?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To explore the efficacy of microwave ablation (MWA) in the treatment of benign thyroid nodules, and analyze related influencing factors.
Methods
The clinical and ultrasound data of 115 patients with 115 benign thyroid nodules treated with MWA were retrospectively analyzed. The volume of nodules at 1, 3, 6, and 12 months after the procedure was obtained, and the volume reduction rate (VRR) at each time point was calculated. With VRR > 90% as the criterion for nodule cure, binary logistic regression was employed to screen the factors that affect the efficacy.
Results
① At 1, 3, 6, and 12 months after the procedure, the volume of nodules continued to decrease, the VRR gradually increased, and the differences at each time point were statistically significant (p < 0.05). A total of 29 (25.21%) nodules disappeared completely at 12 months after the procedure; ② Multivariate stepwise logistic regression showed that there was a statistically significant difference for the internal component of nodules, enhancement mode, and immediate volume after the procedure in determining the ablation efficacy (p < 0.05); ③ The ROC curve was plotted for predicting the efficacy of MWA, with the results showing that the AUC, sensitivity, specificity, and accuracy were 0.82, 67.50, 88.00, 79.10%, respectively; ④ 11 cases (9.56%) had side effects, 10 cases (8.70%) had minor complications, and three cases (2.61%) had major complications.
Conclusion
MWA is safe and effective in the treatment of benign thyroid nodules. The internal component of nodules, enhancement mode, and immediate volume after the procedure are independent factors that affect the efficacy of ablation.
Graphical abstract
1. Introduction
Thyroid nodules are a common clinical disease. With the advancement of imaging inspection technologies, the detection rate of thyroid nodules increases year by year, with the high-frequency ultrasound reaching 19–67% [Citation1]. Although 80–95% of thyroid nodules are benign, aggressive treatment is required in case of large nodules, local compression symptoms, or the possibility of malignancy [Citation2]. At present, surgery is still the main treatment method for thyroid nodules. However, surgery has some drawbacks, such as major trauma, scars, and risk of induced iatrogenic hypothyroidism. In recent years, endoscopic thyroidectomy, chemical ablation, thermal ablation, and other treatment methods have been highly respected. Among them, the thermal ablation guided by ultrasound has been recommended in the clinical treatment of benign thyroid nodules by many guidelines and consensus [Citation3–6]. Thermal ablation is an effective outpatient procedure, with high technical feasibility [Citation7], elevated clinical success rate [Citation8,Citation9], low frequency of complications [Citation7,Citation8,Citation10], and outcomes sustained up to five years [Citation11–13]. Thermal ablation techniques widely applied in clinical practice mainly include laser ablation [Citation14], radiofrequency ablation [Citation15], microwave ablation (MWA) [Citation16,Citation17] and high intensity focused ultrasound (HIFU) [Citation18,Citation19]. However, among them, MWA has a high heating speed and coagulation capacity, and the inactivated necrotic tissues in situ can be gradually absorbed after ablation treatment to achieve the purpose of treatment. MWA was also chosen to combine Radioiodine-131 therapy (RIT) in the treatment of benign thyroid diseases, which significantly increases VRR, and reduces Radioiodine-131 administered activity [Citation20,Citation21]. It has been shown in clinical practice that the degree and speed of lesion resorption vary greatly in different individuals, but few studies have been conducted on related factors affecting lesion resorption. A clear understanding of the relevant factors that influence the efficacy of treatment can help physicians to predict the efficacy of treatment beforehand, and it can also provide a basis for screening the optimal MWA treatment.
