Abstract
Purpose
Hepatic recurrence of liver malignancies is a leading problem in patients after liver resection with curative intention. Thermoablation is a promising treatment approach for patients after hepatic resection, especially in liver-limited conditions. This study aimed to investigate safety, survival, and local tumor control rates of MRI-guided percutaneous thermoablation of recurrent hepatic malignancies following hepatic resection.
Material and methods
Data from patients with primary or secondary hepatic malignancies treated between 2004 and 2018 with MRI-guided percutaneous thermoablation of hepatic recurrence after prior hepatic resection were retrospectively analyzed. Disease-free survival and overall survival rates were calculated using the Kaplan-Meier method.
Results
A total of 57 patients with hepatic recurrence (mean tumor size = 18.9 ± 9.1 mm) of colorectal cancer liver metastases (n = 27), hepatocellular carcinoma (n = 17), intrahepatic recurrence of cholangiocellular carcinoma (n = 9), or other primary malignant tumor entities (n = 4) were treated once or several times with MR-guided percutaneous radiofrequency (n = 52) or microwave ablation (n = 5) (range: 1–4 times). Disease progression occurred due to local recurrence at the ablation site in nine patients (15.8%), non-local hepatic recurrence in 33 patients (57.9%), and distant malignancy in 18 patients (31.6%). The median overall survival for the total cohort was 40 months and 49 months for the colorectal cancer group, with a 5-year overall survival rate of 40.7 and 42.5%, respectively. The median disease-free survival was 10 months for both the total cohort and the colorectal cancer group with a 5-year disease-free survival rate of 15.1 and 14.8%, respectively. The mean follow-up time was 39.6 ± 35.7 months.
Conclusion
MR-guided thermoablation is an effective and safe approach in the treatment of hepatic recurrences in liver-limited conditions and can achieve long-term survival.
Introduction
Hepatic resection is presently the most common therapy option for patients with primary and secondary hepatic malignancies if curative treatment is intended [Citation1–4]. A relevant percentage of patients develop de-novo metastasis or an intrahepatic recurrence after hepatectomy. In ∼60% of patients who underwent resection of hepatic metastases of colorectal cancer, recurrence is diagnosed within two years [Citation5]. In cases of liver-limited recurrence, repeated resection or thermoablation are promising curative therapy approaches [Citation6,Citation7]. The latter has been discussed to be advantageous in cases of difficult locations of the recurrent tumor, the surgery-related loss of relevant liver tissue with the risk of a small-for-size syndrome, or due to the presence of post-operative adhesions [Citation6,Citation8,Citation9]. In general, the most common guidance modalities for percutaneous thermoablation procedures are ultrasound (US) and computed tomography (CT). All present studies concerning thermoablation of recurrences after hepatectomy due to hepatocellular carcinoma (HCC) or colorectal liver metastases (CRLM) were conducted with these guidance modalities and revealed low morbidity levels and favorable clinical outcomes [Citation6,Citation10–12]. Magnetic resonance imaging (MRI) is an alternative modality for interventional procedures and is presently limited to a few specialized institutions, mostly for economic reasons [Citation13], despite its technical advantages. Near-real-time MR-fluoroscopy for accurate applicator placement, free selection of imaging planes, MR-thermometry for evaluation of the therapeutic effect, high sensitivity for small target lesions, and intra-procedural assessment of the ablation process without application of contrast agent are particularly advantageous during MR-guided thermoablation procedures [Citation14–16]. This study aimed to investigate safety, survival, and local tumor control rates of MRI-guided percutaneous thermoablation as first-line treatment of recurrent hepatic malignancies following hepatic resection.
Methods
Patient population
All patients with primary or secondary hepatic malignancies who underwent percutaneous MRI-guided thermoablation [radiofrequency ablation (RFA) or microwave ablation (MWA)] between 2004 and 2018 and had previously undergone liver resection were retrospectively included in this study. Exclusion criteria were benign liver lesions, missing follow-up MRI after ablation, or the initially planned combination therapy of hepatic resection and thermoablation. The local institutional review board approved this retrospective analysis of patient data (approval code: 114/2015BO2).
