Abstract
Purpose
Recommended treatments for muscle-invasive bladder cancer (MIBC) come with considerable morbidity. Hyperthermia (HT) triggered drug release from phosphatidylglycerol-based thermosensitive liposomes (DPPG2-TSL) might prevent surgical bladder removal and toxicity from systemic chemotherapy. We aimed to assess the efficacy of DPPG2-TSL with HT in a syngeneic orthotopic rat urothelial carcinoma model.
Methods
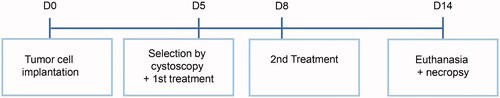
A total of 191 female Fischer F344 rats were used. Bladder tumors were initiated by inoculation of AY-27 cells and tumor-bearing rats were selected with cystoscopy and semi-randomized over treatment groups. On days 5 and 8, animals were treated with DOX in different treatment modalities: intravenous (iv) DPPG2-TSL-DOX with HT, iv free DOX without HT, intravesical DOX without HT, intravesical DOX with HT or no treatment (control group), respectively. Animals were euthanized on day 14 and complete tumor response was assessed by histopathological evaluation.
Results
Iv DPPG2-TSL-DOX + HT resulted in a favorable rate of animals with complete tumor response (70%), compared to iv free DOX (18%, p = .02), no treatment (0%, p = .001), and intravesical DOX with (43%, p = .35) or without HT (50%, p = .41). All rats receiving intravesical DOX with HT and 24% of rats treated with DPPG2-TSL-DOX containing the same DOX dose with HT had to be euthanized before day 14 because of substantial bodyweight loss, which was associated with dilated ureters urine retention in a few rats.
Conclusion
Treatment with DPPG2-TSL-DOX combined with intravesical HT outperformed systemic and intravesical DOX in vivo. There might be a role for DPPG2-TSL encapsulating chemotherapeutics in the treatment of MIBC in the future.
Introduction
Bladder cancer is the fifth most commonly diagnosed cancer in the United States and Europe and represents substantial health, economic and social burden [Citation1]. The predominant histologic type of bladder cancer is urothelial carcinoma (UC) [Citation2]. Muscle-invasive bladder cancer (MIBC) represents approximately 25% of new bladder cancer patient cases, and the remaining 75% non-muscle invasive bladder cancer (NMIBC) patients have a probability of progression to muscle-invasive disease up to 20% in five years [Citation3].
Current treatment modalities for MIBC are associated with high morbidity. The golden standard for stage T2-T4a, a radical cystectomy (RC) with neoadjuvant chemotherapy (NAC) [Citation4] is associated with 90-days complication and mortality rates up to 58% and 7% [Citation5,Citation6]. Patients with advanced age (representing the majority of the MIBC population) are therefore frequently medically unfit or unwilling to undergo RC, causing a risk of undertreatment [Citation7]. This key issue in MIBC management highlights the need for bladder-sparing treatments. ‘Multimodality treatment’ (MMT) involves a transurethral resection of the bladder tumor and subsequent intravenous (iv) chemotherapy with radiotherapy but is also associated with grade 3 and 4 adverse events in 36% of patients [Citation8]. While intravesical chemotherapeutic installations are reserved for NMBIC, the main obstacles of systemic chemotherapy include poor delivery to tumor tissue and systemic toxicity [Citation9]. For doxorubicin (DOX), for example, these adverse effects include heart failure, myelosuppression, and mucosal toxicity [Citation10]. To improve bladder-sparing treatment of MIBC, prevention of systemic toxicity is imperative. Liposome-based chemotherapeutic drug delivery systems which are currently in clinical use have shown a favorable toxicity profile compared to conventional chemotherapy (with unencapsulated drug), but a similar therapeutic effect. Doxil, a well-established liposomal formulation encapsulating DOX, achieves nanoparticle accumulation in the tumor by the enhanced permeability and retention (EPR) effect [Citation11], exploiting the tumor angiogenesis, vascular permeability, and poor lymphatic drainage. Clinical trials have shown that Doxil has a favorable toxicity profile, including reduced heart failure. However, Doxil fails to show improved cancer outcomes compared to systemic free DOX [Citation12–16], possibly by the passive drug release from nanoparticles [Citation17,Citation18].
Thermosensitive liposomes (TSL) might increase efficacy by the local intravascular release of encapsulated drug after being triggered by an external stimulus, i.e., local hyperthermia (HT), leading to a high local concentration of bioavailable drug [Citation19,Citation20]. Additionally, HT (42 ± 2 °C) can boost the effect of chemotherapy because it increases tissue penetration and induces an anti-tumor immune response [Citation21–23]. As such, this TSL is a promising novel cancer treatment modality. A TSL formulation (LTSL, lysolipid thermally sensitive liposome, Thermodox) encapsulating DOX has been studied in combination with local HT in a pig bladder model and has shown increased bladder DOX concentrations, compared to iv free DOX administration [Citation24]. However, when Thermodox, the only TSL-system studied in clinical trials so far, was added to radiofrequency ablation of hepatocellular carcinoma in a phase III trial, PFS and OS were not prolonged [Citation25]. Longer heating appeared to increase tissue concentration of DOX leading to longer OS in a subgroup of patients, demonstrating that therapeutic benefit can be reached, provided adequate heating and heating duration can be achieved. Moreover, TSL stability and pharmacokinetic behavior are important parameters.
