Abstract
Objective
This study aimed to evaluate the clinical safety and efficacy magnetic resonance (MR)-guided percutaneous thermal ablation for the treatment of small liver malignant tumors of segment II and IVa (≤3.0 cm) abutting the heart.
Method
The enrollment of 24 patients with 25 malignant liver lesions located on the II or IVa segment abutting the heart who underwent MRI-guided thermal ablation between August 2010 and February 2020 were retrospectively analyzed. Follow-up MRI was performed to evaluate the curative effect. Local tumor progression-free survival and overall survival rates were also calculated.
Results
The procedures including radiofrequency ablation (RFA) for 15 patients and microwave ablation (MWA) for 9 patients were successfully accomplished (technical success rate of 100%) without major complications. The mean duration time was 78.4 ± 29.4 min (40–140 min), and mean follow-up time was 31.5 ± 22.2 months (6–92 months). The technical efficacy was 100% following one ablation session with MRI assessment after one month. Local tumor progression was observed in one patient with a metastatic lesion located in segment II at 18 months follow-up. The progression-free survival time was 20.1 ± 16.9 months (median: 15 months). The 1-, 3-, and 5-year local tumor progression-free survival rates of this patient were 100%, 94.7%, and 94.7%, respectively. With regards to all the patients, the 1-, 3-, and 5-year estimated overall survival rates were 91.7%, 80.6%, and 50.1%, respectively.
Conclusion
MR-guided thermal ablation is safe and effective for the treatment of small liver malignant tumors located on the II or IVa segment abutting the heart.
Introduction
Image-guided thermal ablation is widely used in the local treatment of malignant liver tumors due to its minimal invasiveness and definite curative effect [Citation1,Citation2]. In the treatment of hepatic malignant tumors of segment II and IVa abutting the heart, thermal ablation has a risk of causing diaphragmatic perforation, cardiac tamponade, pericarditis, arrhythmia, and severe cases that can lead to death [Citation3–5]. Currently, ultrasound (US) and computed tomography (CT) are most commonly used to guide liver tumor ablation. However, due to the interference of gas at the bottom of the lung, liver tumors at the top of the diaphragm often become blind spots for ablation treatment [Citation6]. In addition, CT guidance has ionizing radiation, relatively low resolution of soft tissues, and metal ablation needles have insufficient artifacts such as interference. Therefore, it is often difficult to clearly visualize small liver tumors adjacent to the bottom of the heart using US and plain CT, which remains a huge challenge for guiding thermal ablation of liver tumors abutting the heart.
MRI guidance has the advantages of non-ionizing radiation, excellent soft tissue contrast, multi-parameter and multi-planar imaging functions, and accurate curative effect evaluation. It has been applied to the ablation treatment of liver tumors with good curative effects [Citation7,Citation8]. According to reports in literature, the detection rate of MRI for smaller liver tumors is higher than that of conventional US and CT, especially for patients with liver cirrhosis [Citation9]. However, there are only few reports on the ablation treatment of segment II and IVa malignant liver tumors abutting the heart, most of which are case reports [Citation3]. Thus, this study aimed to evaluate the clinical safety and efficacy of MRI-guided percutaneous thermal ablation for the treatment of small malignant liver tumors of segment II and IVa (≤3.0 cm) abutting the heart.
Materials and methods
This retrospective single-center study was approved by our ethical review committee, and the requirement for a written informed consent was waived. A retrospective analysis of 988 consecutive patients who underwent MR-guided thermal ablation of malignant liver tumors between August 2010 and February 2020 was conducted. The inclusion criteria of the study were as follows: (a) at least one tumor was located in segment II and IVa, with the upper edge of the lesion ≤1.0 cm from the pericardium; (b) there was no macrovascular invasion or extrahepatic metastasis with a maximum diameter ≤3.0 cm and number of metastatic lesions ≤3; (C) a Child-Pugh grade A or B of liver function, prothrombin activity ≥40%, and platelet ≥ 50 × 109/L; (d) ECOG score of 0 or 1.
Of the 988 consecutive patients, a total of 24 patients were enrolled in the study who underwent MRI-guided thermal ablation for segment II and IVa of malignant liver tumors abutting the heart (). Baseline characteristics of the enrolled patients are shown in . All procedures of enrolled patients were followed by Multidisciplinary Tumor Board Decision.