Percutaneous ablation procedure
The decision for recurrence treatment using percutaneous thermoablation was determined by a multidisciplinary tumor board consisting of representatives from the departments of surgery, internal medicine, radiology, pathology, nuclear medicine, and radiotherapy. Decision criteria included tumor size, number, and location of recurrent lesions, the overall state of health, and the presence of extrahepatic tumor load. Percutaneous thermoablation procedures in the liver are routinely conducted under MR guidance at our institution if no contraindications against MRI exist.
Patients were treated in a closed bore 1.5 T MRI system (Magnetom Espree, Siemens Healthineers) with a bore diameter of 70 cm. All treatments were performed in the supine position with an additional elevation of the right body side in cases of tumor localization in the right liver segments. Planning imaging with a standardized MRI protocol of T1-weighted and T2-weighted sequences to confirm tumor number, size, and localization were performed without the use of contrast media if possible. If necessary additional diffusion-weighted imaging sequences (DWI, n = 5) and dynamic contrast-enhanced sequences (n = 4) were acquired after administration of extracellular contrast agent (Gadovist, Bayer HealthCare, Leverkusen, Germany) or hepatocyte-specific contrast agent (Primovist, Bayer HealthCare). Local anesthesia was injected percutaneously in each case. Ablations were conducted with general anesthesia in cases with subcapsular tumor locations or on the explicit request of the patient (n = 19). In all other cases, analgesia (piritramide or pethidine) and sedation (midazolam) were administered. Depending on tumor size 16 G RFA applicators (Celon, Teltow, Germany; length 10–15 cm, active tip 2–4 cm) or MWA antenna (Medwaves Avecure, Medwaves, San Diego, CA, USA; length 16 cm, active tip 2–4 cm) was used. For RFA celon device, two (bipolar mode) or more (multipolar mode) probes are positioned around the target region, and the current flows between all possible electrode combinations. For MWA, one probe is inserted into the center of the lesion. Tumor targeting was executed using steady-state free precession MR-fluoroscopic sequences (BEAT_IRTT) which continuously depicted the applicator path in three dimensions [Citation17].
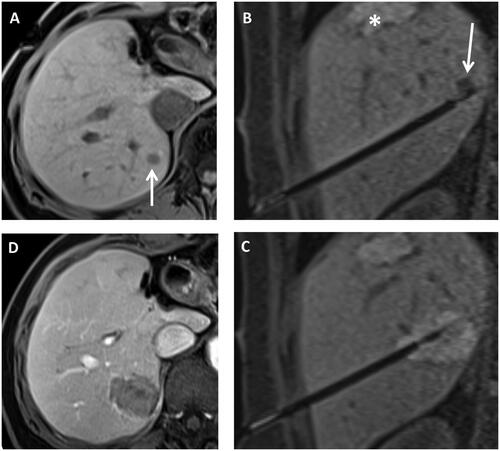
For thermoablation, the applicator was connected to the generator positioned outside the scanner room using an extension cable. T1-weighted sequences were used to monitor the ablation result [Citation18]. Additional ablation with or without repositioning the applicator under MR-fluoroscopy was performed in 48 cases of inadequate ablation zone with suspicious residual tumor tissue. If the ablation zone was sufficiently large with an appropriate safety margin, the applicator was retracted under tract coagulation. Post-interventional control imaging with dynamic contrast-enhanced T1- and T2-weighted sequences was used to assess the technical success and to rule out complications. The average MRI intervention time was 233 ± 59 min (range 142–369 min) and depended on the number and complexity of the lesions. shows an example of the procedure from planning imaging on the day of the intervention to applicator positioning and the sufficiently large ablation zone in contrast-enhanced final control imaging. All patients were routinely hospitalized for at least one night after the procedure and discharged if complete blood count, as well as the ultrasound examination one day after the procedure, ruled out complications [Citation17]. The CIRSE classification system for complications was used [Citation19].
