Abstract
Objective
The aim of this study was to evaluate changes in anti-Müllerian hormone (AMH) levels after ultrasound-guided high-intensity focused ultrasound (USgHIFU) treatment of cesarean scar pregnancy (CSP).
Methods
A retrospective case series study was conducted in the Affiliated Hospital of North Sichuan Medical College. Thirty-two women with cesarean scar pregnancy who met the inclusion criteria were enrolled in the study between January 2018 and December 2019. All patients underwent USgHIFU treatment with or without suction curettage. Intraoperative blood loss in suction curettage and hysteroscopy procedures, time to return of β-human chorionic gonadotropin (β-hCG) to normal levels, and time to recovery of normal menstruation were recorded. AMH levels before and 3 months after HIFU treatment were compared to determine whether USgHIFU treatment affected ovarian reserve.
Results
AMH levels before and 3 months after HIFU ablation were 1.87 ± 1.19 ng/ml and 1.90 ± 1.17 ng/ml, respectively. There was no significant difference in AMH levels between the two-time points (p > .05). The median volume of intraoperative blood loss was 20 ml, the median time for the serum β-hCG level to return to normal was 35.5 days, and the median time of menstruation recovery was 39 days.
Conclusions
USgHIFU treatment for CSP was effective and safe without affecting ovarian reserve.
Introduction
Cesarean scar pregnancy (CSP) is a special type of ectopic pregnancy that refers to the implantation of the gestational sac in a previous cesarean scar, which is a long-term complication of cesarean section [Citation1]. Although the exact incidence of CSP is unknown, it is estimated to affect 1:1800–1:2216 pregnancies [Citation2]. This condition represents 6.1% of all ectopic pregnancies with a history of at least one cesarean section [Citation2,Citation3]. The consequences of the increasing prevalence of cesarean section worldwide in terms of CSP are escalating [Citation4,Citation5]. Since CSP may increase the risk of uterine rupture and catastrophic bleeding, which may be life-threatening and compromise future fertility, early diagnosis and reasonable treatment are essential. However, there are currently no universal standards or guidelines for the diagnosis and treatment of CSP, nor is there high-level evidence-based medicine or any randomized controlled trial with a large sample size [Citation1,Citation6,Citation7].
Safe and effective termination and removal of trophoblastic tissue is the goal of CSP treatment. Direct curettage often results in uncontrollable massive hemorrhage, so preoperative adjuvant therapy is often used to prevent massive bleeding. To date, a number of systematic reviews have recommended uterine artery embolization (UAE) combined with dilatation and curettage as one of the treatment options for CSP [Citation8–10]. However, the influence of UAE on ovarian function and future fertility is also controversial [Citation11–13]. As a non-invasive treatment, high-intensity focused ultrasound (HIFU) has achieved good clinical efficacy for the treatment of gynecological diseases over the last decades [Citation14]. In the treatment of CSP, many studies have confirmed the effectiveness and safety of ultrasound-guided high-intensity focused ultrasound (USgHIFU) [Citation7,Citation15,Citation16]. However, unlike UAE, few studies have investigated whether HIFU treatment of CSP could affect ovarian reserve. Anti-Müllerian hormone (AMH) is an effective indicator to evaluate ovarian reserve in that the value is not affected by the menstrual cycle [Citation17]. The aim of this study was to evaluate changes in AMH levels of patients with CSP 3 months after HIFU treatment.
Materials and methods
This study was approved by the ethics committee of Affiliated Hospital of North Sichuan Medical College (Reference: 2020ER123-1, 28 October 2020). Patient data were suitably anonymized and protected according to national standards.
Patients
In the presence of a positive pregnancy test, a CSP was diagnosed by transvaginal ultrasound using the following criteria [Citation1]: (1) an empty uterus and cervical canal, (2) a gestational sac at the cesarean section scar and the presence of embryonic/fetal pole and/or yolk sac with or without heart activity, (3) thin or absent myometrial tissue between the bladder and the gestational sac, and (4) a prominent and at times rich vascular pattern at or in the area of the cesarean section scar. The included patients who met the above diagnostic criteria were treated with HIFU with or without suction curettage, and follow-up data on AMH levels were obtained before and 3 months after HIFU treatment. The exclusion criteria were as follows: (1) patients who received other treatments for CSP before HIFU and suction curettage (e.g., UAE, MTX, hysterectomy). Upon diagnosis of the CSP, patients had been informed and counseled about the potential risks of the condition and the available treatment plans. The choice of treatment was based on the patients’ informed consent; (2) patients with unstable vital signs, uncontrolled vaginal bleeding, or other conditions not suitable for HIFU; or (3) patients with a history of endocrine diseases, polycystic ovarian disease, ovarian surgery, or a history of radiotherapy and chemotherapy. From January 2018 to December 2019, 32 patients who met the inclusion criteria were enrolled in the study. presents the flow diagram of enrolled patients.