Table 1. Patients’ characteristics.
There were 25 lesions in all 24 patients enrolled, and the average distance from the upper edge to the pericardium was 0.68 ± 0.22 cm (3–10 mm). Of the 21 patients with HCC enrolled, 17 patients relapsed after receiving local treatment (surgery, TACE, or ablation), and 4 patients were treated with thermal ablation as first-line therapy. Four patients with colorectal cancer liver metastasis (CRLM) underwent primary tumor resection combined with systemic chemotherapy before ablation.
MRI equipment and ablation system
MRI equipment: 1.5 T dual gradient MRI scanner (GE Signa Infinity Twinspeed, USA) with a close-bored (inner diameter of 60 cm) was used to guide ablation, and a Torso coil with a rectangular square hole was used to facilitate interventional procedures. Breathing and ECG gating devices were used to monitor vital signs of the patients.
RFA equipment: MRI-compatible monopolar expandable multitined perfusion electrode (StarBurst MRI, RITA, 14 G, shaft length 10/15 cm, USA) with a rigid trocar that included 9 expandable hooks were used. The sub-electrode and the radiofrequency electrode needle were equipped with artificial perfusion holes, and the range of a single ablation was 1–5 cm of spherical coagulation necrosis. To keep the MRI-incompatible RF generator (Model 1500X, RITA, USA) away from the magnet, a 25-foot MR compatible extension cable was necessary. The thermal deposition algorithm was based on the manufacturer’s guidelines for power and duration settings using the temperature-controlled mode.
MWA equipment: MWA (MTC-3C, VISON Medical Equipment Co. Nanjing, China) system was used with a microwave frequency of 2450 MHz, with the host being magnetically shielded. The MRI compatible microwave antenna (14 G, 15 cm, VISON Medical Equipment Co. Nanjing, China) included a built-in water-cooling circulation system. The coaxial transmission cable was 3 cm long, and a choke coil was installed to avoid interference with the MR imaging. The shape of the MWA lesion was spherical.
Ablation procedure
Preoperative preparation
Upper abdominal CE-MRI was performed to confirm the location, size, and number of lesions 2 weeks prior to ablation. Just before the procedure, the patients were intramuscularly injected with 100 mg of bucinnazine. The heart rate, respiration, and blood pressure were monitored during the procedure, and an MR-incompatible RF generator was placed outside the magnet room. The patients were then placed on the MR table in either the supine or left lateral position. Due to the limitation of the magnet bore, the patient’s body needed to be moved to the opposite side in order to facilitate the insertion more easily. The return grounding pads were also placed on the patients’ thighs. Two radiologists with over 5 years of experience in hepatobiliary interventions performed the ablation procedures for all the patients.
MRI sequence and parameters were as follows: 1) 3D dynamic T1 weighted image (3D Dyn-T1WI): thickness of 3.0 mm, FOV was 38 cm × 28 cm, scanning time was 10–14 s; 2) Fat-suppressed fast recovery fast spin echo T2-weighted image (FsFRFSE-T2WI): thickness of 5.0 mm, interval of 1.0 mm, FOV was 38 cm × 28 cm, scanning time was 50–70 s; and 3) Diffusion weighted imaging (DWI): thickness of 5.0 mm, interval of 1.0 mm, b value was 50–800 s/mm2, scanning time was 25–40 s. The 3D Dyn-T1WI sequence used breath-hold scanning, and the fsFRFSE T2WI and DWI sequences used a respiratory gating trigger.
The vitamin E pill or matrix was pasted on the skin as a body surface marker. Fs-T2WI and 3D Dyn-T1WI scans were performed to plan the puncture path, determine the skin needle point, and measure the angle and depth of the needle. The puncture point on the body surface was marked, routinely disinfected, draped, covered the scanning coil, and injected with 1% lidocaine for local infiltration anesthesia. Following the respiration gating control, the patient should breathe calmly and then hold his/her breath. Afterwards, the radiofrequency electrode or microwave antenna was gradually inserted under the guidance of the 3D DynT1WI sequence. Oblique coronal, oblique sagittal, or oblique axis scanning can be used to show the full length of the ablation needle and its spatial relationship with the lesion. As the radiofrequency electrode reached the edge of the lesion, the sub-electrodes gradually expanded according to the size of the lesion. When the microwave antenna penetrated the lesion and protruded for about 5 mm, a repeat scan was performed to confirm the distance between the sub-electrodes or microwave antenna and the lesion, as well as the spatial relationship with structures of clinical importance such as the diaphragm and pericardium ( and ).