Figure 1. Example of ablation procedure in 1.5 T MRI system. Native T1 weighted planning imaging (A) in a patient after left hemihepatectomy and condition after thermal ablation with new colorectal liver metastasis in segment 7 (arrow). (B) Shows the control imaging angulated onto the applicator to determine the position of the applicator at the tumor lesion (arrow). The asterisk marks an old ablation zone. After thermal ablation (C), T1 weighted images were performed again to assess ablation zone size. Finally, contrast-enhanced images were taken (D) to detect bleeding complications and to evaluate potential tumor residuals.
Data collection
Patient characteristics regarding demographics, tumor type, and therapy regimen were obtained from the standardized interdisciplinary tumor board reports. Tumor size and location were defined by measurements using pre-interventional MR-images collected no more than 4 weeks before intervention. Complications, period of hospitalization, systemic tumor therapy, follow-up results, and recurrence therapies were received from institutional medical records and interventional protocol. Complications were classified according to the Clavien-Dindo classification [Citation20]. Technical success was assessed by the contrast-enhanced T1 sequences at the end of the intervention. If the non-enhancing ablation zone covered the target tumor without evidence of residual tumor, the procedure was considered technically successful [Citation21].
According to internal guidelines, the first post-interventional control imaging was intended 4 weeks after thermoablation, followed by MRI examinations every 3 months for one year and extended to every 6 months thereafter. The follow-up period was defined as the time interval between the first thermoablation after liver surgery and the last available control MRI or the date of death for deceased patients.
Statistical analysis
Percentages and mean values were calculated and reported with associated standard deviations. Overall survival and disease-free survival were estimated using the Kaplan–Meier method. Survival data were calculated independently of the censored cases. Group comparisons were conducted with Student’s t-test. Statistical analysis was performed using SPSS (version 26.0; SPSS Inc., Chicago, IL, USA). P-values <0.05 were considered statistically significant.
Results
Clinicopathological characteristics
One hundred and three patients underwent both hepatic resection and percutaneous liver ablation, for primary and secondary hepatic malignancies during 2004 and 2018 and were retrospectively analyzed in the study. Of these, 58 patients who underwent MR-guided thermoablation as first-line treatment of hepatic recurrence following hepatic resection met the inclusion criteria (). One patient with a hepatocellular adenoma was excluded to avoid biases in survival data due to benign tumor types. Therefore, the final analysis included 57 patients, 43 men and 14 women with a median age of 62.2 ± 12.8 years. In total, 17 patients had HCC, 27 patients suffered liver metastases of colorectal cancer, nine patients had cholangiocarcinoma of which four patients had metastases of CCA. Four patients had other primary tumor entities (uveal melanoma, undifferentiated embryonal sarcoma of the liver, inflammatory myofibroblastic tumor, leiomyosarcoma). A total of 71 lesions with an average size of 18.9 ± 9.1 mm (range 6–48 mm) were ablated within 57 RFA or MWA procedures (). The time from resection to ablation was 16.0 ± 14.7 months. One patient was treated for five tumor lesions, two patients were treated for three lesions, six patients for two tumors, and 48 patients for one tumor, respectively. The initial hepatic resection procedure according to the Brisbane classification was right hemihepatectomy (n = 12), atypical resection (n = 11), right trisectionectomy (n = 7), left lateral sectionectomy (n = 5), segmentectomy (n = 3), left hemihepatectomy (n = 2), and others or combinations of different methods (n = 17) in descending frequency.