Ultrasound-guided HIFU ablation
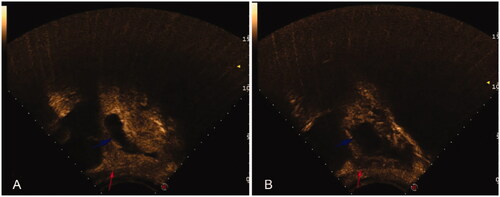
HIFU treatment was performed using the model-JC200 Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). Patients were asked to undergo specific bowel preparation for 3 days before the procedure, which included ingesting liquid food for 3 days, fasting for 12 h, and cleaning the bowel with an enema. The abdominal wall from the umbilicus to the level of the upper margin of the pubic symphysis was shaved and degreased, and a urinary catheter was inserted to control the bladder volume before HIFU surgery. During treatment, midazolam and fentanyl were intravenously injected for sedation and pain control in patients (midazolam hydrochloride 0.03 mg/kg, fentanyl 1 μg/kg, repeated administration every 40 min if necessary.). The treatment plan was made by dividing the gestational sac into different slices with a thickness of 5 mm. The ablation procedure began from the innermost slice with an acoustic output power of 300–400 W. The whole treatment process was carried out under real-time ultrasound monitoring. When color Doppler ultrasound indicated that the blood perfusion signal of pregnancy tissue was significantly reduced or the grayscale image of target tissue was changed, contrast-enhanced ultrasound was performed to determine the end time of treatment. The contrast-enhanced ultrasound images before and after HIFU are shown in . Each patient received only 1 session of HIFU ablation.
Figure 1. Flow diagram of enrolled patients. CSP, cesarean scar pregnancy; HIFU, high-intensity focused ultrasound; UAE, uterine artery embolization; MTX, Methotrexate; AMH, anti-Müllerian hormone.
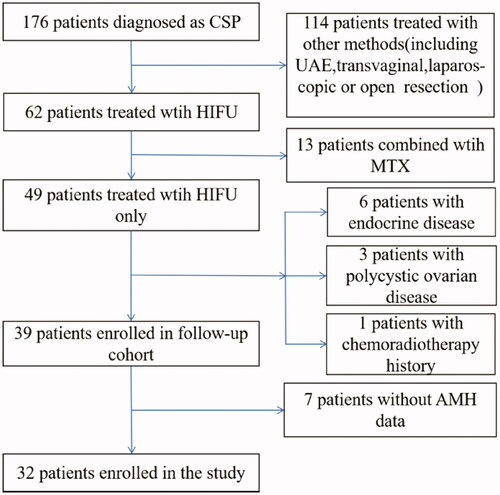
Figure 2. Contrast-enhanced ultrasound images before and after HIFU ablation (sagittal). (A) The gestational sac (blue arrow) and blood flow (red arrow) were presented on transabdominal color Doppler contrast-enhanced ultrasound before HIFU ablation. (B) Contrast-enhanced ultrasound showed the gestational sac was deformed (blue arrow) and blood perfusion was significantly reduced (red arrow) after HIFU ablation.
After HIFU treatment, hysteroscopy or ultrasound-guided suction curettage was performed according to the specific situation, or only follow-up observation was performed.
Follow-up
After discharge, the patients were asked to monitor their serum β-human chorionic gonadotropin (β-hCG) levels weekly until it returned to normal. The patients were requested to return to our department for a color Doppler ultrasound examination 1 month after suction curettage and to return to our department for an AMH test 3 months after HIFU treatment. The time when the serum β-hCG returned to normal and the time of the first menstrual period after treatment were recorded.