The extension cable and negative pads were connected after satisfying the RF electrode layout. The power and target temperatures of the RF generator were set to 150 W and 105 °C, respectively. Afterwards, the appropriate ablation procedure and duration were selected according to different ablation ranges (2–4 cm/5–8 min). The sub-electrode temperature and thermal impedance coefficient were closely monitored during RFA. During RFA, 0.9% normal saline at room temperature was intermittently injected through the water injection hole to enhance heat conduction and reduce tissue carbonization based on temperature and impedance changes.
After confirming that the needle placement was satisfactory, the water-cooling cycle and the coaxial cable were connected, and the corresponding microwave ablation power and time were set (70–90 W, 6–12 min) according to the size of the tumor. The ablation host was turned off after the ablation was completed, and MRI was performed to immediately evaluate efficacy and complications.
Immediate efficacy evaluation after ablation was performed in which complete ablation was considered when the ablated lesion completely covers the original lesion, and the safe ablation margin is 5–10 mm beyond the edge of the lesion. This would imply a successful technique evaluation, and if otherwise, a tumor residue was considered which would prompt supplemental ablation. After ablation was complete, the needle was withdrawn, and a whole liver fs-T2WI sequence scan was performed again to assess immediate complications.
Complication
Complications were evaluated based on the guidelines of the Society of Interventional Radiology. The description of complications follows the proposed standardization of terminology and reporting criteria in this study [Citation10].
Follow-up and clinical results
Liver enhanced MRI was performed to evaluate the ablation efficacy one month after the ablation and every 3 months thereafter. During the follow-up, we observed whether the local tumor had recurred or progressed. Progression-free survival (PFS), 1-year, 3-year, 5-year local tumor progression-free (LPFS) survival, and overall survival rates were observed.
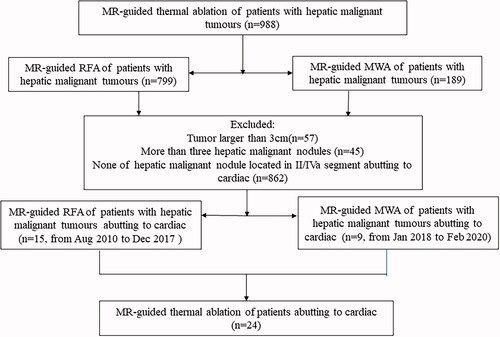
Figure 2. Recurrent HCC in the hepatic dome abutting to heart of a 55-year-old woman treated with MR-guided RFA. (A,B) The recurrent nodule in segment IV (arrow), 3 mm from pericardium, is 7 mm in diameter and appears hypointense in T1WI before RFA. (C) The recurrent nodule is displayed unclearly on plain CT scan. (D) The RF electrode is targeted gradually using the tilting of the puncture path under the coronal 3D-T1WI guidance. When the RF electrode reaches the edge of the nodule, the inner expandable multitined electrodes are expanded to 2.0 cm to overlap the nodule without penetrating the diaphragm. (E–G) After RFA, the nodule is completely overlapped by the rim of the hyperintensity on 3D-T1WI and hypointensity on T2WI (arrow in G). (H,I) The lesion is completely ablated without diaphragm and heart injury at 4-month follow-up by enhanced MRI.
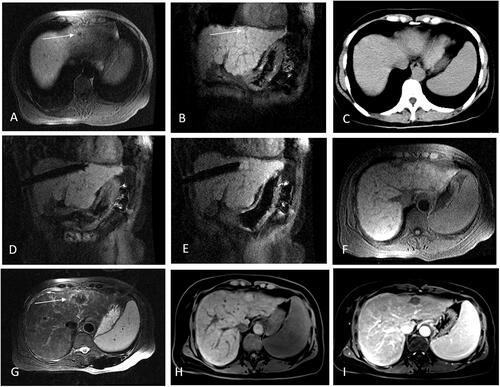
Figure 3. Recurrent HCC after liver resection in the hepatic dome abutting to heart of a 35-year-old man treated with MR-guided MWA. (A,B) The recurrent nodule 4.8 mm from pericardium,13 mm in diameter of segment II appears hyperintense in T2WI and hypointense in T1WI before MWA. (arrow in A,B). (C,D) The MW antenna is gradually targeted and inserted under the coronal T2WI, and the relationship between the antenna and heart is clearly displayed in coronal image. (E–G) A typical ‘target sign’ (arrow) is clearly shown in the ablative zone with the hypointense nodule completely overlapped by the hyperintensity on 3D-T1WI and hypointensity on T2WI, with a safe margin immediately after MWA and without diaphragm and heart injury. (H,I) The ablative zone with the ‘target sign’ is completely ablated without enhancement in MRI at 1-month follow-up.
