Abstract
Background
Several studies have reported the combination of intracavity or cervical lymph node hyperthermia with chemoradiotherapy (CRT) to improve clinical outcomes in nasopharyngeal carcinoma (NPC), but the combination with whole-body hyperthermia (WBH) for treating NPC is unexplored. We aimed to assess the efficacy of the combination of radiotherapy, chemotherapy and WBH in patients with locoregionally advanced NPC.
Methods
Between July 2008 and November 2012, 239 newly diagnosed NPC patients were enrolled in a pre-propensity score-matched cohort, including 193 patients who received CRT (CRT group) and 46 who underwent CRT with WBH (HCRT group). The feasibility and clinical outcomes of both groups were evaluated and toxicities assessed. Survival rates were assessed using the Kaplan–Meier method, log-rank test and Cox regression.
Results
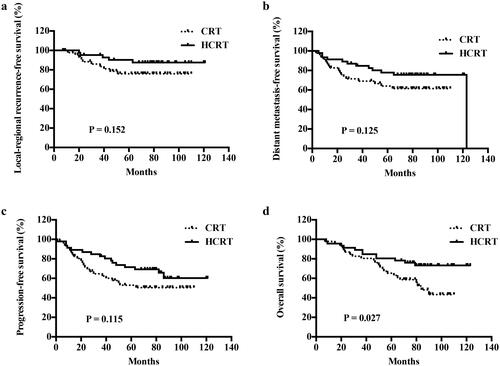
Following propensity score matching, 46 patients from each group were included. The 5-year overall survival (OS) rates were 65.2% in the CRT group and 80.3% in the HCRT group (p=.027). In contrast, the other survival outcomes at 5 years were similar between the groups: locoregional recurrence-free survival (LRRFS), 74.7% vs. 87.6% (p=.152); distant metastasis-free survival (DMFS), 67.4% vs. 77.9% (p=.125); and progression-free survival (PFS), 53.1% vs. 69.2% (p=.115). In the multivariate analyses, the only two independent predictors of OS were clinical stage and HCRT.
Conclusions
These results suggest that WBH, when combined with CRT, can improve the OS of patients with advanced NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare cancer with a striking geographical distribution, predominantly affecting populations in southern China, southeast Asia and northern Africa [Citation1,Citation2]. In China alone, an estimated 42,100 new cases of NPC were reported in 2013, resulting in approximately 21,320 deaths. The crude incidence of NPC was found to be 3.09/100,000 and mortality was 1.57/100,000.
Owing to the anatomic constraints of the disease and its strong radiosensitivity, the primary treatment for NPC is curative radiotherapy. Several trials have shown that intensity-modulated radiotherapy can improve the degree of locoregional control and overall survival (OS) in patients with NPC, especially those with early-stage disease [Citation3–5]. Nevertheless, up to 60% of patients with advanced NPC experience distant metastases [Citation3,Citation4]. For these patients, chemoradiotherapy (CRT) has been widely accepted as the standard treatment modality; yet, the distant relapse rate remains as high as 20–30% [Citation5,Citation6]. For decades, locoregional, intracavity hyperthermia has been used as an adjunct to radiotherapy/chemotherapy for its effect in reducing the degree of hypoxia and increasing local blood flow [Citation7]. There are multiple positive phase III trials showing that locoregional therapies can enhance radiotherapy response for several tumor types. Meta-analyses have shown benefit of hyperthermia with radiotherapy vs. radiotherapy alone for carcinoma of the cervix, head and neck cancer… [Citation7–9]. This beneficial effect of locoregional hyperthermia has raised interest pertaining to whether its application to the whole body could yield benefits in high-risk patients with metastatic NPC. In the case of WBH, the circumstances are more complicated. Typically, peripheral perfusion to the skin is increased during WBH in an attempt to dissipate the heat, but this is not the same for the central parts of the body. Although generally, the head of the patient is outside the heat applicator, the head and neck region is highly perfused due to thermoregulation. Therefore, the mechanism of increasing blood perfusion in WBH may be related to thermoregulation, and may be different from that of local and regional hyperthermia [Citation10–12]. There is, however, no evidence that whole-body hyperthermia (WBH) can improve progression free or OS, in any randomized trial with radiotherapy or chemotherapy.