A recently developed TSL-system using 1,2-distearoyl-sn-glycero-3-phospho-diglycerol as lipid excipient (DPPG2-TSL) showed promising results in the treatment of solid tumors in cats when encapsulating DOX (DPPG2-TSL-DOX) and in combination with regional hyperthermia [Citation26]. Additionally, iv administration of DPPG2-TSL-DOX combined with bladder HT in pigs recently demonstrated that this lead to significantly higher DOX levels and improved DOX distribution in bladder wall tissue compared to free iv and intravesical DOX treatment, while DOX accumulation in the heart and kidneys were lower with DPPG2-TSL-DOX compared to iv DOX [Citation27]. The drug release from DPPG2-TSL in the deeper bladder tissue area, where MIBC resides, suggests that iv treatment with DPPG2-TSL encapsulating chemotherapeutic agent(s) combined with intravesical HT may contribute to a novel treatment approach as a bladder-sparing treatment for MIBC.
The aim of this study was to assess the efficacy of DPPG2-TSL-DOX combined with intravesical HT, compared to free iv and intravesical DOX, in a syngeneic orthotopic rat UC model.
Materials and methods
Ethics statement and general animal conditions
Study design and animal procedures were approved by the national Central Animal Experiments Committee (CCD) and the local Animal Welfare Body (IvD) under the number 2019-0007 and were in compliance with the European Union Directive 2010/63/EU and the Declaration of Helsinki. Experiments were performed at the animal facility of the Radboud University Medical Center, Nijmegen, The Netherlands.
A total of 191 female Fischer F344 rats, 10–12 weeks old and weighing on average 160.0 ± 8.6 (SD) grams were purchased from Charles River Laboratories (Sulzfeld, Germany): 28 for the pilot study, 80 for the dose-finding study, and 83 rats for the comparative therapeutic study. Three to four rats were housed together in standard temperature and lighting conditions with free access to food and water. Rat well-being was monitored daily, with special emphasis on weight loss, self-grooming, urine retention, and hematuria. If predetermined humane endpoints (HEP) were reached, e.g., body weight loss of more than 15%, animals were euthanized using CO2 inhalation.
The day before experimental animal procedures (tumor establishment, cystoscopy, and (intravesical) treatments), 0.1 mg/ml enrofloxacin was dissolved in the drinking water of all rats. All animal procedures were performed under inhalation anesthesia with 2–5% isoflurane in medical air (1 l/min O2). During anesthesia, heart rate and blood oxygen saturation were monitored and maintained by adjusting isoflurane concentration or flow. The body temperature was monitored during anesthesia using a digital infrared ear thermometer and was maintained at 36–37.5 °C by adjusting the temperature of the heat mat when necessary.
Blood samples (from all rats in the comparative study at day 5 and 12; and all rats in the dose-finding study at day 5 but a subset of rats on days 6, 7, and 8) were collected and hemoglobin, leukocytes, and thrombocytes were determined to assess myelosuppression, a common side effect of iv DOX [Citation10].
Tumor establishment
Since the experiments were technically challenging and intravesical HT had not been performed in rats at our department before, a pilot experiment was performed in 28 rats to achieve reproducible tumor formation and to test the study set-up with bladder hyperthermia using the precision pumps (results not shown).
Tumor cell line
The AY-27 rat UC cell line was previously established and was derived from a bladder tumor of N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide (FANFT) fed Fischer F344 rats. AY-27 cells were kindly provided by Dr. Ronald Moore (the University of Alberta and Cross Cancer Institute, Edmonton, Alberta, Canada). Cells with passage 23 were cultured in Roswell Park Memorial Institute 1640 medium with L-glutamine (Invitrogen, Carlsbad, California, USA), supplemented with 10% fetal calf serum (Sigma-Aldrich, St. Louis, Missouri, USA), 1% penicillin/streptomycin (Invitrogen, Carlsbad, California, USA). Cells were cultured in a humidified atmosphere at 37 °C and 5% CO2. Cells were passaged six times to revitalize the cells before tumor cell inoculation.
Tumor cell implantation
The design of this study is depicted in . Orthotopic tumor establishment was performed following a method described by Xiao et al. [Citation28]. The bladder was catheterized under anesthesia with a 17-Gauge (1.4 mm) Venflon plastic cannula (BD Biosystems, Erembodegem-Aalst, Belgium) and urine was drained. A solution of 0.4 ml 0.1 M hydrogen chloride (HCL) was inserted via the cannula to damage the bladder urothelium and after 15 s neutralized by the addition of 0.4 ml 0.1 M potassium hydroxide. The bladder content was drained and the bladder wall was rinsed by three 0.8 ml phosphate-buffered saline (PBS, pH 7.4) instillations. Thereafter, an instillation of 1.5 × 106 freshly harvested isogenic AY-27 cells suspended in 0.5 ml complete medium (described in the previous section) was inserted via the plastic cannula to initiate orthotopic bladder tumors. The cannula was capped and the cells remained in the bladder for 60 min. Rats were rotated 90° every 15 min to optimize exposure of the damaged bladder urothelium to the cells. Then, the cannula was removed and rats were allowed to void.
Selection of tumor-bearing rats by cystoscopy
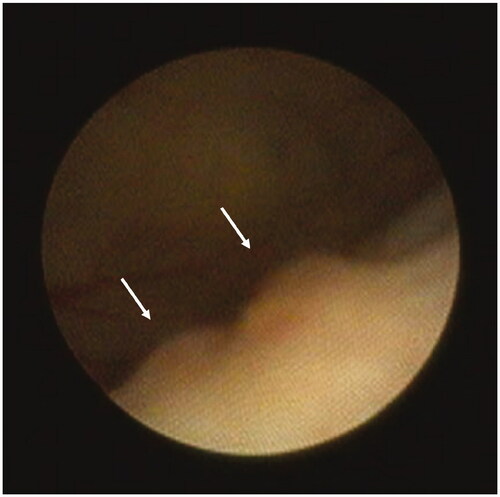
For the selection of tumor-bearing rats, macroscopic tumor formation was assessed by cystoscopy prior to treatment on day 5. A semi-flexible scope (Karl Storz, Tuttlingen, Germany) with a 0° optic, a diameter of 1.1 mm, a working length of 10 cm, and an irrigation channel was used. After the cystoscope was inserted, the bladder was distended with water for injection and the bladder urothelium was inspected systematically. Images were captured with Telepack Vet X (Karl Storz, Tuttlingen, Germany). Tumor burden was roughly estimated by cystoscopic imaging (according to tumor number and size). Only rats with detected bladder tumors were used for this study, which was randomly allocated to treatment groups. Non-tumor-bearing animals were euthanized. In post-treatment analysis, we verified that the tumor burden of the rats based on cystoscopic imaging was evenly distributed over treatment.
