologic outcomes following an imaging-guided TA of T1b kidney tumors in an original cohort of patients. The results had to describe only T1b tumors. Non-segregated laparoscopically and imaging-guided TA and non-segregated T1a and T1b groups were excluded. Then, all duplicates were removed and each abstract was assessed for relevance. We excluded articles which were not relevant and which did not respect the inclusion criteria. Finally, based on the assessment of full-text articles we excluded 4 last articles: 2 in which T1a and T1b groups weren’t segregated at inclusion and 2 which included laparoscopically guided ablations. Nine articles were finally included in our study ranging from 2014 to 2020. This selection process is described in the PRISMA flow diaphragm ().
Analysis technique
The main endpoint extracted measures included technical efficacy (TE), local recurrence (LR) rate, local recurrence-free survival (LRFS), progression to metastatic disease, duration of cancer-specific survival (CSS), duration of cancer-specific mortality (CSM), complications and estimated glomerular filtration rate decrease.
Primary TE was defined as the absence of new contrast enhancement and/or tumor enlargement at the first control. Homogenous, smooth, and low-level enhancement may be seen on post-ablation imaging without corresponding to recurrence [Citation20]. This was assessed by contrast-enhanced CT or MRI between 1 week after the procedure [Citation10] to 6 months after [Citation12]. Secondary TE was defined as TE after multiple treatments of image-guided tumor ablation [Citation21].
LR was defined as a new nodular or irregular enhancement within the ablation zone or enlargement of the ablated tumor in a patient after TE was obtained [Citation20]. LRFS was evaluated depending on studies at 3, 5 or 10 years.
Cancer-specific mortality (CSM) was defined as the rate of patients who died of cancer-related events.
Complications were classified as minor (CIRSE/Clavien-Dindo ≤3) or major (CIRSE/Clavien-Dindo ≥3) [Citation22]. Major complications were those requiring surgical, endoscopic or radiological intervention [Citation23]. Renal function was evaluated with the mean eGFR decrease in percentage.
Statistical analysis
The prevalence of local recurrence and complications were calculated using R software, version 4.0.1, package ‘metaprop’. An individual prevalence double arcsine transformation and random-effects model were used to take the inter-study heterogeneity into account and to pool individual measures of each study [Citation24]. For each endpoint, the pooled data was calculated together with a 95% confidence interval (CI).
Data analysis was performed independently for technical success, technique efficacy, and metastatic progression due to the heterogeneity of the studies. Due to the limited number of studies and patients, subgroup analysis wasn’t performed considering the different ablation techniques. The summation of results and percentages was calculated.
Ablation methods
The most frequently used in daily practice are RFA, CA, and MWA [Citation25]. To be included in this meta-analysis the ablation probe had to be placed percutaneously under US, MRI or CT guidance.
RFA consisted of a heat-based ablation using an alternating electric current applied to the target tissue through the use of an interstitial electrode [Citation26,Citation27]. Two of the included studies used RFA: Takaki et al. [Citation10] and Hasegawa et al. [Citation8] Overall, 44 patients were treated with RFA. Clustered electrodes were used I 4/44 patients ad a single electrode was used in 41/44 patients.
In CA, the freezing process resulted in both intracellular and extracellular ice formation inducing cellular death. The location of ice formation and, therefore, the mechanism of cell death vary with the freezing rate and final tissue temperature [Citation28, Citation29]. Five studies used CA: Atwell et al. [Citation11], Andrews et al. [Citation16], Hasegawa et al. [Citation8], Hebbadj et al. [Citation8], Gunn et al. [Citation17] and Grange et al. [Citation13]. Overall, 204 patients were treated with CA ().
MWA relies on the continuous realignment of polar molecules (primarily water) with the oscillating microwave field, increasing kinetic energy and tissue temperature [Citation30,Citation31]. Two included studies used microwaves: Shapiro et al. [Citation12] and Guo et al. [Citation15]. Sixty-three patients received this treatment.