The purpose of our study was to assess the efficacy of the combination of all three treatments (radiotherapy, chemotherapy and WBH) in patients with locoregionally advanced NPC. We compared patients who were treated with CRT (CRT group) to those who received all three therapies (HCRT group). Since our study was not randomized, we used propensity score matching to reduce the degree of bias due to baseline differences between the two groups.
Methods
Ethics statement
At the time of admission, all patients were sufficiently informed of the risks and potential benefits of CRT and HCRT. All patients signed an informed consent. This retrospective study followed the ethical guidelines of the Declaration of Helsinki (as revised in Brazil in 2013) and was approved by the Ethics Committee of the Affiliated Cancer Hospital of Guangzhou Medical University (NO. P2020-006).
Patients
We retrospectively analyzed the medical data obtained from 239 patients treated at our hospital between July 2008 and November 2012, who satisfied the following inclusion criteria: (1) at least 18 years of age; (2) presence of primary NPC, confirmed by histopathology to be of the non-keratinizing carcinoma subtype, and assigned stage III–IVB based on the American Joint Committee on Cancer (7th edition) staging system; (3) evaluated cardiopulmonary function (ECG, echocardiography, chest X-ray and lung function test) before induction chemotherapy, with a resting left ventricular ejection fraction > 40%; FEV-1 > 70% of the predicted value, and diffusion capacity >80% (subjects with a history of myocardial infarction were excluded); (4) history of receiving two or three cycles of induced chemotherapy and concurrent CRT, with radiotherapy performed according to the intensity-modulated technique, and (5) presence of complete follow-up data.
Patients were classified into the CRT group if they received only CRT, or into the HCRT group if they received CRT combined with WBH ().
Chemotherapy
All patients in this study received induced chemotherapy and concurrent chemotherapy. Induced chemotherapy regimens included PF (80 mg/m2 cisplatin on day 1 and 800 mg/m2/d fluorouracil civ on days 1–5) or TP (75 mg/m2 docetaxel on day 1 and 75 mg/m2 cisplatin on day 1). Both regimens were repeated every 3 weeks for two cycles.
All patients also received concurrent chemotherapy with a dose of 80–100 mg/m2 cisplatin on day 1, every 3 weeks, for 2–3 cycles.
Radiotherapy
All patients received intensity-modulated radiotherapy. Treatment plans were based on contrast-enhanced computed tomography (CT). Target volumes were delineated slice by slice on treatment-planning CT scans, in accordance with Reports 50 [Citation13] and 62 [Citation14] of the International Commission on Radiation Units and Measurements. The primary gross volume (GTVnx) and the involved lymph nodes (GTVnd) included the entire tumor, as defined according to the CT and magnetic resonance imaging (MRI) findings. The following prescribed doses (Gy) were delivered to the planning target volumes: primary gross tumor volume (PTVnx), 68–72; PTV of the GTV of the involved lymph nodes (PTVnd), 66–70; PTV of the high-risk clinical target volume (PTV1), 60–64; and PTV of the low-risk clinical target volume (PTV2), 52–56. Doses were delivered in 31–33 fractions.
Hyperthermia
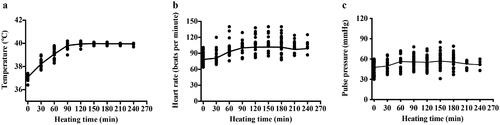
Patients in the HCRT group underwent WBH during induction chemotherapy. During treatment, the body of the patients was inside the heated chamber (electromagnetic, radiofrequency output power 0–800 W, far infrared, Radiant, Xianke SRI, Beijing, China). The hyperthermia machine is heated by dual heat sources (). The main heat source is radiofrequency heating at 13.56 MHz with power of 0–800 W; the other heat source is infrared heating. The power of infrared heating has four levels, 0, 150, 370 and 490 W. The heating power level was manually controlled by an operator depending upon the patients’ condition, such as the patient’s temperature, heart rate and blood pressure. depicts the schematic and circuit diagram of the machine. The patient remained awake throughout the heating process, maintaining a conversation with the operator, and could drink water and move slightly in the treatment cabin. The head was placed outside the chamber. During the treatment, the bed board was covered with a 1 cm thick water pad. Rectal, skin and ambient air temperatures were monitored continuously and recorded at a minimum of 10-min intervals. During each 120 min treatment cycle, the rectal temperature was maintained around 39.5–40.5 °C and skin temperature were kept below 43 °C. Patients’ heart rate, respiratory rate, oxygen saturation and cardiac rhythm were also continuously monitored. Blood pressure was monitored at least once every 10 min. Temperature probes were calibrated at least weekly against defined external standards (0.1 °C). Corrections were made from temperature markings of 37.0–42.0 °C. A typical WBH treatment session lasted for about 5 h, including a median of 100 min (range, 90–120 min) period for target temperature achievement, 2-h duration for body temperature maintenance at 40 °C, and a 1-h cooling phase. shows temperature data from a WBH treatment of one patient. Sedatives and anesthetics were not used. All patients were kept awake during treatment. We prescribed intravenous fluid for all patients (700–1500 mL). Patients were also allowed to drink water freely during the procedure if they felt thirsty. The changes in heart rate and blood pressure were not in a range considered to be a risk for cardiac compromise ().