2. Materials and methods
2.1. Subjects
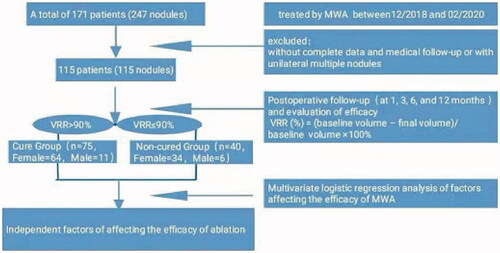
A total of 171 patients (247 nodules) with benign thyroid nodules who underwent MWA in the Department of Ultrasound Medicine of the Second Affiliated Hospital of Harbin Medical University from December 2018 to February 2020 were selected in this research. The local ethics committee approved this study (Ethics Committee of the Second Affiliated Hospital of Harbin Medical University, registry number KY2021-247). Due to the retrospective nature of this study, specific informed consent was waived. Those without complete data and medical follow-up or with unilateral multiple nodules were excluded. As a result, 115 patients (115 nodules) were included, including 17 males ranging in age from 30 to 74, with an average age of (44.4 ± 9.3) years old, and 98 females ranging in age from 19 to 68, with an average age of (43.8 ± 10.8) years old. Inclusion criteria included: ① Patients with no suspicious malignant signs under the ultrasound examination and benign cytological diagnosis twice before MWA; ② Patients with compression symptoms, foreign body sensation or neck discomfort; ③ patients who actively request the thermal ablation treatment due to anxiety about the disease or being worried about the malignant change of the nodule; ④ Patients who refuse traditional surgery or cannot tolerate surgery. Exclusion criteria included: ① Patients with the previous ablation of thyroid nodules; ② Patients with severe cardiac or pulmonary disease or coagulation dysfunction; ③ Patients with severe adhesions between the nodules and esophagus, trachea, and large blood vessels that cannot be effectively separated; ④ Patients with abnormally high calcitonin.
2.2. Research method
2.2.1. Instrument and equipment
Ultrasonic instrument: Esaote MyLab ClassC ultrasonic instrument equipped with LA523 probe (frequency 4–13 MHz) and LA332 probe (frequency 3–11 MHz). Ultrasonic contrast agent: sonovue produced by Italy Bracco, 5 ml 0.9% saline was added to make a suspension, with the amount of 1.5 ml for single use. MWA instrument: KY2000 microwave ablation treatment instrument (Nanjing Kangyou Medical Technology Company limited) equipped with a 16 G disposable sterile ablation needle.
2.2.2. Evaluation before MWA
Preoperative routine blood test, coagulation function test, and thyroid function (FT3, FT4, TSH, anti-TPO, and anti-TG) examinations were performed. Ultrasound was employed to comprehensively evaluate the characteristics of nodules under two-dimensional ultrasound, color Doppler flow imaging (CDFI), elastography, and contrast-enhanced ultrasound (CEUS). The size of nodules was measured, and the volume of them was calculated as per the formula V (mL) = length·width·depth·π/6.
2.2.3. Implementation of MWA
The patient was placed in the supine position with the hyper-extended neck. Electrocardiogram, blood pressure, heart rate, and blood oxygen were monitored in real-time. Local anesthesia was performed between the subcutaneous puncture site and thyroid capsule with 2% lidocaine under ultrasonic guidance. 20–40 ml 0.9% saline was normally injected into the space between the thyroid capsule and the structure of the common carotid artery, trachea, and esophagus to form an ‘isolation zone’ to prevent heat injury, and we may properly add one or two times depending on the actual situation during the operation. The output power of the MWA instrument was set to 30 W. The procedure is performed according to a ‘moving-shot technique’ [Citation6,Citation22–24]. For predominantly cystic thyroid nodules, the cystic fluid was aspirated first and then perform ablation. In case that the target nodule is completely covered by the strong echo gasification zone, or the CDFI shows no blood flow signal within the nodule, the CEUS check is necessary to determine whether ablation is complete. If there is a residual enhancement zone, additional ablation is necessary. All procedures were performed by the same surgeon with 5 years of experience in thermal ablation. After the procedure, a dressing was applied to the wound and the ablated area was treated with local ice for 60 min.
2.2.4. Post-operative follow-up
Ultrasonography was performed 1, 3, 6, and 12 months after the procedure, and thyroid function was reexamined 1 month after the procedure. The recovery from adverse events was recorded at each follow-up time point, the blood perfusion in nodules was observed, and the VRR of the nodule was calculated as per the formula, namely VRR (%) = (baseline volume − final volume)/baseline volume × 100% [Citation25]. The endpoint of follow-up was 12 months after the procedure. The nodule with VRR > 90% was defined as a cure.