Evidence synthesis
Patient demographics at inclusion and study designs are summarized in . In all studies, patients were referred for thermal ablation by a multidisciplinary team comprising urologists and interventional radiologists. Contraindications to surgery, rejection of surgery by the patient or poor renal function genetic syndromes such as Von Hippel Lindau syndrome [Citation14], were the main indications for thermal ablation,
Table 1. Studies, designs and demographics characteristics at inclusion.
All patients in Guo et al. [Citation14], Grange et al. [Citation13], Atwell et al. [Citation11], Hebbadj et al. [Citation14] and Shapiro et al.’s [Citation12] studies had biopsy-proven RCC. Twelve (52%) of patients treated with RFA and 16 (70%) patients in Hasegawa et al.’s [Citation8] article; 24 (50%) patients in Andrews et al.’s [Citation16] article, 23/37 (62%) in Gunn et al.’s article [Citation17] and 10/21 (48%) in Takaki et al. [Citation10] had biopsy-proven RCC. The other patients presented with enhancing T1b renal masses and weren’t biopsied. Overall 221/288 (77%) patients had biopsy-proven RCC.
Technical efficacy (TE)
Apart from Andrews et al. [Citation16], all authors reported TE. In RFA, TE achievement varied from 15/23 (65%) [Citation8] to 45/46 (98%) [Citation11]. Across all studies, 226/263 (86%) procedures achieved TE. Hasegawa et al. [Citation8] found cryoablation to offer a statistically significantly higher rate of TE than RFA.
All studies except Atwell et al. [Citation11] and Hebbadj et al. [Citation14], studied TE after a 2nd percutaneous procedure. Secondary TE (STE) in RFA varied from 91% (21/23) [Citation8] and 31/34 [Citation17] to 100% in the other studies [Citation8,Citation10,Citation12,Citation13,Citation15]. Overall, including Hebadj et al.’s [Citation14] and Atwell et al.’s studies, 94% (247/263) of patients achieved TE after one or two procedures.
Local recurrence
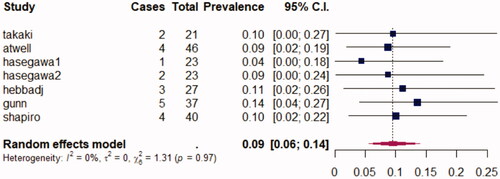
Mean follow-up varied across studies ranging from 14 months in Grange et al. [Citation13] to 6 years in Andrews et al. [Citation16]. Local recurrence rates ranged from 0% in Takaki et al.’s [Citation10] study to 23.5% in Andrews et al.’s [Citation16]. Across all studies, 25 of 293 (9%) patients presented with LR. Pooling all studies, the prevalence of LR following percutaneous ablation, using a random-effects model, was 0.08 (0.04–0.14; 95% CI). A heterogeneity assessment was measured with an I2 calculated here at 48%. No subgroup analysis was performed due to the small size of each population. Moreover, no subgroup analysis was performed in each study making it impossible to make a global subgroup analysis.
Hasegawa et al. [Citation8] found no differences between LR rates following CA or RFA. Takaki et al. [Citation10] found no differences between RFA and RN. Shapiro et al. [Citation12] found no difference concerning local recurrence rates between MWA and radical or partial nephrectomy.
Across studies, LRFS was measured at various lengths of time after ablation. Results were very variable ranging from 3-year LRFS of 60.3% in Hebbadj et al. [Citation14] to 5-year LRFS of 96.4% in Atwell et al. [Citation9]. No difference was noted in CSS among the treatment types between surgery and ablation in Takaki et al. [Citation10] or Shapiro et al. [Citation12].
It is to be noted that Shapiro et al. [Citation12] found 5-year LRFS to be significantly lower for MWA than for RN but not for PN (p = 0.02). In Takaki et al. 10-year LRFS wasn’t statistically different between RFA and RN. represents the random-effects model for prevalence of LR following TA.