Figure 2. Picture of the heat device. (a) Schematic diagram of hyperthermia machine; (b) Patient undergo whole body hyperthermia; (c) The whole body hyperthermia machine we used. The infrared heating equipment is in the arc-shaped part, and the radiofrequency heating plate is in the couch.
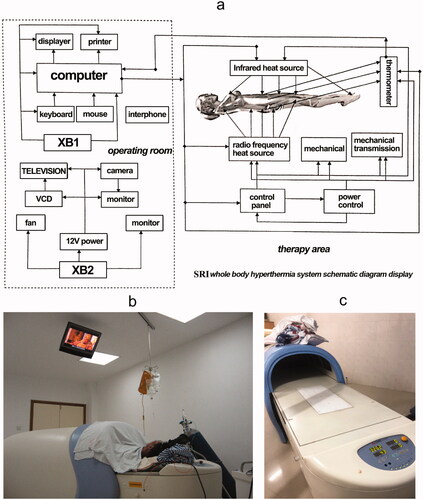
Figure 3. The whole body hyperthermia treatment curve of one patient. The upper figure is the temperature curve. The below figure is the power curve of the same patient.
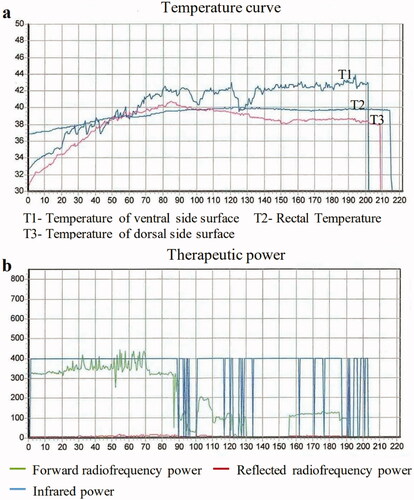
Figure 4. Changes of vital signs during WBH of 46 patients. Each dot represents an average for each patient. (a) Changes of rectal temperature. (b) Changes of Heart rate with time; (c) Changes of pulse pressure.
Intravenous cisplatin was administered when the rectal temperature reached 39.5 °C; fluorouracil and taxol were administered after completion of WBH. In the HCRT group, all patients received two cycles of WBH every 3 weeks.
Follow-up and survival definitions
Patients were followed-up from the first day of therapy to the day of the last examination, or death. All patients were reviewed every 3 months during the first 3 years, every 6 months, the following 2 years, and annually after 3 years. Each follow-up visit included a clinical physical examination, nasopharyngoscopy, ultrasonography of the abdomen and chest radiography, as well as CT or MRI of the head and neck region. At the discretion of the attending physician, additional exams or imaging were performed on patients showing evidence of recurrence.
Survival was assessed from the follow-up data using the following definitions: OS, survival during the follow-up period; locoregional recurrence-free survival (LRRFS), survival without recurrence in the nasopharynx or cervical lymph nodes; distant metastasis-free survival (DMFS), survival without distant metastasis; and progression-free survival (PFS), survival without locoregional failure or distant metastasis.
Statistical analysis and propensity score matching
Statistical analysis was performed using SPSS version 25.0 (IBM, Armonk, NY). Intergroup differences in age were assessed for significance using an unpaired t test, while differences between the categorical variables (sex, T stage, N stage and clinical stage) were assessed using the chi-square test. To reduce the degree of bias or confounding in our results owing to the baseline differences between the HCRT and CRT groups, we performed further analyses on pairs of patients from both groups, generated by propensity score matching in SPSS. Patients from both groups were paired in a 1:1 ratio, and the score scale included five covariates: age, sex, T stage, N stage and clinical stage. In the propensity score-matched patients, the OS, PFS, LRRFS and DMFS rates were determined using the Kaplan–Meier method, and between-group comparisons were performed using log-rank tests. Univariate and multivariate survival analyses were performed using the Cox proportional hazards regression model. Statistical significance was accepted at p<.05 ().
