Abstract
Objective
To assess the long-term outcomes and the factors affecting local recurrence of uterine fibroids after ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation.
Materials and methods
629 patients with a solitary uterine fibroid smaller than 10 cm in diameter treated with USgHIFU at our institutes between January 2011 and December 2016 were retrospectively analyzed. The patients were requested to take pre-HIFU and one day post-HIFU MRI. The patients were asked to return to the hospital every 3 months until January 2020, for imaging evaluation and to check on improvement in symptoms.
Results
Five hundred and thirty-six patients completed follow-up according to our protocol. The median follow-up time was 69 (interquartile range: 48 to 89) months. Among them, local recurrence was detected in 110 patients. 18 (16.4%) patients required additional treatment between 12 and 24 months after USgHIFU treatment, 59 (53.6%) patients required additional treatment 24 months after USgHIFU. Therefore, in total, 77 patients required additional treatment, of which 32 received USgHIFU and 45 underwent myomectomy. The median non-perfused volume (NPV) ratio in patients with recurrence was 73%, compared to 89% among patients without recurrence. Multivariate analysis showed that NPV ratio, maximum fibroid diameter and fibroid enhancement type were the independent factors affecting the recurrence of fibroids after USgHIFU treatment.
Conclusions
Achievement of NPV ratio higher than 70% has led to acceptable re-intervention rate during the follow-up period after USgHIFU. NPV ratio, maximum fibroid diameter, and fibroid enhancement type were the independent factors affecting the recurrence of fibroids after USgHIFU treatment.
Introduction
Uterine fibroids are common among females of childbearing age with prevalence varying among different races. Generally, the prevalence is 20%–40% among reproductive age women, accounting for 52% of all gynecological benign tumors [Citation1]. In patients with uterine fibroids, 50% complained of a variety of symptoms including menorrhagia, lumbosacral pain and constipation [Citation2]. These symptoms seriously affect the quality of life of women. At present, treatments for symptomatic uterine fibroids include medication, myomectomy, hysterectomy, uterine artery embolization (UAE), and high intensity focused ultrasound (HIFU) [Citation3]. Each treatment has its indications, advantages and disadvantages.
Over the past decade, both ultrasound-guided HIFU (USgHIFU) and magnetic resonance-guided focused ultrasound (MRgFUS) have been used as a noninvasive treatment for patients with uterine fibroids. Previous studies have demonstrated that this noninvasive treatment for uterine fibroids is safe and effective [Citation3,Citation4]. Recently, Verpalen et al conducted a long term follow-up study of 123 women treated with MRgHIFU. The median non-perfused volume (NPV) ratio was 37.4% in the group treated using restrictive treatment protocol, while it was 57.4% in the group treated using unrestrictive treatment protocol (aiming for complete ablation). Their results showed the overall re-intervention rate of 33.3% at a mean of 63.5 ± 29.0 months follow-up. The re-intervention rate significantly decreased from 48.8% in the restrictive group to 18.2% (n = 44; follow-up 40.0 ± 22.1 months) in the unrestrictive group [Citation5]. In another study, Funaki et al. found that moderate volume reductions of hypointense and isointense uterine fibroids were observed following MRgFUS, with relatively low re-intervention rates [Citation6]. Li et al. reported their long-term follow-up results of 381 patients treated with USgHIFU. The median NPV ratio of the fibroids was 81.9% (IQR: 70.36%, 91.91%) in the 381 patients. They ascertained that after a mean of 70.0 ± 9.0 months follow-up, 86.4% (329/381) of the patients reported symptomatic relief and the overall re-intervention rate was 20.7% (79/381) [Citation7]. These studies concluded that improvements in NPV ratio may further improve long-term results of patients with uterine fibroids treated with HIFU. On the other hand, there is a possibility of re-intervention when regeneration of the treated fibroid occurs. The long-term outcomes can be improved by studying the factors that affect local recurrence of fibroids and developing appropriate therapeutic strategies. Therefore, the aims of this study were to assess the long-term outcomes and to investigate the factors that affect uterine fibroid local recurrence after USgHIFU.