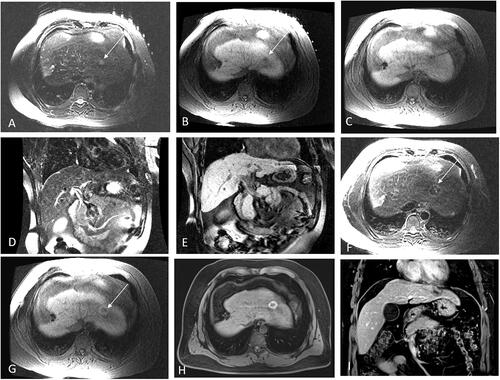
Figure 4. Probability of LPFS and estimated OS in 24 patients with 25 hepatic malignant lesions located on segment II or IVa abutting the heart (mean diameter: 13.4 ± 6.3 mm, range: 7–30 mm), treated with Mrguided thermal ablation after a median follow-up of 28 months. (A) Graph showing 1-, 3-, and 5-year LPFS rates as 100%, 94.7%, and 94.7%, respectively. (B) Graph showing the estimated 1-, 3-, and 5-year OS rates as 91.7%, 80.6%. and 50.1%, respectively.
Table 2. Incidence of complications related to ablation.
Statistical analysis
SPSS software (version 22.0; IBM, Chicago, USA) was used for statistical analysis with data expressed as mean ± standard deviation (SD). Kaplan–Meier survival curves were used to calculate the 1-year, 3-year, and 5-year LPFS and OS.
Results
MRI manifestations before ablation
A total of 24 liver lesions in 23 patients were clearly visible on conventional MRI scans including 3D-T1WI fs-T2WI and DWI sequences as MRI protocol before ablation. The lesions showed slightly hypointense on T1WI and hyperintense on T2WI with a clear boundary, as well as hyperintense on DWI and hypointense on ADC map. The remaining one lesion was invisible on unenhanced MRI. However, it was clearly visible in the hepatobiliary stage using a Gd-BOPTA agent with a clear boundary.
Ablation process
MRI-guided thermal ablation was successful in all patients (15 for RFA, 9 for MWA) with an average duration of 78.4 ± 29.4 min (40–140 min). Step-by-step needle insertion was performed in which the ablation needle showed no signal in any of the MRI sequences. Twenty-four lesions in 23 patients were ablated using conventional plain MRI-guided ablation, and one patient with unclear plain MRI image was ablated under hepatobiliary MR-guided microwave ablation.
3D-T1WI and fs-T2WI sequences were routinely performed as MRI protocol after ablation. Immediate MRI after ablation showed that the hyperintense on T1WI of 22 lesions after ablation were completely surrounded the hypointense original lesion and exceeded the safe ablation margin of 5–10 mm. The ablation area showed hypointense with a slightly hyperintense edema band around it on T2WI (). Additionally, other 14 lesions that were not located abutting the heart were simultaneously ablated. The success rate of the ablation technique was 100% for all procedures.
Complications and side effects
No serious complications were observed in the study. Seven patients (29.2%) developed mild or moderate shoulder pain evaluated by Visual Analogue Scale (VAS), and 4 patients (16.7%) developed precordial discomfort postoperatively which improved after symptomatic and analgesic treatment. Regarding intraoperative complications, one patient (4.2%) had a small amount (pericardial viscera-parietal spacing 13 mm) of asymptomatic pericardial hemorrhage, and 3 patients (12.5%) had a small amount (widest diameter smaller than 10 mm) of crescent-shaped capsular hemorrhage displayed as hyperintense in T2WI and hypointense in T1WI (). There were no cases of massive pneumothorax, pericardial tamponade, arrhythmia, diaphragmatic perforation, liver failure, needle implant transfer, or ablation-related deaths.