Results
A total of 239 patients were identified (), of whom 193 received CRT and 46 received HCRT. All patients completed radiotherapy treatment and two cycles of induced chemotherapy. Before propensity score matching, the CRT and HCRT groups differed significantly only in sex (p = .04). After 92 patients underwent propensity score matching, the resulting groups showed no significant baseline differences.
Table 1. Baseline characteristics of patients before and after propensity score matching.
Survival outcomes
The median follow-up duration of the total population was 75 months (range, 65–121 months). When only the propensity score-matched patients were considered, the 5-year OS rates were 65.2% in the CRT and 80.3% in the HCRT groups (p = .027). In contrast, the other survival outcomes at 5 years were similar between the CRT and HCRT groups: LRRFS, 74.7% vs. 87.6% (p = .152); DMFS, 67.4% vs. 77.9% (p = .125); and PFS, 53.1% vs. 69.2% (p = .115). Similar results were observed for the 8-year outcomes: OS, 45.3% vs. 76.0% (p = .027); LRRFS, 74.7% vs. 87.6% (p = .152); DMFS, 64.6% vs. 75.6% (p = .125); and PFS, 50.2% vs. 60.3% (p = .115); ().
In the multivariate analyses, clinical stage and HCRT were found to be the only two independent predictors of OS ().
Table 2. Multivariable analysis to identify independent predictors of overall survival based on propensity score-matched patients.
Treatment toxicity
Adverse events were evaluated in the propensity score-matched patients according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0. In the HCRT group, five patients presented with grade I/II burns on the torso following hyperthermia; these burns covered an area smaller than 3 cm2 and recovered spontaneously after 3–5 d. Both groups showed no significant differences in the occurrence of grade 3 or 4 toxicities, namely neutropenia, leukopenia, anemia, thrombocytopenia, vomiting, nausea, dermatitis or nausea ().
Table 3. Cumulative grade 3–4 adverse events [n (%)] during treatment, based on propensity score-matched patients.
Discussion
Advances in intensity-modulated radiotherapy have led to improved local control rates in NPC; however, the 5-year OS is still 65–75%, and approximately 60% of all failures are attributed to distant metastasis [Citation3]. For improving control, primary CRT has become the standard of treatment, but its ability to increase the duration of OS remains uncertain [Citation4,Citation15,Citation16]. We examined whether the combination of standard CRT and WBH could improve survival outcomes without increasing the risk of adverse events. In this small study including 92 propensity score-matched patients, we demonstrated significantly better 5-year OS rates in the HCRT group than the CRT group. HCRT was also associated with a tendency toward higher 5-year PFS, DMFS and LRFS values. At the same time, hyperthermia was not associated with any additional risk of grade 3/4 adverse events. To the best of our knowledge, this is the first study to focus on WBH in NPC patients.
Heating can render head and neck squamous carcinoma cells hypersensitive to chemotherapy [Citation17] and several studies in culture [Citation18–23] and mice [Citation24] have shown that heating increases the level of cytotoxicity of cisplatin to 1.59–4.96 [Citation25]. A clinical study showed good response among patients with high-grade neuroendocrine and pancreatic cancers receiving WBH in combination with cisplatin, gemcitabine and interferon-alpha [Citation26]. Another study confirmed that WBH in combination with carboplatin was an active salvage treatment option for patients with advanced ovarian cancer, although significant hematological toxicity was noted [Citation27]. Recently, Lassche et al. reviewed 350 evaluable metastasized solid malignancies in 14 phase II studies and found that a combination of WBH, with chemotherapy achieved promising response rates [Citation28].