Materials and methods
The research protocol for this retrospective study was assessed and approved by the Medical Research Ethics Committee at our institutes (IRB-120319). The requirement for informed consent was waived.
Patients
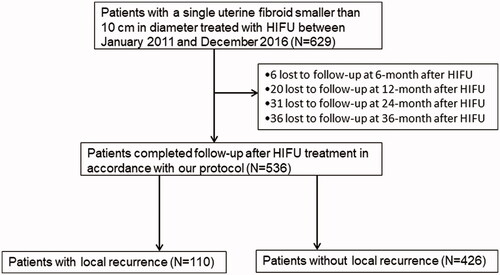
A retrospective study was performed on data collected from 629 patients with a single uterine fibroid smaller than 10 cm in diameter treated with USgHIFU at Yongchuan Maternal and Child Health Care Hospital of Chongqing and Chongqing Haifu Hospital between January 2011 and December 2016. Five hundred and thirty-six patients completed follow-up after USgHIFU treatment in accordance with our protocol, 93 patients lost to follow-up ().
MRI examination
All patients underwent pre- and one day post-HIFU follow-up with a 1.5 T MRI (uMR570, United Imaging Company, China or Siemens Medical Solutions, Erlangen, Germany). A series of standard T1 weighted images (T1WI) and T2 weighted images (T2WI) were performed on all patients. Dynamic enhancement was performed 20 s after an intravenous injection of gadolinium.
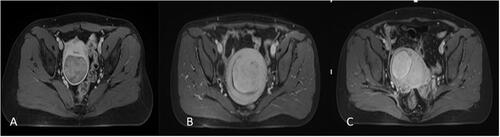
The thickness of the abdominal wall, maximum fibroid diameter, minimum distance from ventral surface of fibroid to skin, maximum distance from dorsal surface of fibroid to skin, and minimum distance from dorsal surface of fibroid to sacrum were measured on MRI (). The position of the uterus (ante-verted, mid-position, retro-verted), location of uterine fibroids (anterior wall, posterior wall, lateral wall, fundus), signal intensity of uterine fibroids on T2WI, and type of uterine fibroids (intramural, submucosal, subserosal) were also determined. Fibroids were classified according to their T2WI signal intensity as homogeneous hypointense, homogeneous isointense and hyperintense. The signal intensity of homogeneous hypointense fibroids was comparable to that of skeletal muscle; homogeneous isointense fibroids had a uniform intensity higher than that of skeletal muscle, but lower than that of the myometrium; hyperintense signaling comprises homogenic and heterogenic hyperintense signals: when compared to that of the myometrium, the signal intensity of uterine fibroids was equal to or higher than that of the myometrium ().
Figure 2. Measurement of distance from ventral side of fibroid to skin, distance from dorsal side of fibroid to skin, distance from dorsal surface of fibroid to sacrum, and the abdominal wall thickness.
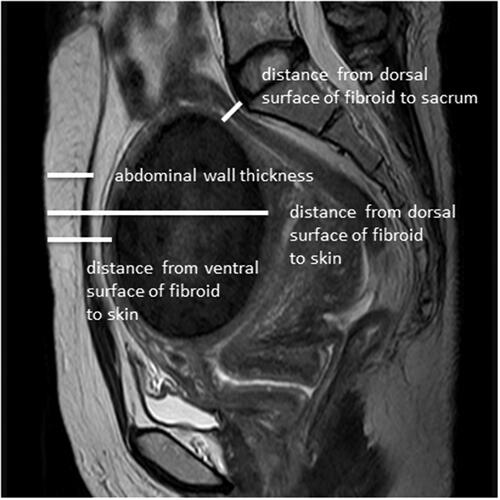
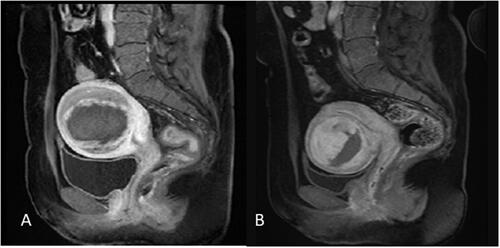
Figure 3. T2-weighted imaging signal intensity of uterine fibroids: (A) homogeneous hypointense. (B) homogeneous isointense. (c) homogeneous hyperintense. (D) heterogeneous hyperintense.