Follow-up and clinical outcome
The average follow-up time was 31.5 ± 22.2 months (6–92 months). According to the MRI evaluation one month after ablation, all 24 cases with 25 lesions were completely ablated, and the technical efficiency rate was 100%. In follow-up, one case of CRLM II lesion showed local tumor progression 18 months after ablation, and the local metastatic cancer was surgically removed after Multidisciplinary Tumor Board Decision. The remaining 23 patients showed no significant recurrence of the local lesions. New liver tumors and extrahepatic metastatic cancers were observed in 12 patients (50%) and 5 cases (20.8%), respectively.
During the follow-up period, 8 patients died including 6 HCC and 2 liver metastases cases. Six patients died of tumor progression, one died of liver failure, and one died of bleeding from varicose veins caused by portal hypertension. The tumor progression-free survival (PFS) was 20.1 ± 16.9 months (median: 15 months), and the local progression-free survival (LPFS) rates at 1, 3, and 5 years after ablation were 100%, 94.7%, and 94.7%, respectively. The estimated overall survival rates (OS) at 1, 3, and 5 years after ablation were 91.7%, 80.6%, and 50.1%, respectively ().
Discussion
Image-guided ablation of segment II and IVa malignant tumors of the liver abutting the heart is challenging with difficulty in balancing the efficacy and safety of the procedure [Citation6,Citation11]. The basis for accurate ablation of liver tumors under image guidance is good visibility. Sometimes small malignant liver tumors abutting the heart are poorer visibility under ultrasound and CT guidance, especially in patients with liver cirrhosis, and sometimes it is impossible to distinguish liver cirrhotic nodules from small hepatocellular carcinoma. Some studies used image fusion technology (such as CT-US or MRI-US fusion) [Citation12,Citation13], artificial assistive technology such as artificial ascites or artificial pleural fluid [Citation14,Citation15], and TACE-labeled lipiodol to improve the visibility of the lesions. The visibility of small lesions can improve the safety of ablation to a certain extent, but it also increases the complexity and number of the operations. Due to excellent soft tissue contrast and multi-parameter imaging, MRI can easily distinguish cirrhotic nodules from hepatocellular carcinoma and small lesions were clearly displayed under MR-guidance, especially those located the bottom of the liver diaphragm [Citation7]. In this study, 96% (24/25) of the lesions were clearly displayed on unenhanced MRI and only 4% (1/25) of the lesions was unclear on unenhanced MRI, otherwise the lesion was clearly displayed in hepatobiliary phase for guiding ablation. RFA electrode and microwave antenna used in this study formed a round-like coagulative necrosis area after ablation. In comparison, smaller round liver tumors near the heart of liver segments II and IVa can achieve better conformal ablation while taking into account safety and efficacy. In this study, only a small amount of hemopericardium displayed as hyperintense on T2WI and hypointense on T1WI occurred after radiofrequency sub-electrodes deployed during the ablation of segment II lesion. Ablation was continued because the patient had no obvious symptoms without hemopericardium increasing. Additionally, our study did not use artificial-assisted technology for isolation, and no serious complications occurred.
Evaluation of the therapeutic effect immediately after ablation is essential for evaluation of the effectiveness of treatment and reduction of the residual rate of tumors. During ultrasound-guided ablation, due to the air bubbles generated by the tissue vaporization intraoperatively, there was a hyperechoic response in the ablation area which obscured the original lesions. The ablation range can only be roughly estimated based on the hyperechoic area. Similar to previous findings, the hyperechoic area produced by ablation can last for 30 min to 6 h, which limited the evaluation of immediate effect of ablation by contrast-enhanced ultrasound [Citation16]. Similar with ultrasound-guided thermal ablation, CT-guided thermal ablation zones showed mixed low-density changes, which cannot visualize a clear boundary of the original lesion and accurately safety margin. A previous study showed that there was a good correlation between MRI signal characteristics and pathological changes in liver tumors after thermal ablation [Citation8]. Koda et al. considered that non-enhanced MRI can accurately assess the immediate treatment response after thermal ablation [Citation17]. In contrast to ultrasound and CT guidance, the original lesion after ablation was still clearly displayed as hypointense on T1WI with the surrounding hyperintense on T1WI for coagulation necrosis of liver tissue, with the typical ‘target sign’ changes and clearly visible safety margin. When the lesion was completely covered by the ablation zone of hyperintense on T1WI with exceeding the safety margin of 5–10 mm, it was evaluated as complete ablation. Otherwise, the ablation site was readjusted and supplemental ablation was performed. Therefore, compared with ultrasound and CT guidance, MRI can provide an accurate evaluation of immediate ablation efficacy and reduce repeated ablation treatments caused by local tumor residues [Citation18]. In this study, all lesions were ablated in one session, and the technical success rate of ablation was 100%. Meanwhile there was not definitely ‘heat sink phenomenon’ caused by the heart leading to incomplete ablation, probably due to the isolation of diaphragm and much thicker myocardium than the vascular wall. The technical efficacy rate was also 100% by enhanced MRI evaluation of 1 month follow-up. Only 1 patient (4%) with liver metastasis from colon cancer showed local tumor progression 18 months after ablation during follow-up. The local progression-free survival (LPFS) rates at 1, 3, and 5 years after ablation were 100%, 94.7%, and 94.7%, respectively.