It appears that one way in which hyperthermia can improve chemotherapy efficacy is by increasing the rate of blood flow and oxygenation in tumors [Citation10]. For example, Sen et al. found that heating results in a sustained reduction in the tumor interstitial fluid pressure, in correlation with increased vascular perfusion rates and reduced rates of tumor hypoxia [Citation11]. Further, Ogoh et al. proved that both cardiac output and external carotid artery blood flow increased after WBH in healthy patients, indicating that WBH re-distributed the blood flow at the extracranial vascular bed because of its effect of increasing the cardiac output [Citation29]. Another mechanism is the enhancement of tumor immunity. Heating triggers the production of heat shock proteins, enhancing the immune recognition of tumor cells [Citation30]. At the same time, it aids in the adherence of cytotoxic T-lymphocytes and natural killer cells to the tumor micro vessels, and their extravasation of the tumor parenchyma [Citation10,Citation31,Citation32]. Systemic hyperthermia may also affect cytokine signaling [Citation33,Citation34]. Clinical studies support the idea that hyperthermia can enhance the degree of immune response [Citation31,Citation34]. The existing literature provides clear evidence that while heating tumors to 39.5–40.5 °C does not efficiently kill them, these temperatures can substantially potentiate the cytotoxicity of chemotherapeutics.
Several studies have reported that a combination of intracavity or cervical lymph node hyperthermia and CRT can improve clinical outcomes in NPC, suggesting that hyperthermia is effective in such settings [Citation35,Citation36]. WBH has been used in many types of cancers. It can heat both the nasopharynx and lymph node at the same time, and we speculate that WBH can prove beneficial in the treatment of NPC as well. Our results indicate that the addition of WBH to NPC treatment can lead to significant improvements in the 5-year OS.
Future studies on optimizing the hyperthermia schedule are warranted. There is currently no standard procedure for WBH. Generally, the higher the temperature, the longer the duration, and the better the therapeutic effect. In our previous study, we observed better survival outcomes when WBH was provided at a temperature of 41.8 °C for 2 h. However, the associated anesthesia risk and costs were high, hampering its widespread use. [Citation37] Other studies used a temperature of 41.8 °C for 2 h [Citation9,Citation38], or 40 ∼ 41.8 °C for 1 h [Citation39], which may not be ideal because of the requirement for general anesthesia that increases the cost and risk associated with the procedure in Lassche’ review; they found that often in heavily pretreated patients, objective responses were observed in several types of advanced malignancies, albeit at the cost of a high proportion of patients experiencing grade 3 and 4 toxicities attributable to chemotherapy, WBH or the combination. However, in our study, there was no significant increase of grade 3 or 4 toxicity in the hyperthermia group, which indicated that it was well tolerated in this patient cohort with locally advanced NPC. Also, the 40 °C target temperature of WBH, and the awake state during treatment may have contributed to the lesser treatment toxicity. Similarly, Bull et al. [Citation26] concluded that the thermochemotherapeutic treatment with 40 °C core temperature was well tolerated and effective. Long-term survival can be achieved in most patients with locoregionally advanced NPC, and when another treatment is added, its safety and feasibility must be ensured. In our study, because the heating technology contains electromagnetic waves, which will interfere with electrocardiogram monitoring, only pulse rate and blood pressure were measured during the treatment. Patients were not administered anesthesia, which was done to maintain communication with them for safety purposes. The pulse rate of most patients increased consistently with temperature, but it was usually below 120 beats/min, and the blood pressure was maintained in the normal range. Some patients felt tired because of lying on their back and enduring heating for a long time. Although we had a few cases, we maintained effective communication and provided adequate education before treatment. These patients were not claustrophobic, but some patients had mild anxiety (they were asking or hoping to finish the treatment early). No obvious acute toxicities were found and no stress diseases (such as large blisters caused by thermal pressure on the back of the heel and head) were found during WBH at 41.8 °C.
Our study findings suggest that all patients can tolerate heating to 40 °C for 2 h without general anesthesia. Indeed, under these conditions, patients were able to complete 2–3 cycles of hyperthermia. Since patients are awake during treatment, they can alert treatment administrators to potentially dangerous discomfort, such as skin overheating.
During local hyperthermia, it is hard to measure the nasopharyngeal temperature. However, it is known that the temperature of the tumor region is associated with treatment efficacy. It is easier to obtain the temperature associated with local hyperthermia than WBH, as the core body temperature can represent the temperature of the nasopharyngeal regions. In our previous study, we placed temperature probes in the nasopharynx, esophagus, lumen of the pulmonary artery, and rectum of anesthetized patients and provided heating as in this study [Citation37,Citation40]. We found that the nasopharyngeal temperature reached the target temperature before the rectum did and tracked well with the core temperature during the treatment plateau. These results suggest that respiration can remove heat from the body, but that blood flow can maintain the nasopharyngeal mucosa at the same temperature as that of the body core.