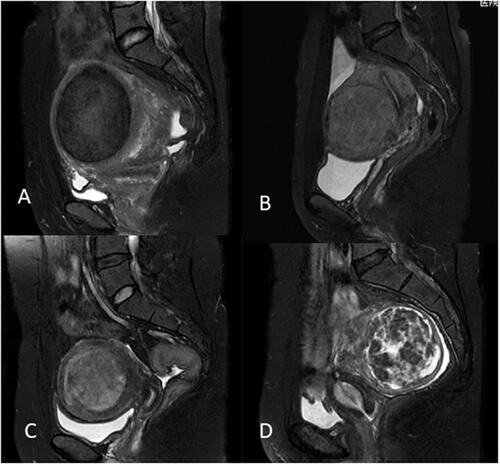
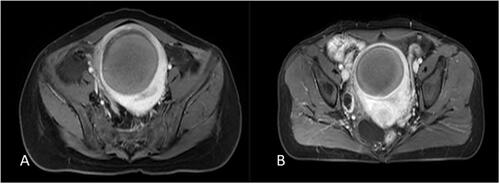
Pre-HIFU contrast enhanced MRI was used to determine the degree of enhancement of uterine fibroids (mild, moderate, significant). Mildly enhanced fibroids had a weaker enhancement compared to that of the myometrium. Moderately enhanced fibroids had an enhancement similar to that of the myometrium. Significantly enhanced fibroids had a stronger enhancement compared to the myometrium ().
Figure 4. The degree of contrast enhancement of uterine fibroids: (A) mild enhancement. (B) moderate enhancement (myometrial grade). (C) significant enhancement.
Post-HIFU and follow-up contrast enhanced MR images were used to measure the NPV and the residual volume of fibroids. NPV indicates the volume of coagulative necrosis ( and ). The volume of fibroids and NPV were obtained by measuring in three dimensions: longitudinal diameter (D1), transverse diameter (D2), and antero-posterior diameter (D3). The volume was calculated according to the following equation: V= 0.5233×D1×D2×D3. NPV ratio is defined as NPV/Vfibroid×100%. Residual volume was calculated using the equation Vresidual=Vfibroid – VNPV. The residual volume of the fibroid between the post-HIFU and each follow-up visit was compared ( and ). Local regeneration or tissue proliferation (regrowth of the residual part) in the treated fibroid detected on the follow-up MRI, and the residual volume increased by 10% was defined as fibroid local recurrence.
USgHIFU ablation
USgHIFU treatment was performed under conscious sedation. The device used was a Model JC200 or JC focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). Therapeutic ultrasound beams were generated by a mono-element transducer with a frequency of 0.8 MHz, a focal length of 15 cm, and a diameter of 20 cm. A Mylab 70 ultrasound imaging device (Esaote, Genova, Italy) was used to provide real-time imaging to localize the fibroid and monitor the treatment. The patients were placed in a prone position on the HIFU table, with the anterior abdominal wall immersed in degassed water. A degassed water balloon was placed between the abdominal wall and the transducer to compress and push the bowel away from the acoustic pathway. The sagittal ultrasound scanning mode was chosen for both pretreatment planning and sonication. The locations of the fibroids and surrounding tissues were identified at ultrasound imaging and the targeted fibroid was divided into a number of sections by using real-time ultrasound, with a distance of 5 mm between the sections. Point scan was used, and power set between 300–400 watts. The distance from focal point to endometrium was at least 1.5 cm, and the distance from focal point to subserosal surface of the uterus was 1 cm. During the procedure of USgHIFU, therapeutic power was adjusted based on patient feedback and changes in grayscale on ultrasonographic imaging. The treatment was terminated when the increased grayscale covered the fibroid or there was an absence of blood supply as assessed by contrast-enhanced ultrasound immediately after HIFU ablation. The patients' vital signs such as heart rate, blood pressure, respiration and oxygen saturation were monitored.