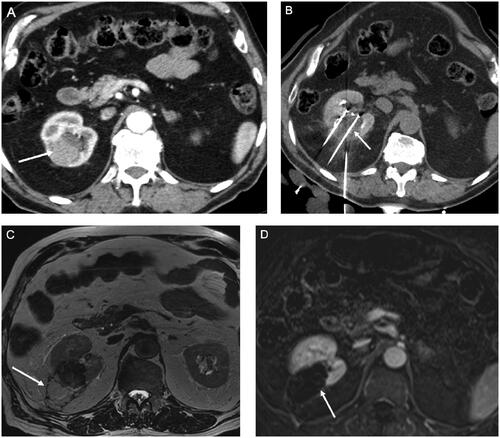
Figure 2. An 83 yo male who presented with a 44 mm clear cell carcinoma of the right kidney confirmed on percutaneous biopsy-proven was treated with cryoablation under computed tomography (CT) guidance. A) Axial contrast-enhanced CT images depicting a T1b CCC measuring 44 mm (arrow) the right kidney. B) Seven cryo-probes were used under CT guidance. CT images acquired intra-procedurally show a large hypodense ice-ball (arrow). C) T2 weighted MRI at 12-month follow-up demonstrates retraction of the ablation zone and a hypointense rim (arrow) around the ablation zone corresponding to cytosteatonecrosis. D) T1 weighted contrast-enhanced MRI obtained at 12 months months follow-up shows no remaining suspicious enhancement in the ablation zone (arrow).
Progression to metastatic disease
Apart from three studies (Hebbadj et al. [Citation14], Guo et al. [Citation15] and Gunn et al. [Citation17]), all authors studied the progression to metastatic disease. Results varied from 0/40 [Citation10] to 9.5% [Citation10]. Across all patients followed for metastatic disease, with follow-up ranging from 2.6 to 86.8 months, 10/176 (6%) patients presented metastatic locations following the ablation. Efficacy and oncologic outcomes of the studies are summarized in . Takaki et al. [Citation10] and Shapiro et al. [Citation12] found no differences between surgical and percutaneous treatments.
Table 2. Technical efficacy, secondary technical efficacy, local recurrence and progression to metastatic disease in all studies.
Cancer-specific mortality
Five articles studied cancer-specific mortality (CSM) [Citation10,Citation12–14,Citation17]. The mean follow-up of these articles ranged from 26 to 46 months. CSM ranged from 0% [Citation12,Citation17] to 4.7% [Citation10]. No differences were found between RFA and RN [Citation10] or between MWA and RN or PN [Citation12].
Tolerance and complications
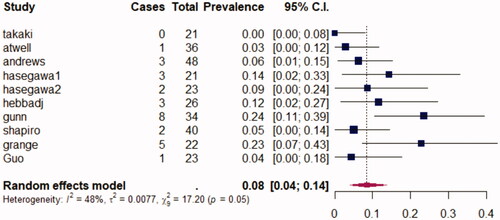
All reviewed articles, except Andrews et al. [Citation16], described adverse effects following ablation. Most articles used the Clavien–Dindo ranking system [Citation23], although Gunn et al. [Citation17] used CIRSE classification [Citation22], Guo et al. [Citation15] and Takaki et al. [Citation10] divided complications into minor and major adverse effects. It is possible, to sum up, those adverse effects by pooling them into minor and major adverse effects. Minor adverse effects correspond to complications Grade <3 in the Clavien–Dindo or CIRSE classifications. Major adverse effects mean complications Grade ≥3 in the Clavien–Dindo or CIRSE classifications. Overall, across 240 procedures, 53 (22%) complications occurred, consisting of 30 minor ones and 23 (10%) high grades ones. In all studies, no ablation-related deaths occurred. Pooling across all studies with a random-effects model, the prevalence of major adverse effects was 0.09 (0.06–0.14; p = 0.05). I2 was at 0%. represents the random-effects model for major adverse effects following percutaneous procedures. Two studies [Citation10,Citation12] compared surgery to ablative therapies, and they found no differences in complication rates, and Hasegawa et al. [Citation8] found no statistical difference between RFA and CA.
Figure 3. Forrest plot of random-effects models for prevalence of local recurrence following percutaneous ablation.
In their meta-analysis on T1b, Jiang et al. found no statistically different complication rates between RN and PN (14% for RN and 25% for PN) [Citation32].