2.3. Statistical analysis
SPSS22.0 statistical software was employed for analysis. The measurement data were expressed as mean ± standard deviation, and the paired samples t-test was employed for comparison of the nodule volume and VRR between each follow-up month after the procedure. Besides, the independent samples t-test was employed for comparison between the two groups in the monofactor analysis. The count data were expressed as frequencies and X2 test was employed for comparison between groups. The cured and non-cured nodules were adopted as dependent variables. clinical data, laboratory examination results, and ultrasonic characteristics of patients before treatment were taken as independent variables. The value assignment of variables is shown in . A univariate analysis was performed for each independent variable and dependent variable, and multivariate stepwise logistic regression analysis was performed for the independent variables with statistically significant differences. Backward LR test was adopted to establish a logistic regression model according to relevant results. The regression model was employed to predict the diagnostic probability logit(p). With logit(p) = 0.5 as the separated value, logit(p) ≥ 0.5 was determined as absorptivity ≤90% and logit(p) < 0.5 was determined as absorptivity >90%. The regression model was employed to plot the receiver operating characteristic (ROC) curve of VRR = 90%, and the area under the curve (AUC), sensitivity, specificity, and accuracy were calculated. p < 0.05 indicated that the difference was statistically significant.
Table 1. Comparison of the single factor for the cure group and the non-cured group.
3. Results
3.1. Evaluation of efficacy
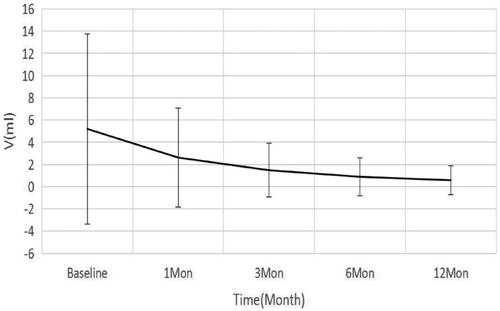
In this group, the initial volume before ablation was 5.30 ± 8.56 ml, and the volume at 1, 3, 6, and 12 months after ablation was 2.62 ± 4.45, 1.48 ± 2.42, 0.89 ± 1.70, and 0.58 ± 1.32 ml, respectively, showing a continuous trend of reduction (), with statistically significant differences at each time point (p < 0.05). At 12 months after the procedure, a total of 29 (25.21%) nodules was completely disappeared. The VRR at 1, 3, 6, and 12 months after the procedure was 41.96 ± 21.66, 68.64 ± 18.85, 83.56 ± 14.05, and 91.43 ± 10.35%, respectively, showing a gradual trend of increase (), with statistically significant differences at each time point (p < 0.05). The difference in thyroid function before and after treatment was not statistically significant (p > 0.05).
Figure 2. Changes in the reduction rate of the volume of thyroid nodules at different follow-up time points.
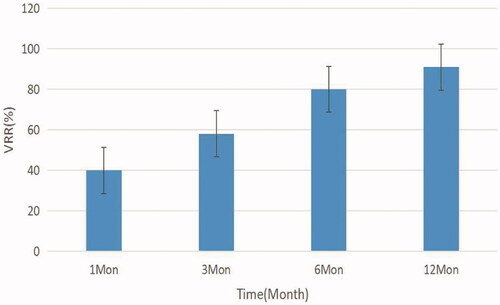
Figure 3. Two-dimensional and CEUS images of thyroid nodules before and after MWA treatment. (a) Before treatment, nodules were oval hypoechoic with well-defined boundaries, and the CEUS showed uniform and high enhancement. (b) Immediately after the operation, nodules were covered by the gasification area, and CEUS showed no enhancement inside nodules. (c–f) The results showed that the volume of the treated area decreased gradually and the VRR increased gradually at 1, 3, 6, and 12 months after the procedure, with the results being 8.7, 44.5, 68.0, and 90.2%, respectively.
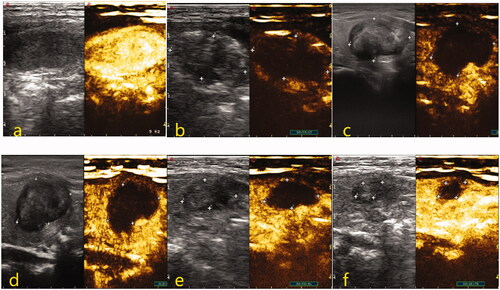
3.2. Factors affecting the efficacy of MWA
As per the univariate analysis, there were no significant differences in age, gender, preoperative thyroid function, nodule hardness, and intra nodule calcification between the cured group and the non-cured group (p > 0.05). The differences in the maximum diameter, preoperative volume, echo, internal component, blood flow distribution, enhancement mode, and immediate volume after ablation, were statistically significant between the cure group and the non-cured group (p < 0.05) ().