Follow-up
In accordance with the follow-up protocols of the institutes, all patients were reviewed every 3 months after USgHIFU treatment to check for symptom improvement until January 2020. The patients were requested to undergo ultrasound every 3 months after USgHIFU for follow-up imaging evaluation. If local recurrence was suspected under ultrasound, the patients were referred for MRI.
Statistical analysis
SPSS 19.0 (IBM Company, Chicago, IL) and MedCalc statistical analysis software were used for data analysis. Normally distributed data were depicted using mean ± standard deviation; skewed distributed data were depicted using median and interquartile range (IQR). The independent two sample t test, Chi-square test and Mann-Whitney U test, were utilized for univariate analysis. Binary logistic regression analysis was utilized for multivariate analysis. The test level was set at α = 0.05, p < 0.05 was considered statistically significant.
Results
Of the 536 patients who completed follow-up, 40 patients were treated in 2011, 66 patients were treated in 2012, 110 patients were treated in 2013, 106 patients were treated in 2014, 95 patients were treated in 2015 and 119 patients were treated in 2016. The follow-up period lasted for a median of 69 (interquartile range: 48 to 89) months and ended in January 2020. Among them, local recurrence was detected in 110 patients, and the other 426 patients didn’t have local recurrence.
Patient information
Based on the follow-up MRI results, the patients were classified into groups with local recurrence and without recurrence. No significant difference was observed between the two groups in terms of age and BMI () (p = 0.135 and p = 0.700).
Table 1. Comparison of baseline characteristics of patients between the group with recurrence and group without recurrence.
Fibroid characteristics
We also compared the fibroid characteristics between the two groups. As shown in , significant differences between the group with recurrence and the group without recurrence were observed in terms of uterine volume, fibroid volume, maximum fibroid diameter, T2WI signal intensity and fibroid enhancement types (p < 0.05). The maximum fibroid diameter, fibroid volume and uterine volume in the patients in the group with recurrence were 65 (interquartile range: 53–84) mm, 106.3 (interquartile range: 66.6–211.1) cm3 and 259.9 (interquartile range: 206.4–437.7) cm3 respectively, compared to 55 (interquartile range: 41–65) mm, 60.0 (interquartile range: 33.7–98.9) cm3, and 199.9 (interquartile range: 145.0–316.0) cm3 respectively, in the group without recurrence. In the group with recurrence, the fibroid diameter, fibroid volume and uterine volume were significantly larger than those in the group without recurrence. There were 29 homogeneous hypointense fibroids, 18 homogeneous isointense fibroids and 63 hyperintense fibroids in the group with recurrence; while there were 293 homogeneous hypointense fibroids, 54 homogenous isointense fibroids and 79 hyperintense fibroids in the group without recurrence. In the group with recurrence, mild enhancement was observed in 21 fibroids, moderate enhancement in 29 fibroids, and significant enhancement in 60 fibroids, while in the group without recurrence, mild, moderate, and significant enhancement were observed in 282, 98, and 46 fibroids respectively. However, there was no significant difference in age, BMI, the abdominal wall thickness, fibroid type, fibroid location, or uterine position between the two groups () (p > 0.05).