Figure 2. Study flowchart. HCC: hepatocellular carcinoma; CRLM: colorectal liver metastases; CCA: cholangiocarcinoma; iCCA: intrahepatic cholangiocarcinoma; cHCC-CC: combined hepatocellular cholangiocarcinoma; UESL: undifferentiated embryonal sarcoma of the liver; RFA: radiofrequency ablation; MWA: microwave ablation; HR: hepatic resection. Patients who have not perceived follow-up MRI after ablation or underwent MRI ex domo were excluded due to insufficient follow-up.
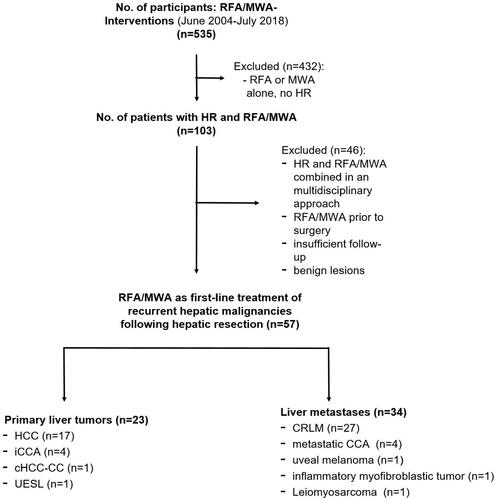
Table 1. Patient characteristics.
Procedures
RFA was performed in 52 patients and five patients received MWA. In the time interval between liver resection and thermoablation, a total of 18 patients received chemotherapy, five patients were treated with transcatheter arterial chemoembolization (TACE), two patients had selective internal radiation therapy (SIRT) and one patient underwent radiotherapy.
The technical success rate of all procedures was 100%. Additional lesions were detected in six patients during intraprocedural planning. The mean hospital stay of all patients after the procedure was 2.7 ± 1.2 days (range: 2–10 days). One patient developed a CIRSE grade 3 complication after the intervention, with irritant peritonitis due to a bile leak that occurred during coil marking in the same session as stereotactic radiation therapy of another liver metastasis [Citation22]. The patient was successfully treated with antibiotics and was discharged from the hospital after an extended stay of 19 days. However, no major complications occurred in any of the other procedures.
Recurrence
A total of 44 patients developed disease progression during follow-up (mean: 39.6 ± 35.7 months). Nine (20.5%) of these patients developed local recurrence at the ablation site. Five of 27 patients (18.5%) with CRLM developed a local recurrence, all of those had the primary tumor located in the left colon. The further patients with local recurrence at the ablation site each had a different tumor entity, including HCC, inflammatory myofibroblastic tumor, metastatic CCA, and intrahepatic CCA. The total local recurrence rate at the ablation site was 15.8% on a per-patient basis and 12.7% on a per-tumor basis. Three of the nine patients with local recurrence at the ablation site also had other de-novo lesions in the liver, and one of these patients also developed extrahepatic disease progression. summarizes the cases with local recurrence at the ablation zone and indicates the therapy after local recurrence.
Table 2. Cases with local recurrence at the ablation zone including therapy after recurrence.
Non-local hepatic recurrences were observed in 33 patients and extrahepatic disease progression in 18 patients. During follow-up, 16 patients underwent further thermoablation procedures and four patients underwent a second resection due to new hepatic tumors.
Survival
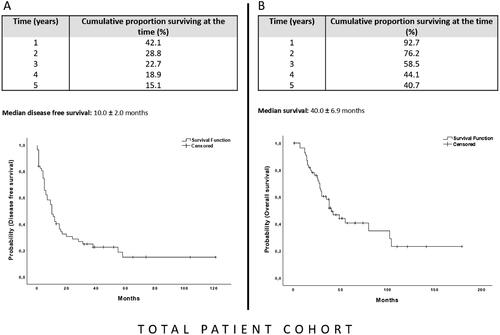
At the last follow-up, 20 patients were alive (five without evidence of disease), 32 patients had died and five were lost to follow-up. Median survival after thermoablation in the total cohort was 40.0 ± 6.9 months and the 1-, 3-, and 5-year overall survival (OS) rates were 92.7, 58.5, and 40.7%, respectively (). The median disease-free survival time in the total cohort was 10.0 ± 2.0 months with 1-, 3-, and 5-year survival rates after thermal ablation of 42.1, 22.7, and 15.1%, respectively ().