We employed cisplatin-based chemotherapy because of its promising effects in NPC patients and its widespread use in combination with WBH. Several studies have revealed that promising therapeutic effects were noted with the combination of cisplatin-based chemotherapy and WHB without severe treatment toxicity [Citation26,Citation41,Citation42]. Furthermore, studies have proven that hyperthermia can increase the efficacy of chemotherapeutic drugs [Citation25,Citation43]. Pre-clinical studies with cisplatin, in particular, favor concomitant administration of heat with drug. Heat has been shown to increase the rate of cisplatin uptake by cells as a result of multimerization of the copper transporter. This mechanism is specific for cisplatin [Citation44]. At present, there is no conclusion pertaining to the proper intervention time for WBH. Most researchers initiate chemotherapy when the WBH temperature reaches the platform stage. Based on a similar protocol, our results showed that the combination of chemotherapy and 40 °C hyperthermia for 2 h did not increase the rate of therapeutic toxicity.
Our study has several limitations. First, although propensity score matching was adopted, patients who accepted WBH might have had higher motivation (compliance) or better health, leading to selection bias. Second, our restricted sample size may have impacted the statistical results. Third, there exists no optimal model for the thermal dose and appropriate timing for the combination of WBH and chemotherapy. Therefore, randomized trials with long-term follow-up are warranted to assess the exact benefit of the combination of WBH in the treatment of locally advanced NPC.
Conclusion
This small retrospective study suggests that WBH, when combined with CRT, can improve the OS of patients with advanced NPC. This appears to be the first study to evaluate the effects of WBH at a temperature of 40 °C for 2 h in such patients. Our findings provide justification for larger prospective studies without the need for propensity score matching.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374(1):22–30.
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):90.
- Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403.
- Lee AWM, Tung SY, Ng WT, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer. 2017;123(21):4147–4157.
- Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595.
- Yi J, Huang X, Gao L, et al. Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2014;9:56.
- Datta NR, Rogers S, Ordonez SG, et al. Hyperthermia and radiotherapy in the management of head and neck cancers: a systematic review and Meta-analysis. Int J Hyperthermia. 2016;32(1):31–40.
- Datta NR, Stutz E, Gomez S, et al. Efficacy and safety evaluation of the various therapeutic options in locally advanced cervix cancer: a systematic review and network Meta-Analysis of randomized clinical trials. Int J Radiat Oncol Biol Phys. 2019;103(2):411–437.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- Xu Y, Choi J, Hylander B, et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int J Hyperthermia. 2007;23(6):513–527.
- Sen A, Capitano ML, Spernyak JA, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011;71(11):3872–3880.
- Rich LJ, Winslow TB, Alberico RA, et al. Enhanced tumour perfusion following treatment with water-filtered IR-a radiation to the thorax in a patient with head and neck cancer. Int J Hyperthermia. 2016;32(5):539–542.
- ICRU. Prescribing‑recording‑and‑reporting‑photon‑beam‑therapy‑report‑50. Available from: http://www.icru.org/home/reports/ICRUreport50.pdf.
- ICRU. Prescribing‑recording‑and‑reporting‑photon‑beam‑therapy‑report‑62. Available from: http://www.icru.org/home/reports/ICRUreport62.pdf
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC Meta-analysis. Lancet Oncol. 2015;16(6):645–655.
- Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024.
- Ren G, Jv H, Tian Z, et al. Ultrasound hyperthermia induces apoptosis in head and neck squamous cell carcinoma: an in vitro study. Med Oral Patol Oral Cir Bucal. 2017;22(3):e289–0.
- Herman TT, Cathcart KN, et al. Effect of hyperthermia on cis-Diamminedichloroplatinum(II) (rhodamine 123) 2[tetrachloroplatinum(II)] in a human squamous cell carcinoma line and a cis-Diamminedichloroplatinum(II)-resistant subline. Cancer Res. 1988;48(18):5101–5105.
- Raoof M, Zhu C, Cisneros BT, et al. Hyperthermia inhibits recombination repair of gemcitabine-stalled replication forks. J Natl Cancer Inst. 2014;106(8):dju183.
- Alavizadeh SH, Gheybi F, Nikpoor AR, et al. Therapeutic efficacy of cisplatin thermosensitive liposomes upon mild hyperthermia in C26 tumor bearing BALB/c mice. Mol Pharm. 2017;14(3):712–721.