Study agents
DPPG2-TSL-DOX with a DOX concentration of 1.8 mg/ml, a mean particle size (z average) of ∼120 nm, and a polydispersity index of <0.10 was provided by Thermosome GmbH. Doxorubicin HCl solution (2 mg/ml,) was purchased from Accord Healthcare BV.
Experimental groups
Because it was unknown whether dose-limiting toxicity would occur and to study DPPG2-TSL-DOX dose-response we first conducted a dose-finding study. Rats received 0.5, 1, 2, or 4 mg/kg iv DPPG2-TSL-DOX combined with intravesical HT. One group received intravesical HT only, to assess the influence of HT and another control group was included which did not receive any treatment. We defined the optimal dose as the highest dose that is expected to be tolerated without leading to life-threatening toxicity during the study. Only the first batch of tumor-bearing rats of the dose-finding study was directly allocated to treatment with 0.5 and 1 mg/kg DPPG2-TSL-DOX with HT (not randomized), all rats from subsequent batches (including for the comparative therapeutic study) were randomly allocated to treatment groups.
The comparative therapeutic study was performed, after deciding to use the dose 2 mg/kg DOX in different treatment modalities (see results section). The five experimental groups consisted of 1. control group (no treatment), 2. iv DPPG2-TSL-DOX with HT, 3. iv free DOX without HT, 4. intravesical DOX without HT, and 5. intravesical DOX with HT. In compliance with the 3 R guideline, we did not include a group subjected to iv DPPG2-TSL-DOX without HT as this has shown to result in negligible DOX release in a previous report [Citation27,Citation29].
Treatment
Animal procedures
Rats from all treatment groups were treated twice, at day 5 and day 8, and were treated per 2–5 depending on the group size. Rats treated with iv medication received a 17-Gauge (1.4 mm) iv Venflon plastic cannula (BD Biosystems, Erembodegem-Aalst, Belgium) in the tail vein before treatment. Bladders of rats that received intravesical HT were catheterized with an 18-Gauge (1.2 mm; outflow) and a 24-Gauge (0.6 mm; inflow) Venflon plastic cannula. Intravesical DOX (without HT) treated animals received one 17-Gauge transurethral plastic cannula.
Iv medication was dissolved in PBS to reach a total injection volume of 0.4 ml. Animals receiving DPPG2-TSL-DOX and HT were administrated 0.5–4 mg/kg (dose-response study) or 2 mg/kg DPPG2-TSL-DOX (group 2 of the comparative study) via the iv cannula, as soon as the bladder temperature was stable at 41 °C (see next section for the heating protocol). Rats of other groups received 2 mg/kg iv free DOX via the tail cannula (group 3) or an intravesical instillation of 2 mg/kg DOX dissolved in 1 ml water for injection via the transurethral cannula (group 4). Rats treated with intravesical DOX with HT (group 5) received intravesical instillation with a similar concentration DOX as group 4 (of 2 mg/kg/ml DOX) dissolved in water for injection, but via continuous circulation in the bladder to achieve HT. All rats receiving intravesical treatment were rotated 90° every 15 min to optimize intravesical HT and/or DOX exposure.
After treatment, all cannulas were removed and rats were allowed to void freely. Rats in the control groups (group 1, and control group of dose-finding experiments) were not subjected to treatment.
Experimental heating protocol
Sixty minutes of hyperthermia was achieved by conductive heat exchange, by circulating water for injection (group 2) or DOX solution (group 5) from a warm water bath in the bladder. Precision pumps were used to pump the warm water or DOX solution from the water bath (at 60 °C) via tubing and the transurethral in-flow cannula into the bladder. The bladder content passively leaked out the bladder via the outflow cannula into a container, and the fluid ran back through the tubing into the water bath. Thermometry was carried out using a noninvasive T-type temperature probe of 0.6 mm in diameter inserted in the outflow cannula connected to a Keithley Multiplexer device (CN Rood, Zoetermeer, The Netherlands). The pump speed was adjusted to maintain a bladder temperature of 41 °C (mean temperature at the distal tip of the outflow cannula [about 1.5 cm away from the urethra/bladder] was 40.2 ± 1.4 °C) and ranged from 10 to 15 ml/min.
Necropsy and histopathology
Euthanasia was performed 14 days after tumor inoculation by asphyxiation by CO2. Organs were globally inspected for metastases and the bladder was harvested for histological evaluation. Bladders were weighed, fixed in 3.7% formaldehyde, and embedded in paraffin. Sections were cut at 4–5 μm thickness prior to standard hematoxylin and eosin (HE) staining. Histopathology was performed by two observers (with proper blinding of MS, dedicated genitourinary pathologist, and ISGB), including assessment of tumor stage according to the 2009 TNM classification and tumor grade according to the 1973 and 2004/2016 WHO classifications. The thickness of the bladder wall was measured (>1 mm distance from any tumor) and the presence of inflammation and atypia was assessed.
Statistical analysis
The complete tumor response rate was defined as the percentage of tumor-bearing rats (selected with cystoscopy at day 5) that were tumor-free (T0 at histopathological evaluation of the bladder and no macroscopic metastases at necropsy) after two treatment cycles. We were interested in the comparisons between iv DPPG2-TSL-DOX combined with HT (group 2) and the other four groups. Differences incomplete tumor response rates between groups were compared with a Chi-square test. Moreover, the proportion of tumor stage (T0, T1, T2) between groups were compared using a Mann-Whitney U test. In all analyses, we corrected for multiple comparisons with the posthoc Bonferroni Holm method. Analysis was performed using SPSS (Version 25, IBM), with a two-sided p < .05 considered statistically significant.