Multivariate logistic regression analysis was performed for the variables with statistically significant differences. The results showed that the internal component (X7), the enhancement mode (X10), and the immediate volume after ablation (X12) were statistically significant in the determination of the ablation efficacy ().
Table 2. Logistic regression analysis for multiple factors affecting the efficacy of MWA.
3.3. Roc curve analysis of the predictive ability of regression model
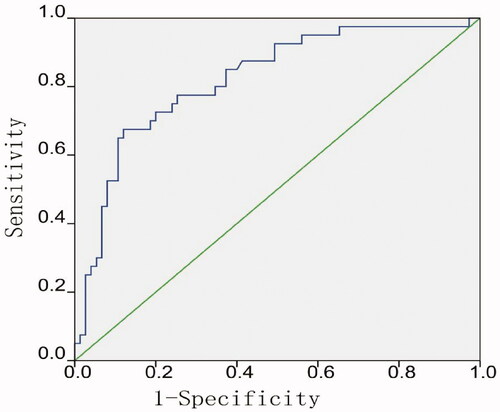
The associated variables were substituted into the logistic regression equation, Logit(p) = −1.242 + 1.997X7 (cystic predominance) − 1.9X7 (solid predominance) − 0.386X10 (equally enhanced) + 1.171X10 (highly enhanced) + 0.077X12. The ROC curve of the predicted efficacy of MWA was plotted according to the regression model, with the results showing that the AUC was 0.82, the sensitivity was 67.50%, the specificity was 88%, and the accuracy was 79.10% ().
3.4. Adverse events
Side effects: 11 (9.56%) cases had significant neck pain radiating to the face, ear, teeth, or shoulder during or after the procedure, which resolved completely within 5 days after the procedure.
Minor complications: 3 (2.61%) cases had a hoarse voice, which gradually resolved within 1 day after the procedure. Four (3.48%) cases had subcutaneous or intramuscular hematoma, which resolved 2–7 days after the procedure. Two (1.74%) cases occurred post-operative fever, which resolved 3 days after the procedure. One (0.87%) case developed mild skin burn.
Major complications: 2 (1.74%) cases had a long-term hoarse voice, which resolved 3 months after the procedure. One (0.87%) case had an esophagus burn.
4. Discussion
In MWA, the thermal energy generated by the oscillation of water molecules in the tissue during ablation is employed to cause apoptosis and coagulative necrosis of tumor cells, and the necrotic tissue is finally degraded by the body immune system [Citation28]. The results of this study show that the volume of thyroid nodules gradually decreases and the VRR gradually increases with time after MWA, which is consistent with the findings in previous research [Citation29–32]. By 12 months, about 1/4 of nodules have completely disappeared. Comparison of thyroid function before treatment and 1 month after MWA shows that the difference is not statistically significant, which indicates that the treatment with MWA does not affect the thyroid function of patients consistent with the study of Liu [Citation33].
To address the clinical problem that the degree and rate of lesion resorption vary greatly between individuals after MWA, multifactorial stepwise logistic regression analysis has been employed to identify the internal composition, enhancement mode, and immediate post-operative volume as independent factors that would affect the nodule resorption. In this study, those nodules have been divided into three groups according to their internal components, with the cystic-predominance nodules having a significantly greater post-operative VRR at 12 months than the other two groups, which is consistent with the conclusion of Dobnig and Amrein [Citation25]. There is no significant difference in VRR between solid nodules and solid primary nodules, which may be related to the treatment method of MWA used in this study. For the cystic-predominance nodules, cystic fluid is first aspirated and then the parenchyma is ablated by microwave. The immediate post-operative volume of most cystic-predominance nodules is significantly smaller than that before treatment. In a small number of predominantly cystic nodules, fresh bleeding may occur within the cystic cavity during aspiration, mostly due to blood leaking from the cystic wall or the solid part of the nodule, which is often difficult to manage when the bleeding is rapid, the bleeding volume is large, and the point of bleeding cannot be accurately identified. For predominantly cystic nodules, the needle tip should be placed in the middle of the cystic cavity when the cystic fluid is pumped to avoid the puncture of the parenchymal part of the cystic wall resulting in bleeding, and the pumping speed should not be too fast to avoid the sudden drop of pressure in the cystic cavity increasing the risk of bleeding. If the solid part is rich in blood or has irregular wall nodules, the solid part can be ablated first, and then the cystic wall can be ablated after aspiration, which can reduce the occurrence of fresh intracapsular bleeding.