Figure 3. The Kaplan–Meier curve for disease-free survival (A) and overall survival (B) of the total patient cohort after MR-guided thermoablation of recurrent hepatic malignancies following hepatic resection. The starting point for the calculation is the date of the first ablation after resection.
Median survival in the CRLM-group was 49.0 ± 14.2 months and the 1-, 3-, and 5-year OS rates were 96.3, 63.5, and 42.5%, respectively ().
Figure 4. The Kaplan–Meier curve for disease-free survival (A) and overall survival (B) after MR-guided thermoablation of recurrent colorectal liver metastases following hepatic resection. The starting point for the calculation is the date of the first ablation after resection.
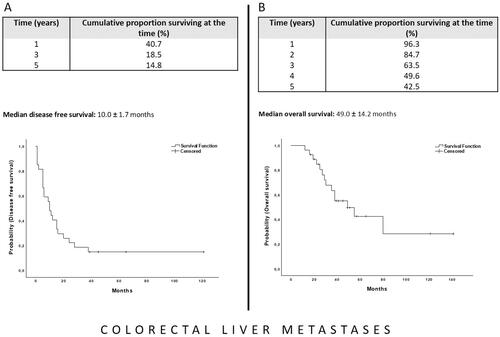
Median disease-free survival in the CRLM-group was 10.0 ± 1.7 months and the 1-, 3-, and 5-year survival rates were 40.7, 18.5, and 14.8%, respectively ().
Discussion
Hepatic resection is the gold standard in the treatment of primary and metastatic liver tumors, but recurrence is common. Treatment options range from medical to surgical and interventional options, depending on the underlying disease [Citation6,Citation23–27]. Thermoablation is a well-established, effective, and promising technique for the treatment of liver tumors, especially in liver limited conditions and in difficult local conditions. Few studies have investigated the role of thermal ablation as an equivalent, the alternative procedure to surgery in patients with recurrent liver tumors after primary hepatic resection [Citation6,Citation10,Citation11,Citation25–27]. In our study, survival was examined in conditions of one or more hepatic recurrences of primary and secondary liver tumors after hepatic resection. The unique attributes of this study are firstly the long-term observation period of up to 15 years, which allows the investigation of possible long-term survivors, and secondly the use of MRI as a guidance modality for the interventional procedure. The latter offers an immediate assessment of the ablation process and the target tumor, usually without administration of contrast medium. In contrast, with CT-guided thermoablation, the target tumor and the ablation zone can be difficult to distinguish [Citation28]. With ultrasound guidance, the evaluation of the ablation process is challenging due to the physically induced gas formation in the ablation zone [Citation29]. Furthermore, MR fluoroscopy has advantages regarding real-time monitoring by free angulation of the imaging slices and reconfiguration of applicator position in difficult anatomical situations [Citation17]. Finally, MRI has a higher sensitivity than CT in the detection of small liver lesions [Citation30]. Particularly in our patient population, these are important requirements to detect, safely reach and monitor recurrences in already operated livers. In six of our 57 patients, planning imaging at the beginning of the thermoablation revealed one or more new suspicious liver lesions. Five patients showed between one and three new lesions which could be successfully treated in the same intervention. In one case with a history of two successful ablation procedures of a hepatic recurrence of uveal melanoma, the planning imaging during the third intended MR-guided radiofrequency ablation of singular liver metastasis revealed new disseminated liver metastases which resulted in terminating the intervention. As the intra-procedurally detected new liver lesions in these cases had a minimum diameter of 6 mm, it is questionable if they would have been detected under a CT-guided procedure. One patient remained tumor-free to date after this ablation and a second patient remained tumor-free to date after liver resection of a subsequent hepatic progression so that MR-guided ablation had at least a supportive role in tumor control in these cases. Other promising approaches to successful ablation of lesions invisible on CT or ultrasound include fusion of CT images with contrast-enhanced ultrasound or pre-interventional MRI images [Citation31,Citation32]. This approach is advantageous because it is radiation-free, imaging is repeatable and certainly less expensive than MRI-guided interventions [Citation31].