Results
Selection of tumor-bearing rats prior to treatment
Cystoscopy revealed tumors in 70.0% (56/80) of the rats at day 5 in the dose-finding study, and 65.0% (54/83) in the comparative study. Tumor-bearing rats had typically 1–3 tumors with a diameter of 1.0–2.5 mm. A representative cystoscopy image of bladder tumors is depicted in . No signs of a urine tract infection were detected in any rat.
Macroscopic evaluation following treatment
At necropsy, tumor-bearing rats showed bladder tumors that were confined to the bladder (thus without extravesical growth), and no distant metastases were observed in any of the rats. In some of the rats treated with intravesical DOX combined with HT (n = 3, from the comparative study) and iv DPPG2-TSL-DOX combined with HT (n = 4, dose 2 mg/kg from the dose-finding study), dilated ureters were observed (Supplementary Figure 1(A)). After removing and opening the bladder, considerable edema of all (n = 7) bladders treated with intravesical DOX combined with HT was seen, and of a subset of bladders treated with DPPG2-TSL-DOX combined with HT (n = 4 in the comparative study, and n = 13 in the dose-finding study [of which n = 9 were treated with dose 4 mg/kg, n = 2 with 2 mg/kg and n = 2 with 1 mg/kg]). Histological images of bladder edema are presented in Supplementary Figure 1(B,C). Other internal organs showed no abnormalities at inspection.
Histopathology following treatment
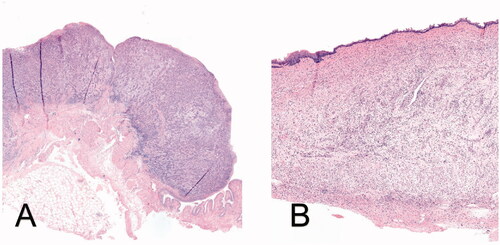
Histopathology revealed that all tumors (unresponsive to treatment or from rats in the control group), were high-grade UC with stage T1 or T2 (). Without treatment, 85% of the rats had stage T2 (MIBC) at day 14. No T3 or T4 bladder tumors were detected in any of the rats. The therapeutic effect derived from histopathology is discussed in the section below. Mild to moderate inflammation with edema was observed in rats that received intravesical DOX with HT or DPPG2-TSL-DOX with HT, which was more pronounced in the intravesical DOX with HT group compared to iv DPPG2-TSL-DOX with HT. Focally, the inflammatory response was further increased, showing mixed inflammatory infiltrates and occasional fibrosis, possibly indicating prior tumor localization (). Epithelial atypia, considered reactive to intravesical treatment or pre-conditioning, was seen in almost all rat bladders, including those in the control group. Squamous metaplasia and hyperplasia, was observed occasionally and randomly divided over groups. Tumor-related necrosis and ulcers were often observed. Mean bladder wall thickness was 1.0 mm (SD 0.4) and mean tumor invasion was 1.1 mm (SD 0.8).
Figure 3. (A) HE-staining (50× magnification) showing high-grade urothelial carcinoma invading the muscularis propria (T2) in a rat of the control group; (B) HE-staining (40× magnification) showing edema and inflammatory cells in the bladder submucosa in a rat of DPPG2-TSL-DOX with HT group, indicating possible prior tumor localization.
Therapeutic effect
Dose-finding study
Histopathology showed that treatment with 1, 2, and 4 mg/kg iv DPPG2-TSL-DOX combined with HT yielded a complete tumor response in 70–75% of the rats (). In contrast, treatment with the lowest DPPG2-TSL-DOX dose resulted in a complete tumor response rate of 50%. When rats were treated with HT alone, the complete tumor response rate was only 10%. All rats in the control group had tumors at day 14. Tumor stage proportion (T0/T1/T2) was favorable in the treatment groups that received 2 and 4 mg/kg iv DPPG2-TSL-DOX combined with HT: in both groups, no rats had T2 tumor, and only three rats had T1 tumor.
Table 1. Therapeutic effect and reached humane endpoints in the dose-response study.
Four out of eleven rats (36%) and two out of 11 rats (18%) treated with 4 mg/kg or 2 mg/kg iv DPPG2-TSL-DOX with HT, respectively, reached HEP before day 14 (e.g., weight loss >15%). Because the bodyweight of these two rats was low at study onset (144 and 146 gram), this presumably largely contributed to the observed toxicity. For this reason in combination with favorable tumor stage proportion, we defined that the dose of DPPG2-TSL-DOX that should be used for rats with normal body weight was 2 mg/kg. The specific side effects from treatment are described in the animal well-being section.
Comparative therapeutic study
The therapeutic effect per treatment group based on histopathology is shown in and . Complete tumor response rate in the iv DPPG2-TSL-DOX combined with HT group was significantly higher compared to the iv-free DOX group (70% vs. 18.2%, p = .02) and the control group (70% vs. 0%, p = .001). The proportion of tumor stage (T0/T1/T2), was also significantly better in the DPPG2-TSL-DOX iv with HT group, compared to iv free DOX without HT and the control group (p = .01 and p = .001, respectively; ). Complete tumor response rate and tumor stage proportion appeared moderately better in rats treated with iv DPPG2-TSL-DOX with HT compared to intravesical DOX (with and without HT), but this difference was not significant (p = .35 and p = .41, respectively).
Figure 4. Percentage of rats with tumor after treatment per group, including the proportion of tumor stage as determined by histopathological evaluation. Green-colored parts of bars represent T1 tumors and purple T2. The oblique p-value responds to the difference between groups in tumor proportion (T0/T1/T2) as determined by the Mann-Whitney U test and the straight p-value to the difference in complete tumor response (tumor yes/no) as determined by the chi-square test.