Concerning eGFR decrease, results varied from a mean decrease of 27% after RFA (Hasegawa [Citation8]) (11% in CA) to no modification in Gunn et al. [Citation17]. In Takaki et al. [Citation10] and Shapiro et al. [Citation12] studies, eGFR decrease was significantly lower in the ablation group compared to RN. Complications and eGFR decrease following ablations are presented in .
Table 3. Complications and eGFR decrease following thermal ablations.
Follow-up
Median follow-up was variable across studies. The longest mean follow-up was found in Andrews et al. [Citation16] at 6 years (ranging from 4 to 7.2 years) and the shortest was in Grange et al.’s study at 14 months [Citation13].
Discussion
All articles included in this analysis focused on thermal ablation of T1b CCR. Overall ablation therapies appeared to be safe, effective treatments for T1b. Katsanos et al. [Citation33] and Rivero et al. [Citation34] have published meta-analyses on T1 RCC comparing ablation to surgery concluding that ablative therapies are associated with a lower complication rate and cause less of a decrease in eGFR compared with PN. These meta-analyses concerned all cT1 renal masses [Citation35] without segregating T1a from T1b. More recently, Yu et al. [Citation36] found that there were no significant differences regarding oncologic outcomes and complications between percutaneous microwave ablation and laparoscopic partial nephrectomy for patients with cT1a renal cell carcinoma. Percutaneous MWA led to smaller renal function changes and lower blood loss. To date, a review on T1b RCC ablation was published [Citation37], however, no meta-analysis has been published. Although TE isn’t always reached after the first treatment (primary success: 86%), a successful second ablation for a further 8% of patients is possible. All articles found a cumulative technical success greater than 91%.
In this review, we included all percutaneous ablations techniques. Only Hasegawa et al. [Citation8] compared ablation techniques (CA vs. RFA) and no efficacy differences were found. CA appeared to be the most used ablation tool with 6/9 studies and 204/311 (66%) patients. Only Shapiro et al. [Citation12] and Guo et al. [Citation15] reported results with MWA.
Among the articles recording pre-ablation embolization [Citation8,Citation10,Citation11,Citation13,Citation14], none found it to be predictive of success. Overall, 41/200 patients (21%) received pre ablation embolization. In the CIRSE Guidelines on Percutaneous Ablation of small RCC, pre-ablation embolization was mentioned in order to reduce heat sink effects, risk of bleeding and assist in sparing healthy parenchyma but no recommendation as a systematic procedure was noted [Citation38].
All studies found TA to be a safe technique. High-grade complications were uncommon (9% overall). Concerning complications, no statistical differences were found with surgery; that is, between RFA and RN [Citation10], between MWA and PN or RN [Citation12], or between RF and CA [Citation8]. The prevalence of major adverse effects was 0.09 (0.06–0.14; CI 95%) with very low heterogeneity between studies (I2 0%). Katsanos [Citation33] meta-analysis about TA of smaller renal tumors (mean size 2.5cm) found a 7.4% prevalence of adverse effects and 2.3% prevalence of major adverse effects. In a recent cohort study on cT1b tumors treated by surgery, Jiang et al. found a complication rate of 26% for PN and 14% for RN [Citation32]. No statistical differences were found between RN and PN. eGFR decrease was significantly lower with ablations compared to RN [Citation10,Citation12]. No article evaluated the cost of the procedure, however, a study found that for small renal masses, ablation therapies were less expensive and required shorter hospital stays than their surgical equivalents [Citation39].
Shapiro et al. [Citation12] compared length of hospitalization between surgery and ablations, they found a shorter median length of hospitalization for the MW group than for the PN or RN groups, 1 day (IQR 1–1) versus 4 days (IQR 3–6) or 4 days (IQR 3–4), respectively (p < 0.0001).
LR following ablation was variable between studies. Summing up all results, 25 out of 293 (9%) patients presented with local recurrence. LR rate following percutaneous ablation was 0.08 (0.04–0.14; p = 0.05) with intermediate heterogeneity (I2 48%). It is, however, difficult to pool these results due to the variable lengths of mean follow-up.
No differences in rates of local recurrence between surgery and ablation were noted in any of those studies but follow-up was longer for surgery patients due to higher comorbidities among ablation patients. Shapiro et al. [Citation12] found 5-year LRFS significantly lower for ablation compared to PN but not compared to RN. No differences were found between TA techniques.