AMH test
Blood samples of 3 ml were collected from patients before and 3 months after HIFU treatment. The samples were centrifuged at 2000 r/min for 20 min, and then serum was separated and stored at −20 °C. The hormones were then measured by an electrochemiluminescence immunoassay method (ECLIA, Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Statistical analysis was performed using SPSS Version 22.0 (IBM, Armonk, NY, USA). Continuous variables with a normal distribution are described as the mean ± standard deviation. Continuous variables with a skewed distribution are presented as the median, and categorical variables are expressed as frequency (percentage). AMH levels before and after HIFU treatment were compared using a paired t-test. A value of p < .05 was considered statistically significant.
Results
Demographic characteristics
The mean age of these patients was 32.3 ± 4.9 years (range, 25–44 years). The median number of pregnancies in these patients was 4 (range, 2–10). Twenty (62.5%) patients had one previous cesarean delivery, 11 (34.4%) patients had two previous cesarean deliveries, and 1 (3.1%) patient had three previous cesarean deliveries. Except for 1 patient who had one low-segment cesarean section diagnosed with CSP for the second time, the others were diagnosed with CSP for the first time. The median interval from the last cesarean section (CS) to CSP was 51 months (range, 35–73 months). The average gestational age was 47.2 ± 6.2 days (range, 37–60 days). Among these patients, the median serum β-hCG level before treatment was 5,6250.0 IU/L (range, 4276–181,180 IU/L), the mean largest diameter of the sac/mass was 26.8 ± 10.0 mm (range, 8.0–47.0 mm), and fetal cardiac activity was observed in 16 cases (50%). According to relevant literature, we divided the thickness of the intervening myometrium between the gestational sac and the bladder into more than 3 mm and less than or equal to 3 mm [Citation6,Citation18,Citation19]. There were 9 (28.1%) patients with a myometrial thickness >3 mm and 23 (71.9%) patients with a myometrial thickness ≤3 mm. ().
Table 1. Demographic characteristics of the study population.
Treatment results
All 32 patients successfully completed 1 session of HIFU ablation therapy. The HIFU treatment time (mean ± standard deviation), HIFU ablation time, and treatment energy were 41.2 ± 9.2 min, 456.2 ± 162.9 s, and 180,725.0 ± 64,877.5 Joules, respectively. During HIFU treatment, 3 patients complained of sacral pain, 5 patients complained of lower abdominal pain, 3 patients complained of a ‘‘hot’’ skin sensation, and 1 patient had a small amount of vaginal bleeding, which was less than one-half of the menstrual volume. All patients tolerated the treatment process well, and no serious complications, such as intestinal, nerve or skin damage, occurred.
Twenty patients received suction curettage under hysteroscopic guidance, and 10 patients received ultrasound-guided suction curettage after HIFU treatment. The median time from HIFU treatment to suction curettage was 2 days (range, 1–7 days). The median volume of intraoperative blood loss was 20 ml (range, 5–400 ml). Two patients did not undergo suction curettage after HIFU, and only follow-up observations were made.
Follow-up result
The median hospitalization time was 8 days (range, 5–18 days). One patient who did not undergo suction curettage had the longest hospital stay of 18 days, and the other patient who did not undergo suction curettage was hospitalized for 10 days. All patients complained of a small amount of vaginal bleeding after suction curettage, but no catastrophic hemorrhage or infection was observed. The median time for the serum β-hCG level to return to normal was 35.5 days (range, 20–83 days), and the median time of menstruation recovery was 39 days (range, 22–72 days). Four patients complained that the amount of menstruation after recovery was less than that before the CSP, but it did not reduce to 1/2 of the original menstrual volume. Other patients did not complain that the menstrual volume or menstrual period after recovery of menstruation were significantly changed. The results of treatment and follow-up are shown in .
Table 2. Treatment results of the study population (n = 32).