- Zhang JF, Yan XM, Lan B, et al. Molecular mechanisms of synergistic induction of apoptosis by the combination therapy with hyperthermia and cisplatin in prostate cancer cells. Biochem Biophys Res Commun. 2016;479(2):159–165.
- Schaaf L, Schwab M, Ulmer C, et al. Hyperthermia synergizes with chemotherapy by inhibiting PARP1-Dependent DNA replication arrest. Cancer Res. 2016;76(10):2868–2875.
- Ng GPRSDLDDSCE. A comparison of hyperthermia cisplatin sensitization in human ovarian carcinoma and glioma cell lines sensitive and resistant to cisplatin treatment. Cancer Chemother Pharmacol. 1996;37:574–580.
- Dunne M, Dou YN, Drake DM, et al. Hyperthermia-mediated drug delivery induces biological effects at the tumor and molecular levels that improve cisplatin efficacy in triple negative breast cancer. J Control Release. 2018;282:35–45.
- Urano M, Kuroda M, Nishimura Y. Invited review for the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 1999;15(2):79–107.
- Bull JMC, Scott GL, Strebel FR, et al. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: a description of a phase I-II protocol. Int J Hyperthermia. 2008;24(8):649–662.
- Atmaca A, Al-Batran SE, Neumann A, et al. Whole-body hyperthermia (WBH) in combination with carboplatin in patients with recurrent ovarian cancer – a phase II study. Gynecol Oncol. 2009;112(2):384–388.
- Lassche G, Crezee J, Van Herpen CML. Whole-body hyperthermia in combination with systemic therapy in advanced solid malignancies. Crit Rev Oncol Hematol. 2019;139:67–74.
- Ogoh S, Sato K, Okazaki K, et al. Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab. 2013;33(12):1915–1920.
- Dewhirst MW, Lee CT, Ashcraft KA. The future of biology in driving the field of hyperthermia. Int J Hyperthermia. 2016;32(1):4–13.
- Kobayashi Y, Ito Y, Ostapenko VV, et al. Fever-range whole-body heat treatment stimulates antigen-specific T-cell responses in humans. Immunol Lett. 2014;162(1 Pt A):256–261.
- Repasky EA, Evans SS, Dewhirst MW. Temperature matters! and why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1(4):210–216.
- Fisher DT, Chen Q, Skitzki JJ, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121(10):3846–3859.
- Sulyok I, Fleischmann E, Stift A, et al. Effect of preoperative fever-range whole-body hyperthermia on immunological markers in patients undergoing colorectal cancer surgery. Br J Anaesth. 2012;109(5):754–761.
- Hua Y, Ma S, Fu Z, et al. Intracavity hyperthermia in nasopharyngeal cancer: a phase III clinical study. Int J Hyperthermia. 2011;27(2):180–186.
- Huilgol NG, Gupta S, Dixit R. Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperthermia. 2010;26(1):21–25.
- Shao X. Comparison the temperature variety of different point inside body during whole body hyperthermia(WBH). St. Louis (MO); 2004; (9th international congress on hyperthermia oncology[C]; 89).
- Zhao C, Dai C, Chen X. Whole-body hyperthermia combined with hyperthermic intraperitoneal chemotherapy for the treatment of stage IV advanced gastric cancer. Int J Hyperthermia. 2012;28(8):735–741.
- Atanackovic D, Pollok K, Faltz C, et al. Patients with solid tumors treated with high-temperature whole body hyperthermia show a redistribution of naive/memory T-cell subtypes. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R585–94.
- Zhi -Rong ZHU, Yw C. Hemodynamic changes and oxygen metabolism in patients with malignant tumor during whole-body hyperthermia therapy. Chin J Anesthesiol. 1994;5(1):311–328.
- Engelhardt R, Muller U, Weth-Simon R, et al. Treatment of disseminated malignant melanoma with cisplatin in combination with whole-body hyperthermia and doxorubicin. Int J Hyperthermia. 1990;6(3):511–515.
- Douwes F, Bogovi CJ, Douwes O, et al. Whole-body hyperthermia in combination with platinum-containing drugs in patients with recurrent ovarian cancer. Int J Clin Oncol. 2004;9(2):85–91.
- Mohamed F, Marchettini P, Stuart OA, et al. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10(4):463–468.
- Landon CD, Benjamin SE, Ashcraft KA, et al. A role for the copper transporter Ctr1 in the synergistic interaction between hyperthermia and cisplatin treatment. Int J Hyperthermia. 2013;29(6):528–538.