This study confirms the curative approach of thermoablation in liver recurrence and shows long-term survival in a 15-year observation. Dupré et al. reported a median overall survival of 33.3 months and progression-free survival of 4.3 months after CT-scan and ultrasound-guided ablation of recurrent colorectal liver metastases [Citation6]. The results of our study revealed higher median overall and disease-free survival rates of 10.0 and 49.0 months, respectively. During a median follow-up of 22 months, a study conducted by Sofocleous et al. showed during a median follow up of 22 months a median overall survival of 31 months and 1-, 2-, and 3-year overall survival rates of 91, 66, and 41%, respectively, after CT-guided thermoablation of colorectal metastases after hepatectomy [Citation26]. Compared to our results, it confirms our above-average results of 1- and 3-year overall survival rates of 96.3 and 63.5%, respectively. As discussed in the literature, the pathogenesis differs between right- and left-sided colorectal cancers and therefore, the overall survival of right-sided colon cancer was noticeably poorer than that of left-sided colon cancer [Citation33,Citation34]. A possible reason for our favorable results compared to the literature could be the proportionally larger number of left-sided colorectal cancer (22/27). Unfortunately, the distribution of left and right-sided colorectal cancer is missing in the studies by Sofocleous and Dupré, so the comparison to our results is not possible in this respect.
The following limitations of our work need to be addressed: due to the retrospective design, some minor complications might have not been documented in the medical reports, and this information was therefore not available for our evaluation. The patient population was rather small and heterogeneous, so subgroup analysis of HCCs or other rare entities was not possible and cannot be compared with other results in the literature [Citation27]. Consequently, the results of the entire cohort must be viewed with caution. Due to the selected patient population with a long history of the disease, previous liver surgery and often other local liver therapies, local primary tumor therapies, and often other systemic therapies, these interindividual factors as well as other influences, such as patient age at diagnosis, patient compliance and comorbidities should be considered in the evaluation of the results. Finally, due to the small number of MWA cases, an evaluation comparing the effects of the two different ablation techniques was not possible.
Conclusion
MR-guided thermal ablation is an effective therapeutic option for liver tumor recurrence after primary tumor resection, which is associated with low morbidity and potentially long-term survival. In addition, thermal ablation offers the advantage of multiple treatments in mainly liver-limiting conditions with correspondingly long tumor control of the disease.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157(1):54–64.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Frilling A, Clift AK. Surgical approaches to the management of neuroendocrine liver metastases. Endocrinol Metab Clin North Am. 2018;47(3):627–643.
- Bacalbasa N, Balescu I, Ilie V, et al. The impact on the long-term outcomes of hormonal status after hepatic resection for breast cancer liver metastases. In Vivo. 2018;32(5):1247–1253.
- de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–448.
- Dupre A, Jones RP, Diaz-Nieto R, et al. Curative-intent treatment of recurrent colorectal liver metastases: a comparison between ablation and resection. Eur J Surg Oncol. 2017;43(10):1901–1907.
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338.
- Elias D, De Baere T, Smayra T, et al. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89(6):752–756.
- ten Broek RP, Issa Y, van Santbrink EJ, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ. 2013;347:f5588.
- Schullian P, Johnston EW, Putzer D, et al. Stereotactic radiofrequency ablation (SRFA) for recurrent colorectal liver metastases after hepatic resection. Eur J Surg Oncol. 2021;47(4):866–873.