Distance measurements from fibroid to adjacent structures
We further compared the distance measurements from fibroid to adjacent structures between the two groups. As shown in , significant differences between the group with recurrence and the group without recurrence were also observed in terms of minimum distance from the ventral surface of the fibroid to the skin, the maximum distance from the dorsal surface of the fibroid to the skin, the minimum distance from the dorsal surface of the fibroid to the sacrum. The distance from the ventral surface of the fibroid to the skin in the patients with recurrence and the patients without recurrence were 42 (interquartile range: 29–60) mm and 50 (interquartile range: 34–69) mm, respectively. The distance from the dorsal surface of the fibroid to the skin was 110.9 ± 20.7 mm and 100.0 ± 21.5 mm in the patients with recurrence and the patients without recurrence respectively, and the distance between the dorsal surface of the fibroid to the sacrum was 16 (interquartile range: 9–27) mm and 23 (interquartile range: 12–46) mm respectively. The fibroids in the group with recurrence were closer to the sacrum and the ventral surface of the skin compared to those of the group without recurrence (p < 0.05).
Post-HIFU assessment
All patients completed USgHIFU treatment in one session. As shown in , a significant difference was observed between the two groups in terms of NPV, NPV ratio, treatment time, sonication time, and the amount of therapeutic energy used (p < 0.05). In the group with recurrence, NPV and NPV ratio were 74.3 (interquartile range: 48.0–170.7) cm3 and 73% (interquartile range: 58%–87%) respectively; the median treatment time was 106 (interquartile range: 71–165) minutes, the median sonication time was 1,150 (interquartile range: 623–1,805) seconds and the therapeutic energy required was 458,000 (interquartile range: 248,700–720,000) Joules (J). In the group without recurrence, NPV and NPV ratio were 55.0 (interquartile range: 26.4–98.9) cm3 and 89% (interquartile range: 79%–100%) respectively; the median treatment time was 62 (interquartile range: 36–102) minutes, the median sonication time was 720 (interquartile range: 410–1,200) seconds, and the therapeutic energy required was 290,000 (interquartile range: 162,250–461,300) J.
Table 2. Comparison of HIFU treatment results between the group with recurrence and the group without recurrence.
Evaluation of the independent factors that affect recurrence
Factors that may affect recurrence, including uterine volume, fibroid volume, maximum fibroid diameter, distance from ventral side of fibroids to skin, distance from dorsal side of fibroids to skin, distance from dorsal side of fibroids to sacrum, T2WI signal intensity, fibroid enhancement type, treatment time, sonication time, therapeutic energy, NPV, and NPV ratio, were further analyzed to test whether they are independent factors affecting recurrence. A multivariate analysis demonstrated that the independent factors influencing recurrence were fibroid enhancement type, maximum fibroid diameter and NPV ratio () (p < 0.05). Fibroids with mild or moderate enhancement were less likely to relapse compared to significantly enhanced fibroids. The maximum fibroid diameter is positively correlated with recurrence, while NPV ratio is negatively correlated with recurrence.
Table 3. Binary logistic regression analysis results of independent factors affecting recurrence.
Clinical outcome follow-up
After 3 months, all the USgHIFU treated patients reported symptom relief.
110 patients, who had responded to requested follow-up visits, had local recurrence. Of these 110 patients, 52 reported that symptoms had returned whilst the other 58 patients did not have symptoms.
The 52 patients, who experienced a return in symptoms, and 25 of those patients who did not experience a return in symptoms, required additional treatment.
During the median of a 69 month follow up, between 12 and 24 months after USgHIFU treatment, 18 (16.4%) patients required additional treatment. 59 (53.6%) patients required additional treatment 24 months after USgHIFU. These additional treatments consisted of 32 USgHIFU treatments and 45 myomectomies.
There was no further treatment for the group without recurrence. The overall reintervention rate in all 536 patients during the median of 69 months follow-up was 14.4%. No patient required a hysterectomy.