AMH level result
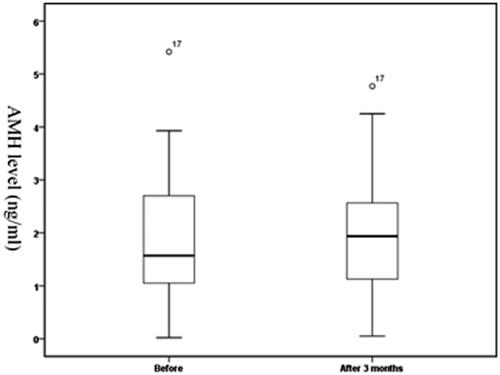
AMH levels before and at 3 months after HIFU treatment were 1.87 ± 1.19 ng/ml (range, 0.02–5.42 ng/ml) and 1.90 ± 1.17 ng/ml (range, 0.05–4.77 ng/ml), respectively. There was no significant difference in AMH levels between the two-time points (t = −0.416, p = .680, shown in ).
Discussion
CSP is a potentially long-term and serious complication that occurs after cesarean delivery [Citation20]. Although there are currently no standard treatment guidelines, once CSP is diagnosed, medical advice should be given to terminate the pregnancy, and the basic principles and methods for terminating early pregnancy should be followed to minimize damage and preserve the patient’s fertility as much as possible [Citation6] . Systemic methotrexate (MTX) and dilatation and curettage (D&C) alone are associated with high complication and hysterectomy rates [Citation10]. Transvaginal, laparoscopic, or even open resection of pregnancy tissue is effective but more traumatic [Citation9]. Uterine artery embolization (UAE), as a minimally invasive nonsurgical treatment, is widely used before uterine surgery to prevent excessive bleeding in uterine myomas, cervical pregnancies, or postpartum hemorrhage. Previous studies have shown that UAE followed by suction curettage achieved a high success rate in the treatment of CSP, and it appeared to have more advantages and maybe a priority option [Citation8,Citation21,Citation22]. However, many studies have evaluated the effects of UAE for uterine fibroids on ovarian reserve based on AMH and fertility after UAE. The results showed that ovarian reserve was affected by UAE and that the pregnancy rate was lower and the miscarriage rate was higher after UAE [Citation11,Citation23,Citation24]. Although the long-term complications of UAE for CSP are rarely reported, the severe adverse effects of UAE, such as ovarian dysfunction and infertility, are our concern.
Over the last few years, as a non-invasive treatment, ultrasound-guided high-intensity focused ultrasound (USgHIFU) has been widely used in the management of uterine fibroids and adenomyosis [Citation25,Citation26]. Recently, USgHIFU has also been used in the management of CSP, and the safety and effectiveness of HIFU in the treatment of CSP have been confirmed by many studies [Citation7,Citation15,Citation27]. Zhu et al. compared the outcomes of HIFU and UAE treatment for CSP, and the results showed that HIFU and UAE were equally safe and effective in the treatment of CSP; however HIFU treatment had the advantages of a lower pain score and fewer adverse effects [Citation28]. Zhang et al. reported the pregnancy outcomes after HIFU treatment of CSP, showing a rate of the successful conception of 82.14% (23/28) [Citation29]. However, the effect of HIFU for CSP on ovarian reserve function has not been reported.
AMH is produced by the granulosa cells of preantral and small antral follicles, and its levels can be assessed in serum [Citation30]. AMH levels correlate well with the number of antral follicles measured by ultrasound and are believed to be the best representation of the gradual decline in reproductive capacity among women [Citation31]. Unlike estradiol, follicle-stimulating hormone (FSH), or luteinizing hormone (LH), AMH is a cycle-independent marker for ovarian reserve [Citation17]. We measured changes in AMH levels before and 3 months after HIFU treatment to evaluate the effect of HIFU treatment on ovarian reserve. The results showed that there was no significant difference in AMH levels between the two-time points (1.87 ± 1.19 vs. 1.90 ± 1.17 ng/ml, p > .05). Our findings were consistent with reported findings in the literature. Lee et al. reported that there was no significant difference in AMH levels before treatment and 6 months after HIFU ablation for adenomyosis and uterine fibroids (2.11 ± 2.66 vs. 1.84 ± 2.57 μg/l, p > .05) [Citation32]. Qu et al. found no significant change in the AMH level after HIFU treatment for uterine fibroids in the group of patients younger than 35 years and a group older than 40 years at 6 months [Citation33]. Patients with CSP tend to be younger than those with uterine fibroids or adenomyosis. It is particularly important to choose treatments that are less traumatic and have less impact on ovarian function and future fertility. Because the blood supply to the ovaries is partially from the uterine arteries, UAE for CSP may also affect the collateral blood supply to the ovaries and has the potential risk of unintended embolization to the ovarian arteries [Citation34]. However, HIFU treatment selectively ablates pregnancy tissues, and the ovary and its vessels are not included in the treatment area. Thus, HIFU ablation does not damage the blood supply of the ovaries.