It has been shown from the single factor comparison that the differences in nodule maximum diameter, preoperative volume, and immediate post-operative volume are statistically significant between the cured group and the non-cured group. Although the diameter or volume grouping criteria vary in different studies, it is consistent that the VRR for smaller nodules is more significant than larger nodules [Citation25,Citation32]. After ablation, the necrotic tissue is wrapped and engulfed by macrophages, lymphocytes, and other inflammatory cells, and finally cleared by the body [Citation34]. The greater the volume of the nodule, the more necrotic tissue of the lesion after MWA, the longer the time required for phagocytosis. It has been shown from the multivariate regression analysis that immediate post-operative volume is an independent factor affecting nodule resorption. It is suggested that for benign thyroid nodules, based on ensuring the complete ablation of nodules, the surgeons should minimize the ablation area to facilitate the resorption of nodules. However, the question of how to achieve conformal ablation of thyroid nodules, especially in small nodules without ablating too much of the normal tissue surrounding the nodule, deserves further discussion.
There is no report on the correlation between the enhancement mode of nodules and the efficacy of MWA in the literature, but the results of this study suggest that highly enhanced nodules have a better outcome 12 months after the procedure compared to those with other enhancement modes. The possible reasons are that the enhancement mode of nodules reflects the microcirculation perfusion in nodules. There is a lower proportion of fibrous tissue and interstitial tissue and a higher proportion of blood and more water molecules in highly enhanced nodules. More water molecules could contribute to the energy delivered, thus generate more heat in a shorter period enhancing the thermal coagulation process. In the study of Trimboli and Deandrea [Citation36], the energy delivered is significantly correlated with nodule resorption. In addition, shorter ablation times can reduce the production of carbonized zones and conduce to the resorption of nodules. DeAndrea et al. [Citation35] has conducted a multicenter study on radiofrequency ablation for benign thyroid nodules, with the results showing that nodules with rich peripheral and internal blood flow respond better to ablation, which is also in line with the conclusion of this study. Eleven cases with side effects were neck pain. 10 cases with minor complications included transient hoarse voice, localized hematoma, post-operative fever, and mild skin burn. Transient hoarse voice is caused by the anesthetic effect of lidocaine in the isolation fluid and the compression of the recurrent laryngeal nerve by tissue edema, and it is usually resolved naturally in 1 h to a few days after the procedure. The case with skin burn in this group was due to a misconfiguration of the ablation device connection, which was detected in time without serious consequences. Therefore, it shall be noted that the operator shall test the ablation needle in vitro for proper functioning after it has been connected. In addition, there are three cases with major complications, including long-term hoarse voice and esophagus burn. The time for recovery of long-term hoarse voice that is directly caused by thermal damage to the laryngeal nerve will be longer. In this study, two patients gradually recover in three months after the procedure, which may be that the function of the affected vocal cords is gradually compensated by the contralateral vocal cords. There is also 1 patient with esophageal serosal layer injury, which is caused by the large nodule extrusion narrowing of the esophagus and insufficient observation of the tip during the operation. The patient develops foreign body sensation during swallowing after the procedure and then recovers one month after the procedure. To minimize the occurrence of adverse events, first, there is a sufficient understanding of the neck anatomy, followed by an accurate assessment of the target nodules and adjacent structures. Besides, the appropriate puncture position and adoption path shall be selected, the position of the tip shall be observed in real-time, and a ‘liquid isolation method’ shall be adopted.
There are several limitations in this study: Firstly, subject to the patient's compliance, the amount of sample with complete follow-up data is slightly small, and there is a lack of long-term follow-up data. Secondly, the energy used per unit volume of the nodule is not analyzed due to the lack of effective quantitative methods.