Among patients followed for progression to metastatic disease, ranging from 2.6 months to 86.8 months follow-up, 9/153 (6%) patients presented metastatic locations following TA.
However, it should be noted that patients referred to percutaneous techniques tended to be more prone to a genetic predisposition to cancer, such as Von Hippel Lindau syndrome, or were poor surgical candidates due to past RN and/or PN with poor renal function [Citation40]. No differences were found with surgical techniques [Citation10,Citation12], concerning progression to metastatic disease or cancer-specific mortality.
There are several limitations to our study. Firstly, the retrospective nature of all studies and the absence of randomization should be considered. Secondly, the studies utilized various TA tools. Moreover, not all patients had biopsy-proven RCC (232/267: 87%) and follow-up imaging protocols were different. No subgroup analysis was performed due to the small size of each population. The heterogeneity between studies concerning LR was due to different ablation tools used, different teams of radiologists, and variable follow-up durations. Further, non-biopsy proven lesions may bias results. Finally, limited and variable follow-up between the studies made it more difficult to pool the results.
Overall, this analysis shows that mean follow-up after percutaneous ablation was shorter compared to surgery cohorts [Citation32]. It is to be noted that in Matin et al.’s study on local tumor progression following RFA or CA for kidney tumors, it was found that in most cases (69.8%) disease was detected within the first 3 months of surveillance imaging and the majority (92.1%) within the first 12 months, with a few cases detected after 1 year [Citation41]. Hence a larger cohort of patients with RCCs proved on biopsy and longer and systematic imaging follow-up is necessary.
Conclusions
Thermal ablation appears to be a feasible, safe and effective way to treat T1b RCC across these studies with very low local recurrence rates. Oncologic outcomes appear to be excellent on both local control and distant progression. More trials are necessary to determine the rate of the evidence of the benefit.
Acknowledgements
The authors thank Pippa McKelvie-Sebileau for medical editorial services and B. Naffrechoux for the help with the statistics.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84.
- Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63(3):486–492.
- Sanchez A, Feldman AS, Hakimi AA. Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermal ablation. J Clin Oncol. 2018;36(36):3591–3600.
- Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(6):668–680.
- Filippiadis D, Mauri G, Marra P, et al. Percutaneous ablation techniques for renal cell carcinoma: current status and future trends. Int J Hyperthermia. 2019;36(2):21–30.
- Xing M, Kokabi N, Zhang D, et al. Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a surveillance, epidemiology, and end results (SEER)–medicare-linked population study. Radiology. 2018;288(1):81–90.
- Morris CS, Baerlocher MO, Dariushnia SR, et al. Society of interventional radiology position statement on the role of percutaneous ablation in renal cell carcinoma: endorsed by the Canadian Association for Interventional Radiology and the Society of Interventional Oncology. J Vasc Interv Radiol. 2020;31(2):189–194.e3.
- Hasegawa T, Yamanaka T, Gobara H, et al. Radiofrequency ablation versus cryoablation for T1b renal cell carcinoma: a multi-center study. Jpn J Radiol. 2018;36(9):551–558.
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
- Takaki H, Soga N, Kanda H, et al. Radiofrequency ablation versus radical nephrectomy: clinical outcomes for stage T1b renal cell carcinoma. Radiology. 2014;270(1):292–299.
- Atwell TD, Vlaminck JJ, Boorjian SA, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol. 2015;26(6):792–799.
- Shapiro DD, Wells SA, Best SL, et al. Comparing outcomes for patients with clinical T1b renal cell carcinoma treated with either percutaneous microwave ablation or surgery. Urology. 2020;135:88–94.
- Grange R, Tradi F, Izaaryene J, et al. Computed tomography-guided percutaneous cryoablation of T1b renal tumors: safety, functional and oncological outcomes. Int J Hyperth. 2019;36(1):1065–1071.
- Hebbadj S, Cazzato RL, Garnon J, et al. Safety considerations and local tumor control following percutaneous image-guided cryoablation of T1b renal tumors. Cardiovasc Intervent Radiol. 2018;41(3):449–458.