Discussion
Previous studies have demonstrated that the long-term symptom relief of patients with uterine fibroids after HIFU/MRgFUS correlates with the NPV ratio of the treated fibroids [Citation5,Citation6]. The high re-intervention rate after HIFU/MRgFUS is mainly as a result of the low NPV ratio [Citation5]. In this study, the median non-perfused volume (NPV) ratio in patients with recurrence was 73%, compared to 89% among patients without recurrence. After a median of 69 months follow-up, local recurrence was detected in 110 patients and only 77 patients required re-intervention. Thus, the NPV ratio achieved in this study was significantly higher than those in previous studies, lowering the overall re-intervention rate. Several studies have also shown that a larger NPV ratio is easier to achieve in hypointense fibroids or isointense fibroids than hyperintense fibroids [Citation6,Citation8]. In clinical practice, we noted that multiple factors besides signal intensity on T2WI were also related to NPV ratio, and thus related to recurrence. In this study, our results showed that the NPV ratio in the recurrent group was over 70%, but was significantly lower than in the non-recurrent group. We further compared the baseline characteristics and treatment results of the two groups and found that maximum fibroid diameter, fibroid volume and uterine volume were significantly larger in the recurrent group compared to that in the group where recurrence did not occur. The distance from the ventral surface of fibroids to skin and the distance from the dorsal surface of uterine fibroids to the sacrum were significantly shorter in the group with recurrence than that in the group without recurrence; however, the distance from the dorsal surface of uterine fibroids to the skin was larger in the group with recurrence. In addition, patients in the group with recurrence were found to have more hyperintense fibroids; alternatively, patients in the group without recurrence had more hypointense fibroids. Simultaneously the group with recurrence contained more significantly enhanced fibroids than the group without recurrence. During treatment, uterine fibroids in the group with recurrence required higher ultrasonic intensity, longer treatment time, longer sonication time and greater therapeutic ultrasound energy than fibroids in the group without recurrence. However, the NPV ratio was lower in the group with recurrence. We further performed a multivariate analysis to investigate the independent factors affecting recurrence. The results showed that recurrence was affected by the enhancement type of the fibroids, maximum fibroid diameter, and NPV ratio.
Following HIFU treatment, local recurrence of uterine fibroids occurred less frequently in fibroids with mild or moderate enhancement than in fibroids with significant enhancement (). The possibility of recurrence of fibroids with mild enhancement was 0.059 times that of fibroids with significant enhancement; while the incidence of recurrence of fibroids with moderate enhancement was 0.151 times that of fibroids with significant enhancement. Therefore, the possibility of recurrence increases with greater enhancement. This may be because fibroids with significant enhancement are more vascular; blood flow in vascular fibroids could dissipate some heat and therefore the acoustic energy deposition in these fibroids is less than that of avascular uterine fibroids [Citation9,Citation10]. Recently, Nieuwenhuis et al. found that the growth rate of fibroids was related to the proportion of blood vessels within the fibroid tissue [Citation11]. Therefore, after HIFU, the residual tumor tissue in vascular fibroids (significant enhancement) is more likely to grow faster than that in avascular fibroid tumors (less enhancement).
In this study, we found that the maximum fibroid diameter correlated with recurrence. The possibility of recurrence increases by 1.039 times with each 1 mm increase in fibroid diameter, if the fibroids are larger than 60 mm in diameter. The reason for this could be because large uterine fibroids have large feeding blood vessels, or it could be because of some other physiological or pathological feature of large uterine fibroids. Often more therapeutic ultrasound energy is required to ablate large fibroids completely. If the therapeutic energy deposited in the fibroids is insufficient, vascularization of fibroids may occur. Further studies to explore the mechanisms of recurrence of large uterine fibroids after HIFU are required.