5. Conclusion
MWA is safe and effective in the treatment of benign thyroid nodules, and it can be employed as a useful supplement to surgical treatment. The internal component of nodules, enhancement mode, and immediate volume after surgery are independent factors that affect the efficacy of ablation. A thorough understanding of the factors that influence the efficacy of MWA in nodules contributes to surgeons predicting the efficacy before surgery, thereby choosing the best treatment and improving the success rate of the surgery.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kuo LE, Kelz RR. Management of thyroid nodular disease: current cytopathology classifications and genetic testing. Surg Oncol Clin N Am. 2016;25(1):1–16.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(5):1–639.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016;17(3):370–395.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally invasive treatments for benign thyroid nodules: a delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Pacella CM. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20(4):347–349.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multi-center study. Korean J Radiol. 2018;19(1):167.
- Døssing H, Bennedbaek FN, Hegedus L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules – a randomised study. Eur J Endocrinol. 2005;152(3):341–345.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multi-center study on 1531 patients. J Clin Endocrinol Metab. 2015;100(10):3903–3910.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99(10):3653–3659.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460–466.
- Amabile G, Rotondi M, Pirali B, et al. Interstitial laser photocoagulation for benign thyroid nodules: time to treat large nodules. Lasers Surg Med. 2011;43(8):797–803.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: Safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–e16.
- Korkusuz H, Happel C, Grünwald F. Ultrasound guided percutaneous microwave ablation of hypofunctional thyroid nodules: evaluation by scintigraphic 99mTc-MIBI imaging. Nuklearmedizin. 2013;52:205–257.
- Korkusuz H, Sennert M, Fehre N, et al. Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Fortschr Röntgenstr. 2015;187(11):1011–1015.
- Trimboli P, Pelloni F, Bini F, et al. High-intensity focused ultrasound (HIFU) for benign thyroid nodules: 2-year follow-up results. Endocrine. 2019;65(2):312–317.
- Korkusuz H, Happel C, Koch DA, et al. Combination of ultrasound-guided percutaneous microwave ablation and radioiodine therapy in benign thyroid disease: a 3-month follow-up study. Fortschr Röntgenstr. 2016;188:60–68.
- Happel C, Korkusuz H, Koch DA, et al. Combination of ultrasound guided percutaneous microwave ablation and radioiodine therapy in benign thyroid diseases. A suitable method to reduce the 131I activity and hospitalization time? Nuklearmedizin. 2015;54(3):118–124.
- Kim J-H, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Wu W, Gong X, Zhou Q, et al. US-guided percutaneous microwave ablation for the treatment of benign thyroid nodules. Endocr J. 2017;64(11):1079–1085. Nov
- Dobnig H, Amrein K. Monopolar radiofrequency ablation of thyroid nodules: a prospective Austrian Single-Center study. Thyroid. 2018;28(4):472–480.
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a Mayo clinic case series. Mayo Clin Proc. 2018;93(8):1018–1025.
- Lyshchik A, Higashi T, Asato R, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237(1):202–211.
- Zheng B-W, Wang J-F, Ju J-X, et al. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine. 2018;62(2):307–317.
- Cheng Z, Che Y, Yu S, et al. US-Guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554.
- Liu Y-J, Qian L-X, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med. 2017;242(15):1515–1523.
- Erturk MS, Cekic B, Celik M, et al. Microwave ablation of symptomatic benign thyroid nodules: short- and long-term effects on thyroid function tests, thyroglobulin and thyroid autoantibodies. Clin Endocrinol. 2020;10:1–7.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Liu S-Y, Guo W-H, Yang B, et al. Comparison of stress response following microwave ablation and surgical resection of benign thyroid nodules. Endocrine. 2019;65(1):138–143.
- Korkusuz Y, Mader OM, Kromen W, et al. Cooled microwave ablation of thyroid nodules: initial experience. Eur J Radiol. 2016;85(11):2127–2132.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Trimboli P, Deandrea M. Treating thyroid nodules by radiofrequency: is the delivered energy correlated with the volume reduction rate? A pilot study. Endocrine. 2020;69(3):682–687.