In terms of the effectiveness and safety of HIFU treatment for CSP, our study is in line with other studies. Zhu et al. reported that 53 patients with definite CSP were treated with one session of HIFU ablation followed by suction curettage under hysteroscopic guidance [Citation15]. The median volume of blood loss in the procedure and the average hospital stay were similar to our study (20 ml vs. 20 ml, 7.8 ± 1.5 days vs. 8 days, respectively). A possible reason that the time for serum β-hCG to return to normal and time to menstruation recovery in our study were slightly longer than those reported by Zhu et al. is that the median serum β-hCG level before treatment was 56,250.0 IU/L in our study but was 36,645 mIU/ml in the study by Zhu et al. We found that all patients tolerated the HIFU treatment process well, no serious complications occurred, and no catastrophic hemorrhage, infection, or need for further treatment were observed during the postoperative follow-up.
A comparative study of UAE and systemic MTX followed by suction curettage in the treatment of CSP showed that the bleeding volumes were 36.93 ± 6.01 ml in the UAE group and 415.63±.37 ml in the MTX group (p < .001). The hospitalization time was 11.73 ± 0.80 days in the UAE group and 39.63 ± 4.57 days in the MTX group (p < .001) [Citation21]. Shen et al. retrospectively reviewed management with bilateral uterine arterial chemoembolization with MTX in 46 cases of CSP. The results showed that the meantime until normalization of serum β-hCG was 37.7 days and that the mean hospitalization time was 10.5 days [Citation22]. Our results demonstrated that HIFU followed by suction curettage is effective and safe in treating CSP and similar to UAE in combination with suction curettage. Meanwhile, in our study, despite a relatively small sample size and a relatively short follow-up time, no significant difference in AMH level was found between pretreatment and 3 months after HIFU ablation. From this point of view, HIFU seems to be superior to UAE.
The main limitations of this study included the small cohort of enrolled patients and the lack of long-term follow-up. Furthermore, as a retrospective study, selection biases and missing data should also be considered. Therefore, future studies require a larger sample size, longer follow-up time, and randomized controlled studies with UAE. More attention should be paid to subsequent reproductive outcomes after treatment because these outcomes reflect not only ovarian function but also the condition of the uterus.
In conclusion, this preliminary study is the first report to demonstrate that there was no change in AMH levels after HIFU treatment of CSP. Although further prospective studies are needed to explore the long-term outcomes, the results of our study provide evidence that USgHIFU ablation followed by suction and curettage is an effective and safe treatment for CSP that does not affect ovarian reserve.
Author Contributions
Wenping Wang: data acquisition; analysis and interpretation; drafting the article and final approval of the version to be published. Jing Jiang, Yan Chen: performance of USgHIFU ablation of the patients. Chengzhi Li: data analysis and interpretation. Honggui Zhou, Zhibiao Wang: responsible for the initial concept, data acquisition and final review of the manuscript.
Acknowledgments
We are grateful to the patients who participated in this study and acknowledge the contribution of clinical staff from the Affiliated Hospital of North Sichuan Medical College for the treatment of patients and the delivery of clinical care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Timor-Tritsch IE, Monteagudo A, Santos R, et al. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(44):e1–e13.
- Seow KM, Huang LW, Lin YH, et al. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247–253.
- Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2013;25(6):456–461.
- Gibbons L, Belizan JM, Lauer JA, et al. Inequities in the use of cesarean section deliveries in the world. Am J Obstet Gynecol. 2012;206(4):331 e1–19.
- O'Neill SM, Khashan AS, Kenny LC, et al. Caesarean section and subsequent ectopic pregnancy: a systematic review and meta-analysis. BJOG. 2013 May;120(6):671–680.
- Jin L, Chen W, Zhou Y. Expert opinion of diagnosis and treatment of cesarean scar pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2016;51(8):568–572.