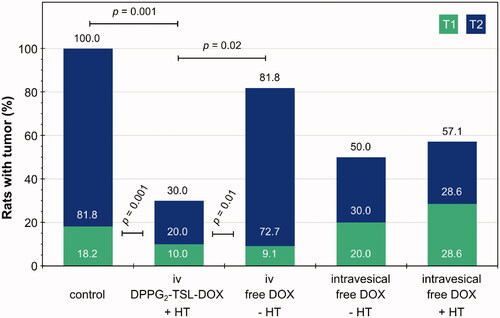
Table 2. Therapeutic effect and reached humane endpoints in the comparative study.
Because five rats in the intravesical DOX with HT group had not received two treatments due to technical difficulties during treatment, this group contained only seven rats. These problems included: inability to reach an intravesical temperature of 40–41 °C (two rats), inability to place a cannula in the bladder (at necropsy we observed a stiff bladder with extended edema; two rats), and death from too high water pressure from the pump into the bladder (at necropsy black kidneys with blood cloths were observed; one rat).
We performed a sub-analyses including only rats that survived the pre-planned study design of 14 days, thus excluding the rats that reached HEP before day 14 (i.e., the three rats treated with iv DPPG2-TSL-DOX combined with HT group, and all seven rats treated with intravesical DOX without HT; see animal well-being section). This sub-analysis showed that the complete tumor response rate in the group treated with iv DPPG2-TSL-DOX combined with HT remained significantly higher compared to the iv free DOX group (85.7% vs. 18.2%, p = .01) and the control group (85.7% vs. 0%, p < .001). Also, tumor stage proportion (T0/T1/T2) DPPG2-TSL-DOX iv with HT group was significantly better compared to iv free DOX without HT and the control group (p = .01 and p = .001, respectively) in this sub-analysis. There was no significant difference between iv DPPG2-TSL-DOX combined with HT and intravesical DOX without HT regarding complete response or tumor stage proportion.
Animal well-being
In the dose-finding study, 6/38 rats were treated with different doses of DPPG2-TSL-DOX (2/11 with dose 2 mg/kg and 4/11 with dose 4 mg/kg) in combination with HT reached HEP before day 14 (; on day 10–12). Another 3/10 rats treated with DPPG2-TSL-DOX and HT in the comparative study reached HEP before the end of the study (; on day 10–12). All of these rats lost over 15% of their body weight (which we defined as a HEP) and exhibited less self-grooming. From these rats, four rats had dilated ureters at necropsy, one rat had urine retention and in one rat cloudy and mucous urine was observed.
All seven rats treated with intravesical DOX in the comparative study reached HEP before the end of the study (on day 9–12), because of body weight loss of more than 15% and poor self-grooming. Three (out of seven) rats had dilated ureters at necropsy, two rats showed urine retention, and one rat had hematuria. In the other rats, no side effects or abnormalities at necropsy were observed.
Serum diagnostics showed that thrombocytes were elevated at day 12 compared to day 5 in the comparative study in the iv-free DOX group, DPPG2-TSL-DOX with HT group, and to a smaller extent the intravesical DOX with HT group (an increase of 202%, 223%, and 136%, respectively). In the dose-finding study, thrombocytes were slightly elevated in the 2 mg/kg DPPG2-TSL-DOX with HT group at day 8 compared to day 5 (122%), but not in the 4 mg/kg group. Hemoglobin and leukocytes were within the normal range in all rats.
Discussion
The aim of this study was to assess the efficacy of DPPG2-TSL-DOX combined with intravesical HT, compared to free iv and intravesical DOX, in a syngeneic orthotopic rat UC model. Tumor-bearing rats were selected with cystoscopic imaging and randomized over treatment groups. The dose-finding study showed high response rates and limited toxicity at 2 mg/kg DPPG2-TSL-DOX, and this dose was therefore further explored in the comparative therapeutic study. In the latter study, we show that treatment with DPPG2-TSL-DOX with HT resulted in the highest number of animals with complete tumor response (70%), which was significantly better than iv free DOX (18%, p = .02) and no treatment (0%, p = .001), and moderately better than intravesical DOX with or without HT (43% vs. 50%, respectively, not significant). Also, tumor stage proportion (T0/T1/T2) was significantly better in the group treated with DPPG2-TSL-DOX plus HT compared to iv-free DOX and no-treatment groups.
The imposing complete tumor response rate from DPPG2-TSL-DOX with HT can be explained by the mechanism of action of DPPG2-TSL with intravascular drug release after being triggered by heat, causing high local drug concentrations [Citation19,Citation20]. The great difference in efficacy compared to iv-free DOX is supported by a recently published study in pigs, in which application of DPPG2-TSL-DOX with intravesical HT led to a 7.5-fold higher DOX concentration in the bladder muscle layer, compared to iv free DOX [Citation27]. HT might also contribute to the high response rate observed, since it may work synergistically with chemotherapeutic agents and can promote an immune response [Citation30].
Compared to intravesical DOX (with or without HT), DPPG2-TSL-DOX with HT performed only marginally better (, ). This was explained by additional experiments which showed that bladder mucosa DOX levels were similar in non-tumor bearing animals subjected to DPPG2-TSL-DOX with HT or intravesical free DOX without HT, and detrusor (muscle) DOX levels were slightly higher after DPPG2-TSL-DOX with HT, compared to intravesical DOX (results not shown) [Citation31]. However, the mean bladder wall thickness of rats is only 1.0 mm, likely to be thin enough for sufficient drug penetration and consequential efficacy from intravesical drug instillation, whereas the thickness of the human bladder wall ranges between 3 and 5 mm [Citation32]. A clinical study showed that, after intravesical DOX instillation, only 4% of the DOX concentration measured in the superficial bladder wall reaches the deeper bladder detrusor [Citation33]. Also in pigs, with bladder wall thickness similar to humans, DOX was only visualized in the most superficial 1 mm of the bladder wall after intravesical free DOX application, whereas more homogeneous DOX distribution was observed in the bladder wall after DPPG2-TSL-DOX with HT [Citation27]. Drug penetration from intravesical drug application might even be poorer in larger solid MIBC tumors, presumably resulting in dissatisfactory efficacy in human MIBC.