Previous studies have shown that high re-intervention rate was related to a small NPV ratio [Citation5,Citation12]. In our study, the results showed that the NPV ratio correlated negatively with recurrence. When the NPV ratio was greater than 88.26%, for every 1% increase in the NPV ratio the likelihood of recurrence decreased by a factor of 0.944. When treating a given uterine fibroid, the characteristics of the fibroid cannot be changed, but the joint score could be changed by increasing the NPV ratio, thus lowering the risk of recurrence. The NPV ratio is related to a variety of factors. Firstly, HIFU treatment is more effective in treating hypointense and isointense fibroids, due to the relatively poor blood supply and more fibrous tissue in these fibroids [Citation13–16]. Hyperintense fibroids are difficult to treat, resulting in a lower NPV ratio, and thus the 5-year intervention rate will be higher [Citation5]. Secondly, the fibroid’s location is also related to the NPV ratio: fibroids located in the posterior wall of the uterus are more difficult to treat with HIFU than those located in the anterior wall. Mindjuk et al. reported that the NPV ratio can be significantly increased when the distance between the fibroids and the spine is greater than 3 cm [Citation17]. Lastly, other factors such as sonication power, sonication time and treatment intensity may also affect the NPV ratio. However, multivariate analysis did not show that any of these were independent factors.
This study is limited because it is a retrospective study and some bias may occur. This study is also limited because we only enrolled patients with a solitary fibroid with size smaller than 10 cm in diameter. The patients with multiple fibroids or the size larger than 10 cm in diameter may have different results. Future studies, with randomized, enrolled patients with a tumor larger than 10 cm in diameter or with multiple fibroids, are required.
Conclusion
In our study, during the follow-up period, the overall re-intervention rate for patients with a solitary fibroid smaller than 10 cm in diameter, treated with USgHIFU, was 14.4%. Achievement of a NPV ratio higher than 70% has led to acceptable re-intervention rates for these patients. Based on our results, multiple factors affect the recurrence of fibroids after HIFU treatment. The maximum diameter of fibroids, degree of fibroid enhancement, and the NPV ratio were the independent factors affecting fibroid recurrence. Aiming for complete ablation could reduce the risk of re-intervention.
Acknowledgment
The authors are grateful to Wesley Zhang for helping us edit and revise this paper.
Disclosure statement
Zhibiao Wang and Lian Zhang are senior consultants to Chongqing Haifu. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
References
- Wang J, Zhang G, Shi H, et al. Dextran uterine artery embolization to treat fibroids. Chin Med J. 2002;115(8):1132–1136.
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73(2):396–403.
- Tempany CM, Stewart EA, McDannold N, et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905.
- Verpalen IM, de Boer JP, Linstra M, et al. The focused ultrasound myoma outcome study (FUMOS); a retrospective cohort study on long-term outcomes of MR-HIFU therapy. Eur Radiol. 2020;30(5):2473–2482.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589.
- Li W, Jiang Z, Deng X, et al. Long-term follow-up outcome and reintervention analysis of ultrasound-guided high intensity focused ultrasound treatment for uterine fibroids. Int J Hyperthermia. 2020;37(1):1046–1051.
- Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82(12):2265–2269.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16(6):765–778.
- Nieuwenhuis LL, Keizer AL, Stoelinga B, et al. Fibroid vascularisation assessed with three-dimensional power doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG. 2018;125(5):577–584.
- Froeling V, Meckelburg K, Scheurig-Muenkler C, et al. Midterm results after uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for symptomatic uterine fibroids. Cardiovasc Intervent Radiol. 2013;36(6):1508–1513.
- Oguchi O, Mori A, Kobayashi Y, et al. Prediction of histopathologic features and proliferative activity of uterine leiomyoma by magnetic resonance imaging prior to GnRH analogue therapy: correlation between T2-weighted images and effect of GnRH analogue. J Obstet Gynaecol (Tokyo 1995)). 1995;21(2):107–117.
- Funaki K, Fukunishi H, Funaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196(2):184.e1-6–184.e6.
- Gong C, Setzen R, Liu Z, et al. High intensity focused ultrasound treatment of adenomyosis: the relationship between the features of magnetic resonance imaging on T2 weighted images and the therapeutic efficacy. Eur J Radiol. 2017;89:117–122.
- Zhao WP, Chen JY, Chen WZ. Effect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol. 2015;41(2):423–431.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single Centre. Eur Radiol. 2015;25(5):1317–1328.