- Guo J, Arellano RS. Percutaneous microwave ablation of stage T1b renal cell carcinoma: short-term assessment of technical feasibility, short-term oncologic outcomes, and safety. J Endourol. 2020;34(10):1021–1027.
- Andrews JR, Atwell T, Schmit G, et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2019;76(2):244–251.
- Gunn AJ, Joe WB, Salei A, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: safety, technical results, and clinical outcomes. Cardiovasc Intervent Radiol. 2019;42(7):970–978.
- Gunn AJ, Parikh NS, Bhatia S. Society of interventional radiology quality improvement standards on percutaneous ablation in renal cell carcinoma. J Vasc Interv Radiol. 2020;31(2):195–201.e3.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLOS Med. 2013;10(4):e1001419.
- Lum MA, Shah SB, Durack JC, et al. Imaging of small renal masses before and after thermal ablation. Radiographics. 2019;39(7):2134–2145.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–1146.
- Filippiadis DK, Binkert C, Pellerin O, et al.https://doi.org/10.13140/RG.2.2.27199.00161
- Hinshaw JL, Lubner MG, Ziemlewicz Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Naike W. How to conduct a meta-analysis of proportions in R: a comprehensive tutorial. 2018 [cited 2020 Sep 15]. Available from: http://rgdoi.net/https://doi.org/10.13140/RG.2.2.27199.00161
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation – what should you use and why? Radiographics. 2014;34(5):1344–1362.
- Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S179–S86.
- Schramm W, Yang D, Haemmerich D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. In: 2006 International Conference of the IEEE Engineering in Medicine and Biology Society [Internet]. New York (NY): IEEE; 2006 [cited 2020 Sep 15]. p. 5013–6.
- Baust JG, Gage AA, Bjerklund Johansen TE, et al. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology. 2014;68(1):1–11.
- Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60(2 Suppl 1):40–49.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–S203.
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38(1):65–78.
- Jiang Y-L, Peng C-X, Wang H-Z, et al. Comparison of the long-term follow-up and perioperative outcomes of partial nephrectomy and radical nephrectomy for 4 cm to 7 cm renal cell carcinoma: a systematic review and meta-analysis. BMC Urol. 2019;19(1):48.
- Katsanos K, Mailli L, Krokidis M, et al. Systematic review and meta-analysis of thermal ablation versus surgical nephrectomy for small renal tumours. Cardiovasc Intervent Radiol. 2014;37(2):427–437.
- Rivero JR, De La Cerda J, Wang H, et al. Partial nephrectomy versus thermal ablation for clinical stage T1 renal masses: systematic review and meta-analysis of more than 3,900 patients. J Vasc Interv Radiol JVIR. 2018;29(1):18–29.
- Maxwell AWP, Baird GL, Iannuccilli JD, et al. Renal cell carcinoma: comparison of RENAL nephrometry and PADUA scores with maximum tumor diameter for prediction of local recurrence after thermal ablation. Radiology. 2017;283(2):590–597.
- Yu J, Zhang X, Liu H, et al. Percutaneous microwave ablation versus laparoscopic partial nephrectomy for cT1a renal cell carcinoma: a propensity-matched cohort study of 1955 patients. Radiology. 2020;294(3):698–706.
- Welch B, Shah P, Thompson R, et al. The current status of thermal ablation in the management of T1b renal masses. Int J Hyperthermia. 2019;36(2):31–37.
- Krokidis ME, Orsi F, Katsanos K, et al. CIRSE guidelines on percutaneous ablation of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2017;40(2):177–191.
- Piechaud-Kressmann J, Bellec L, Delchier-Bellec M-C, et al. [Treatment of small renal masses: effectiveness and cost-comparison analysis]. Prog Urol. 2016;26(2):89–95.
- Joly D, Méjean A, Corréas J-M, et al. Progress in nephron sparing therapy for renal cell carcinoma and von Hippel–Lindau disease. J Urol. 2011;185(6):2056–2060.
- Matin SF, Ahrar K, Cadeddu JA, et al. Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol. 2006;176(5):1973–1977.