We chose this syngeneic orthotopic rat UC model to resemble human MIBC as much as possible. Importantly, these rats possess an intact immune system, taking into account the supposed anti-tumor immunostimulatory effect from HT [Citation30,Citation34] and DOX [Citation35]. Moreover, it is feasible to perform cystoscopy for assessment of tumor establishment and bladder instillations via a urinary cannula in female rats. High-grade invasive bladder tumors (≥T1) develop directly from three days after tumor cell inoculation on, likely because the bladder urothelium is damaged due to bladder preconditioning with HCL [Citation36,Citation37]. Histopathological examination and immunochemistry confirmed that tumors maintained features of UC. Despite that previous studies with the same tumor model report primarily stage T2 and T3 bladder tumors in rats that did not receive treatment 14 days after tumor cell inoculation [Citation38,Citation39], the current study reports only (15%) T1 and (85%) T2 bladder tumors in the control group. In humans, T2-T4 bladder tumors are generally found on the lateral (39%) or posterior wall (19%) [Citation40] and T2 tumors have a median tumor size of 3.0 cm [Citation41]. Despite obvious differences between rats and humans, we believe that the application of DPPG2-TSL with HT has potential for MIBC T2-T4a because the working mechanism of the nanocarriers is exploiting heat-triggered vascular drug release [Citation42]. Up to date, experience with HT for MIBC only exists using the deep regional HT method (only with very small sample sizes) [Citation43,Citation44], not yet with intravesical HT. Which HT method is most suitable for (homogeneous) heating of MIBC in combination with DPPG2-TSL remains to be elucidated.
Animal well-being was most impaired by treatment with intravesical DOX with HT (7/7 reached HEP), followed by DPPG2-TSL-DOX with HT (9/48 reached HEP). All rats that reached HEP had over 15% body weight loss and practiced less self-grooming, both general signs of illness. From these rats, a substantial part had dilated ureters at necropsy and/or urine retention. Moreover, both treatments caused considerable bladder edema and inflammation, as observed by necropsy and histopathological evaluation. Since no toxicity was observed in rats treated with intravesical DOX without HT or with HT only, we hypothesize that DOX at higher doses (2 mg/kg or more) in combination with HT caused a severe inflammatory response, resulting in edema of the bladder wall. These local effects may in turn lead to urine retention and/or ureteral obstruction at bladder level and cause kidney dysfunction [Citation45], explaining the symptoms of illness. Clinical studies investigating treatment with instillations of chemotherapeutic agents combined with local bladder HT in NMIBC patients show that this treatment is safe and severe adverse events are rare [Citation46,Citation47]. Moreover, no urine retention has been reported. In retrospect, using 1 mg/kg DOX for the comparative study, half of the ultimately used dose DPPG2-TSL-DOX, might have been a better choice since it resulted in similar efficacy in the dose-finding study without leading to animal sacrifice due to animals reaching a defined HEP before day 14. However, because the proportion of tumor stage (T0/T1/T2) was somewhat better and two rats treated with 2 mg/kg that reached HEP had very low body weight at study onset, we envisioned that that dose would have the greatest effect and be safe for animals with normal body weight at that time.
Regarding systemic toxicity, myelosuppression was excluded by blood analysis which showed that hemoglobin, leukocytes, and thrombocytes were not reduced in any rat. The thrombocytosis detected in the iv DOX, DPPG2-TSL-DOX with HT, and intravesical DOX with HT groups was likely chemotherapy-related [Citation48], or from sterile inflammation from HT or other experimental procedures [Citation49]. Whether toxicity was due to the interaction of liposomes with the MPS (macrophages in liver and spleen) and renal system [Citation18,Citation50] was not assessed by blood diagnostics, but no abnormalities of these organs were observed at necropsy. Importantly, no infusion reaction was observed in rats treated with DPPG2-TSL-DOX, which is a notorious adverse event observed in the application of liposomal formulations in pigs and less severe and frequent in humans [Citation51].
Drug delivery systems have not extensively been studied in the treatment of bladder cancer. Panteliadou et al. reported a significant overall survival benefit in MIBC patients when Doxil was added to an aggressive radiotherapy scheme, with two-year overall survival rates of 58.7% vs. 86.5%, p = .04, respectively [Citation52]. However, whether Doxil or other liposomal formulations are superior to systemic free chemotherapy in the treatment of MIBC patients is unknown. Currently, a Chinese study group is conducting a phase II trial in MIBC patients whom are treated with PD-1 with or without ‘pegylated liposomal doxorubicin’ (clinicaltrials.gov, NCT04101812). However, it is unclear which liposomal formulation is used.
The results from in vivo studies with DPPG2-TSL-DOX with HT might compare favorably to those with Doxil and Thermodox, although a head-to-head comparison would be more conclusive. Iv administration of free DOX was much more effective in reducing tumor volume than Doxil in a bladder cancer mouse model (70% vs. 33%, respectively) [Citation53], whereas we report significantly better complete tumor response rates from DPPG2-TSL-DOX with HT compared to systemic free DOX (70% vs. 18%, respectively). Comparison of DPPG2-TSL-DOX with Thermodox, both investigated in combination with bladder HT in pigs [Citation24,Citation27], showed that a ∼1.5-fold higher dose of DPPG2-TSL-DOX resulted in ∼3-fold higher bladder wall DOX levels. The dissimilar DOX release might be based on differences in phospholipid composition of the liposome systems [Citation19] as well as the heating method. Although these studies were performed in healthy pigs without bladder tumors, this observation supports the hypothesis that iv DPPG2-TSL-DOX with HT may have the potential to outperform the treatment outcome of Thermodox with HT.