Despite these findings, this study had certain limitations. First, MRI-guided thermal ablation of liver tumors spent more time than ultrasound and CT guidance. Second, this study did not use MR noninvasive temperature measurement technology to monitor the thermal field distribution during the ablation. Third, this was a single-center retrospective study with a small sample size. Thus, multi-center, large-samples, randomized controlled studies is needed to further verify the efficacy and safety of the procedure and improve these shortcomings in the future.
In conclusion, MR-guided thermal ablation is safe and effective for small liver malignant tumors located on the II and IVa segments abutting the heart, and it is expected to achieve a higher complete ablation rate and a lower local tumor progression rate in one session.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nault J, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909.
- Silverman ER, Lai YH, Osborn IP, et al. Percutaneous radiofrequency ablation of hepatocellular lesions in segment II of the liver: a risk factor for cardiac tamponade. J Clin Anesth. 2013;25(7):587–590.
- Hsu JC, Tsai HL, Lin YL, et al. Acute pericarditis following treatment of a metastatic liver tumor with radiofrequency ablation: a case report. Bmc Cardiovasc Disor. 2018;18:1–5.
- Moumouh A, Hannequin JM, Chagneau C, et al. A tamponade leading to death after radiofrequency ablation of hepatocellular carcinoma. Eur Radiol. 2005;15(2):234–237.
- Li M, Yu X, Liang P, et al. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia. 2012;28(3):218–226.
- Weiss J, Hoffmann R, Rempp H, et al. Feasibility, efficacy, and safety of percutaneous MR-guided ablation of small (≤12 mm) hepatic malignancies. J Magn Reson Imaging. 2019;49(2):374–381.
- Stephan C, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J Magn Reson Imaging. 2010;27:421–433.
- Nam CY, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(2):161–167.
- Image-Guided tumor ablation: standardization of terminology and reporting criteria 10-Year update: Supplement to the consensus document. Radiology. 2014;273(1):241–260.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.
- Song KD, Lee MW, Rhim H, et al. Percutaneous US/MRI fusion-guided radiofrequency ablation for recurrent subcentimeter hepatocellular carcinoma: technical feasibility and therapeutic outcomes. Radiology. 2018;288(3):878–886.
- Lee MW, Rhim H, Cha DI, et al. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1–3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013;24(7):958–965.
- Nam SY, Rhim H, Kang TW, et al. Percutaneous radiofrequency ablation for hepatic tumors abutting the diaphragm: clinical assessment of the heat-sink effect of artificial ascites. AJR Am J Roentgenol. 2010;194(2):W227–231.
- Zhang D, Liang P, Yu X, et al. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia. 2013;29(7):663–670.
- Choi D, Lim HK, Kim SH, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: usefulness of power Doppler US with a microbubble contrast agent in evaluating therapeutic response-preliminary results. Radiology. 2000;217(2):558–563.
- Koda M, Tokunaga S, Miyoshi K, et al. Assessment of ablative margin by unenhanced magnetic resonance imaging after radiofrequency ablation for hepatocellular carcinoma. Eur J Radiol. 2012;81(10):2730–2736.
- Clasen S, Rempp H, Hoffmann R, et al. Image-guided radiofrequency ablation of hepatocellular carcinoma (HCC): is MR guidance more effective than CT guidance? Eur J Radiol. 2014;83(1):111–116.