- Schullian P, Laimer G, Putzer D, et al. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur J Surg Oncol. 2020;46(8):1503–1509.
- Mao R, Zhao JJ, Bi XY, et al. Resectable recurrent colorectal liver metastasis: can radiofrequency ablation replace repeated metastasectomy? ANZ J Surg. 2019;89(7-8):908–913.
- Maurer MH, Schreiter N, de Bucourt M, et al. Cost comparison of nerve root infiltration of the lumbar spine under MRI and CT guidance. Eur Radiol. 2013;23(6):1487–1494.
- Wijlemans JW, Bartels LW, Deckers R, et al. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) ablation of liver tumours. Cancer Imaging. 2012;12(2):387–394.
- Fischbach F, Lohfink K, Gaffke G, et al. Magnetic resonance-guided freehand radiofrequency ablation of malignant liver lesions: a new simplified and time-efficient approach using an interactive open magnetic resonance scan platform and hepatocyte-specific contrast agent. Invest Radiol. 2013;48(6):422–428.
- Salem U, Kumar VA, Madewell JE, et al. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19(1):65.
- Rempp H, Waibel L, Hoffmann R, et al. MR-guided radiofrequency ablation using a wide-bore 1.5-T MR system: clinical results of 213 treated liver lesions. Eur Radiol. 2012;22(9):1972–1982.
- Rempp H, Unterberg J, Hoffmann R, et al. Therapy monitoring of magnetic resonance-guided radiofrequency ablation using T1- and T2-weighted sequences at 1.5 T: reliability of estimated ablation zones. Invest Radiol. 2013;48(6):429–436.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the Cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–1146.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196.
- Kierans AS, Elazzazi M, Braga L, et al. Thermoablative treatments for malignant liver lesions: 10-year experience of MRI appearances of treatment response. Am J Roentgenol. 2010;194(2):523–529.
- Weiss J, Winkelmann MT, Gohla G, et al. MR-guided microwave ablation in hepatic malignancies: clinical experiences from 50 procedures. Int J Hyperthermia. 2020;37(1):349–355.
- Lintoiu-Ursut B, Tulin A, Constantinoiu S. Recurrence after hepatic resection in colorectal cancer liver metastasis. J Med Life. 2015;8(Spec Issue):12–14.
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722; discussion 22–24.
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–825; discussion 25–27.
- Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–761.
- Liu J, Zhao J, Gu HAO, et al. Repeat hepatic resection VS radiofrequency ablation for the treatment of recurrent hepatocellular carcinoma: an updated meta-analysis. Minim Invasive Ther Allied Technol. 2020:1–10.
- Clasen S, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J Magn Reson Imaging. 2008;27(2):421–433.
- Chiou SY, Liu JB, Needleman L. Current status of sonographically guided radiofrequency ablation techniques. J Ultrasound Med. 2007;26(4):487–499.
- Maegerlein C, Fingerle AA, Souvatzoglou M, et al. Detection of liver metastases in patients with adenocarcinomas of the gastrointestinal tract: comparison of (18)F-FDG PET/CT and MR imaging. Abdom Imaging. 2015;40(5):1213–1222.
- Schullian P, Johnston E, Laimer G, et al. Thermal ablation of CT ‘invisible’ liver tumors using MRI fusion: a case control study. Int J Hyperthermia. 2020;37(1):564–572.
- Bo XW, Xu HX, Wang D, Guo LH, et al. Fusion imaging of contrast-enhanced ultrasound and contrast-enhanced CT or MRI before radiofrequency ablation for liver cancers. Br J Radiol. 2016;89(1067):20160379.
- Baran B, Mert Ozupek N, Yerli Tetik N, et al. Difference between left-sided and right-sided colorectal cancer: a focused review of literature. Gastroenterology Res. 2018;11(4):264–273.
- Yahagi M, Okabayashi K, Hasegawa H, et al. The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg. 2016;20(3):648–655.