While the DPPG2-based TSL-system might have the potential to overcome limitations of current drug delivery systems encapsulating chemotherapeutic agents, DOX is not the ideal chemotherapeutic agent for the treatment of MIBC. Neoadjuvant cisplatin-based combination chemotherapy results in the greatest therapeutic benefit in MIBC patients compared to other (combinations of) chemotherapeutic agents [Citation54,Citation55], and is therefore recommended by urology guidelines [Citation4]. A PEGylated liposomal formulation encapsulating cisplatin showed promising results in a rat bladder cancer model [Citation56]. Future studies investigating DPPG2-based TSL-system in MIBC should focus on (a combination with) cisplatin.
Conclusion
Treatment with DPPG2-TSL-DOX combined with intravesical HT resulted in the highest number of rats with complete tumor response and the most effective downgrading, compared to systemic and intravesical DOX, in a syngeneic orthotopic rat UC model. There might be a role for DPPG2-TSL encapsulating chemotherapeutics as bladder-sparing treatment of MIBC in the future.
Ethics approval
The authors state that they have obtained approval of the study design and animal procedures by the national Central Animal Experiments Committee (CCD) and the local Animal Welfare Body (IvD) under the number 2019-0007. Animal procedures were in compliance with the European Union Directive 2010/63/EU and the Declaration of Helsinki.
Author contributions
ISGB: Conceptualization, Methodology, Formal analysis, Investigation, Writing Original Draft. MS: Methodology, Investigation, Writing – Review & Editing. LHL: Conceptualization, Writing – Review & Editing. SK: Conceptualization, Methodology, Investigation, Writing – Review & Editing. SdJ: Methodology, Investigation, Writing – Review & Editing. MH: Conceptualization, Writing – Review & Editing. JAW: Supervision, Conceptualization, Methodology, Writing – Review & Editing. EO: Supervision, Conceptualization, Methodology, Writing – Review & Editing.
Supplemental Material
Download PDF (133.4 KB)Acknowledgments
We would like to thank Bianca Lemmers-van de Weem, Kitty Lemmens-Hermans, Karin Haas-Cremers and Irith Bergsma for their support during the experiment implementation, and Mirjam de Weijert and Kees Jansen for the biotechnical support. We would also like to thank Gerard van Ooijen for technical support with the thermocouples, Steven Teerenstra for the statistical support, and Pascal Schweizer for the warm contact with Thermosome GmbH.
Disclosure statement
LHL and MH hold shares in Thermosome GmbH. The other authors have no relevant financial or non-financial interests to disclose.
Data availability statement
Protocols and data are available at the request of the authors.
Additional information
Funding
References
- GLOBOCAN estimated cancer incidence, mortality, and prevalence worldwide in 2018; [cited 2020 August 26]. Available from: http://globocan.iarc.fr
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388(10061):2796–2810.
- Cambier S, Sylvester RJ, Collette L, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in Non-Muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette-Guérin. Eur Urol. 2016;69(1):60–69.
- Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104.
- Hautmann RE, de Petriconi RC, Volkmer BG. Lessons learned from 1,000 neobladders: the 90-day complication rate. J Urol. 2010;184(3):990–994; quiz 1235.
- Nielsen ME, Mallin K, Weaver MA, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the national cancer data base. BJU Int. 2014;114(1):46–55.
- Parker WP, Smelser W, Lee EK, et al. Utilization and outcomes of radical cystectomy for high-grade non-muscle-invasive bladder cancer in elderly patients. Clin Genitourin Cancer. 2017;16(1):e79–e97.
- James ND, Hussain SA, Hall E, BC2001 Investigators, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–1488.
- Senapati S, Mahanta AK, Kumar S, et al. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7.
- Doxorubicin (Farmacotherapeutisch Kompas); [cited 2018 December]. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/d/doxorubicine
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.
- Bhowmik S, Bhowmick S, Maiti K, et al. Two multicenter phase I randomized trials to compare the bioequivalence and safety of a generic doxorubicin hydrochloride liposome injection with Doxil® or Caelyx® in advanced ovarian cancer. Cancer Chemother Pharmacol. 2018;82(3):521–532.
- O’Brien MER, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449.
- Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2001;37(7):870–877.
- Rifkin RM, Gregory SA, Mohrbacher A, et al. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: a phase III multicenter randomized trial. Cancer. 2006;106(4):848–858.
- Dimopoulos MA, Pouli A, Zervas K, Greek Myeloma Study Group, et al. Prospective randomized comparison of vincristine, doxorubicin and dexamethasone (VAD) administered as intravenous bolus injection and VAD with liposomal doxorubicin as first-line treatment in multiple myeloma. Ann Oncol. 2003;14(7):1039–1044.
- Golombek SK, May JN, Theek B, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38.
- Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014.
- Kneidl B, Peller M, Winter G, et al. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9:4387–4398.
- Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–5575.
- van der Heijden AG, Verhaegh G, Jansen CF, et al. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol. 2005;173(4):1375–1380.
- Hurwitz MD. Hyperthermia and immunotherapy: clinical opportunities. Int J Hyperthermia. 2019;36(sup1):4–9.
- van Valenberg FJP, van der Heijden AG, Lammers RJM, et al. Intravesical radiofrequency induced hyperthermia enhances mitomycin C accumulation in tumour tissue. Int J Hyperthermia. 2018;34(7):988–993.
- Mikhail AS, Negussie AH, Pritchard WF, et al. Lyso-thermosensitive liposomal doxorubicin for treatment of bladder cancer. Int J Hyperthermia. 2017;33(7):733–740.
- Tak WY, Lin SM, Wang Y, et al. Phase III HEAT study adding Lyso-Thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res. 2018;24(1):73–83.