- Xiao J, Zhang S, Wang F, et al. Cesarean scar pregnancy: noninvasive and effective treatment with high-intensity focused ultrasound. Am J Obstet Gynecol. 2014;211(4):e3511–e3517.
- Qiao B, Zhang Z, Li Y. Uterine artery embolization versus methotrexate for cesarean scar pregnancy in a Chinese population: a meta-analysis. J Minim Invasive Gynecol. 2016;23(7):1040–1048.
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, et al. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016;105(4):958–967.
- Glenn TL, Bembry J, Findley AD, et al. Cesarean scar ectopic pregnancy: current management strategies. Obstet Gynecol Surv. 2018;73(5):293–302.
- Kim CW, Shim HS, Jang H, et al. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016;206:172–176.
- McLucas B, Voorhees WD 3rd, Elliott S. Fertility after uterine artery embolization: a review. Minim Invasive Ther Allied Technol. 2016;25(1):1–7.
- El Shamy T, Amer SAK, Mohamed AA, et al. The impact of uterine artery embolization on ovarian reserve: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99(1):16–23.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284.
- Zhu X, Deng X, Wan Y, et al. High-intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine. 2015;94(18):e854.
- Chen L, Xiao S, Zhu X, et al. Analysis of the reproductive outcome of patients with cesarean scar pregnancy treated by high-intensity focused ultrasound and uterine artery embolization: a retrospective cohort study. J Minim Invasive Gynecol. 2019;26(5):883–890.
- Hehenkamp WJ, Looman CW, Themmen AP, et al. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–4063.
- Sun Q-L, Wu X-H, Luo L, et al. Characteristics of women with mixed mass formation after evacuation following uterine artery chemoembolization for cesarean scar pregnancy. Arch Gynecol Obstet. 2018;297(4):1059–1066.
- Sun QL, Luo L, Gao CY, et al. Scoring system for the prediction of the successful treatment modality in women with cesarean scar pregnancy. Int J Gynaecol Obstet. 2019;146(3):289–295.
- Silver RM. Delivery after previous cesarean: long-term maternal outcomes. Semin Perinatol. 2010;34(4):258–266.
- Zhuang Y, Huang L. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted within a cesarean scar. Am J Obstet Gynecol. 2009;201(152):e1–3.
- Shen L, Tan A, Zhu H, et al. Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(5):386.e1–386.e6.
- Hehenkamp WJ, Volkers NA, Broekmans FJ, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22(7):1996–2005.
- Karlsen K, Hrobjartsson A, Korsholm M, et al. Fertility after uterine artery embolization of fibroids: a systematic review. Arch Gynecol Obstet. 2018;297(1):13–25.
- Tempest N, Hapangama D. Should we be putting our scalpels down? Is HIFU the answer to fertility-sparing fibroid treatment? BJOG. 2018;125(3):366.
- Zhang C, Jacobson H, Ngobese ZE, et al. Efficacy and safety of ultrasound-guided high intensity focused ultrasound ablation of symptomatic uterine fibroids in black women: a preliminary study. BJOG. 2017;124(Suppl 3):12–17.
- Huang L, Du Y, Zhao C. High-intensity focused ultrasound combined with dilatation and curettage for cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2014;43(1):98–101.
- Zhu X, Deng X, Xiao S, et al. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. Int J Hyperthermia. 2016;32(2):144–150.
- Zhang C, Zhang Y, He J, et al. Outcomes of subsequent pregnancies in patients following treatment of cesarean scar pregnancy with high intensity focused ultrasound followed by ultrasound-guided dilation and curettage. Int J Hyperthermia. 2019;36(1):926–931.
- van Rooij IA, Broekmans FJ, Te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071.
- van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–987.
- Lee JS, Hong GY, Lee KH, et al. Changes in anti-müllerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high-intensity focused ultrasound treatment of adenomyosis and uterine fibroid. BJOG. 2017;124(Suppl 3):18–22.
- Qu K, Mao S, Li J, et al. The impact of ultrasound-guided high-intensity focused ultrasound for uterine fibroids on ovarian reserve. Int J Hyperthermia. 2020;37(1):399–403.
- Ouyang Z, Liu P, Yu Y, et al. Role of ovarian artery-to-uterine artery anastomoses in uterine artery embolization: initial anatomic and radiologic studies. Surg Radiol Anat. 2012;34(8):737–741.