- Zimmermann K, Hossann M, Hirschberger J, et al. A pilot trial of doxorubicin containing phosphatidylglycerol based thermosensitive liposomes in spontaneous feline soft tissue sarcoma. Int J Hyperthermia. 2017;33(2):178–190.
- Van Valenberg FJP, Brummelhuis ISG, Lindner LH, et al. DPPG2-Based thermosensitive liposomes with encapsulated doxorubicin combined with hyperthermia lead to higher doxorubicin concentrations in the bladder compared to conventional application in pigs: a rationale for the treatment of muscle-invasive bladder cancer. Int J Nanomedicine. 2021;(16):75–88.
- Xiao Z, McCallum TJ, Brown KM, et al. Characterization of a novel transplantable orthotopic rat bladder transitional cell tumour model. Br J Cancer. 1999;81(4):638–646.
- Guideline on the principles of regulatory acceptance of 3Rs (replacement, reduction, refinement) testing approaches; [cited 2018 September]. Available from: https://www.ema.europa.eu/en/regulatory-acceptance-3r-replacement-reduction-refinement-testing-approaches
- Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15(11):667–685.
- Limmer S. Zielgerichtete chemotherapie solider tumoren durch thermosensitive liposomen in kombination mit doxorubicin., Gemcitabin und Mitomycin C. Faculty of Veterinary Medicine: Ludwig-Maximilians-Universität München; 2014.
- Kim SH, Cho JY, Lee HJ, et al. Ultrasound of the urinary bladder, revisited. J Med Ultrasound. 2007;15(2):77–90.
- Wientjes MG, Badalament RA, Au JL. Penetration of intravesical doxorubicin in human bladders. Cancer Chemother Pharmacol. 1996;37(6):539–546.
- Arends TJ, Falke J, Lammers RJ, et al. Urinary cytokines in patients treated with intravesical mitomycin-C with and without hyperthermia. World J Urol. 2015;33(10):1411–1417.
- Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73.
- Hendricksen K, Molkenboer-Kuenen J, Oosterwijk E, et al. Evaluation of an orthotopic rat bladder urothelial cell carcinoma model by cystoscopy. BJU Int. 2008;101(7):889–893.
- Arentsen HC, Hendricksen K, Oosterwijk E, et al. Experimental rat bladder urothelial cell carcinoma models. World J Urol. 2009;27(3):313–317.
- Falke J, Parkkinen J, Vaahtera L, et al. Curcumin as treatment for bladder cancer: a preclinical study of Cyclodextrin-Curcumin complex and BCG as intravesical treatment in an orthotopic bladder cancer rat model. Biomed Res Int. 2018;2018:9634902.
- van Valenberg FJP, Strauss-Ayali D, Agmon-Gerstein Y, et al. Assessment of the efficacy of repeated instillations of mitomycin C mixed with a thermosensitive hydrogel in an orthotopic rat bladder cancer model. Ther Adv Urol. 2018;10(7):213–221.
- Weiner AB, Desai AS, Meeks JJ. Tumor location may predict adverse pathology and survival following definitive treatment for bladder cancer: a national cohort study. Eur Urol Oncol. 2019;2(3):304–310.
- Cheng L, Neumann RM, Scherer BG, et al. Tumor size predicts the survival of patients with pathologic stage T2 bladder carcinoma: a critical evaluation of the depth of muscle invasion. Cancer. 1999;85(12):2638–2647.
- Hossann M, Hirschberger J, Schmidt R, et al. A heat-activated drug-delivery platform based on phosphatidyl-(oligo)-glycerol nanocarrier for effective cancer treatment. Adv NanoBio Res. 2021;1(6):2000089.
- Datta NR, Stutz E, Puric E, et al. A pilot study of radiotherapy and local hyperthermia in elderly patients with muscle-invasive bladder cancers unfit for definitive surgery or chemoradiotherapy. Front Oncol. 2019;9:889.
- Merten R, Ott O, Haderlein M, et al. Long-term experience of chemoradiotherapy combined with deep regional hyperthermia for organ preservation in high-risk bladder cancer (Ta, Tis, T1, T2)). Oncologist. 2019;24(12):e1341–e50.
- Ucero AC, Gonçalves S, Benito-Martin A, et al. Obstructive renal injury: from fluid mechanics to molecular cell biology. Open Access J Urol. 2010;2:41–55.
- Arends TJ, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette-Guerin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol. 2016;69(6):1046–1052.
- Arends TJ, van der Heijden AG, Witjes JA. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J Urol. 2014;192(3):708–713.
- Ahmed S, Shahid RK, Bhatt H, et al. Chemotherapy-related thrombocytosis: does it increase the risk of thromboembolism? Oncology. 2012;82(6):327–332.
- Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913–922.
- Attia MF, Anton N, Wallyn J, et al. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198.
- Szebeni J, Simberg D, Gonzalez-Fernandez A, et al. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat Nanotechnol. 2018;13(12):1100–1108.
- Panteliadou M, Touloupidis S, Giatromanolaki A, et al. Concurrent administration of liposomal doxorubicin improves the survival of patients with invasive bladder cancer undergoing hypofractionated accelerated radiotherapy (HypoARC). Med Oncol. 2011;28(4):1356–1362.
- Mazurchuk R, Glaves D, Raghavan D. Magnetic resonance imaging of response to chemotherapy in orthotopic xenografts of human bladder cancer. Clin Cancer Res. 1997;3(9):1635–1641.
- Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) Meta-analysis collaboration. Eur Urol. 2005;48(2):202–205. discussion 5–6.
- Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361(9373):1927–1934.
- Ghaferi M, Asadollahzadeh MJ, Akbarzadeh A, et al. Enhanced efficacy of PEGylated liposomal cisplatin: in vitro and in vivo evaluation. Int J Mol Sci. 2020;21(2):559.