?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The choice of the most appropriate therapeutic approach for a diagnosed papillary thyroid microcarcinoma (PTMC) remains controversial. The present study aimed to evaluate the efficacy of microwave ablation (MWA) for unifocal PTMC with a diameter of ≤0.6 cm.
Methods
A total of 63 consecutive patients with PTMC treated with MWA were studied retrospectively. MWA was performed using the hydrodissection technique and multidimensional fixed-needle principle. We analyzed the absorption of the MWA area and evaluated the prognosis over a follow-up period of 24 months. In addition, 83 patients with PTMC who underwent surgery were selected. The operating room characteristics and procedural complications of the two groups were compared.
Results
In the MWA group, the volume of nodules (p < 0.05) decreased from 0.04 ± 0.03 cm3 to 0.0001 ± 0.0004 cm3 at the 24-month follow-up after MWA, and the volume reduction rate (p < 0.05) was 99.43 ± 1.58%. The incidence of temporary reactive hyperplastic lymphadenectasis was higher and that of other complications was lower in the MWA group than in the surgery group. One percent of the patients in the surgery group had recurrence or metastasis, but none were detected in the MWA group. The loss of thyroid tissue volume (p < 0.001), operating room time (p < 0.001), and the mean length of hospital stay (p < 0.001) were significantly lower in the MWA group than in the surgery group.
Conclusion
Ultrasound-guided MWA is an effective treatment strategy for unifocal PTMC with a diameter of ≤0.6 cm.
Introduction
Thyroid carcinoma is one of the most common endocrine malignancies worldwide. Differentiated thyroid carcinoma (DTC) is a histological type of thyroid carcinoma [Citation1]. The disease-specific mortality rate for papillary carcinoma, which accounts for 85% of DTC, has been reported to be <3% [Citation2]. Papillary thyroid microcarcinoma (PTMC) has a diameter of ≤1 cm and has a clinically silent course, without obvious growth or metastasis for decades. The American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), Associazione Medici Endocrinologi (AME), and National Comprehensive Cancer Network (NCCN) guidelines suggest surgical resection as the preferred treatment for malignant nodules diagnosed using fine-needle aspiration (FNA) [Citation1,Citation3]. The American Thyroid Association (ATA) and European Society for Medical Oncology (ESMO) guidelines indicate that the thyroid and cervical lymph nodes should be monitored every 6–12 months by ultrasound (US) to detect unifocal papillary microcarcinoma without evidence of extracapsular expansion or lymph node metastasis [Citation2,Citation4]. Several patients experience physical and psychological pressure and prefer to eradicate cancer to resolve their anxiety of active surveillance [Citation5]. However, because of the high risk, post-operative pain, or cosmetic issues, several patients are hesitant to undergo surgery. Hence, determining an alternative and effective treatment is important [Citation6].
With the progress of modern medical technology and the development of evidence-based medicine [Citation7], minimally invasive treatment is gaining more acceptance in clinical treatment [Citation8]. The principle of microwave ablation (MWA) is to target the solid tumor under real-time US guidance, place the ablation needle percutaneously into the lesion, and use the local heating effect of microwaves to produce a large amount of heat in the focus tissue within a short period. Consequently, the tumor coagulates and is driven to necrosis instantly due to the high temperature. In addition, transmural damage occurs in the blood vessel wall in the tissue, which further stimulates the autoimmune response. This, in turn, results in continuous degradation and absorption of the necrotic tissue, such that the tumor is significantly reduced or completely disappears. Initially, thermal ablation is a relatively novel procedure for liver, lung, kidney, and adrenal tumors [Citation9–12]. In recent years, MWA has been widely applied to benign thyroid nodules [Citation13–19], but only a few studies have reported its use for PTMC. Although favorable results have been obtained [Citation5,Citation20–24], there is no international consensus on its long-term clinical value. The present study aimed to analyze and evaluate the efficacy of US-guided MWA for unifocal PTMC with a diameter of ≤0.6 cm.
Materials and methods
This retrospective study was approved by the Institutional Review Board (QYFYWZLL26554) of the Affiliated Hospital of Qingdao University, Qingdao, China. All patient identities were protected.
Patients
From January 2016 to December 2018, patients who met the inclusion criteria were included in the MWA group and compared to selected patients with PTMC who were referred to traditional open surgery. The inclusion criteria were as follows: (1) single-focal nodule, excluding benign nodules; (2) papillary carcinoma confirmed by fine-needle aspiration biopsy (FNAB); (3) nodule measuring ≤0.6 cm along its greatest dimension; (4) no evidence of invasion of the surrounding tissue or capsule; (5) no evidence of extrathyroidal extension; (6) no evidence based on imaging studies or biopsy evaluation indicating lymph node involvement; (7) no other special treatment, such as radiotherapy and chemotherapy; and (8) no severe disease affecting the patient's life. The exclusion criteria were as follows: (1) multiple malignant nodules; (2) nodules measuring >0.6 cm along its greatest dimension; (3) evidence of another type of carcinoma or a coexisting thyroid malignancy confirmed by FNAB or other biopsy methods; (4) ultrasonographic manifestations suggestive of macrocalcification; (5) invasion of surrounding tissues or capsules; (6) evidence of external organ extension; (7) imaging or pathological evidence of lymph node metastasis; (8) pregnancy or other severe diseases; (9) other special treatments; (10) coagulation disorders or other bleeding tendency diseases; and (11) fear or anxiety of the disease treatment.
Definitions and equations
Throughout the MWA procedure and after surgery, the patients were asked to rate their level of pain, according to the World Health Organization Numerical Rating Scale (NRS) (0–10), where 0, 1–3, 4–6, and 7–10 were no, mild, moderate, and severe pain, respectively [Citation25]. Complications were grouped into intraoperative (pain and hoarseness) and post-operative (pain, cervical hematoma, dysphagia or laryngeal edema, temporary/permanent hoarseness, lymphatic fistula, reactive hyperplastic lymphadenectasis, parathyroid dysfunction, and thyroid dysfunction). Parathyroid dysfunction was defined as abnormal parathyroid hormone and serum calcium levels during reexamination within 1 month after the procedure, whereas thyroid dysfunction was defined as at least one abnormal thyroid hormone level within 6 months after the procedure. The maximum diameter (MD) of the ablation area was defined as the long axis length of the quasi-elliptical ablation area. The ablation area volume (AAV) was calculated using the following equation:
(1)
(1)
a/b/c: the three-dimensional diameter of the ablation area
The volume reduction rate (VRR) was calculated as follows:
(2)
(2)
Complete absorption of the ablation area was defined as the complete disappearance of the ablation area on US examination.
Equipment
The ultrasonic diagnostic instruments used in this study were Healthineers (SIEMENS), EPIQ Elite (PHILIPS), and LOGIQ (series) (GE). The MWA instrument, ECO-100A1 microwave therapeutic instrument (Nanjing YiGao Microwave System Engineering Co., Ltd.), had 2450 ± 20 MHz with an output power of 0–150 W (continuously adjustable) and was connected to a disposable MWA needle (16G/17G) microwave cable interface, through the output interface. To prevent the shaft from overheating, distilled water was circulated through dual channels inside the antenna continuously cooling the shaft.
MWA procedures
Pre-procedural evaluation
All included patients underwent a pre-procedural physical examination. Serum thyroid-stimulating hormone, thyroglobulin, antithyroglobulin antibodies, and thyroid hormones were normal within 7 days. The features of the tumor, including site, composition, echogenicity, morphology, aspect ratio, margin, calcification, and vascularity, were identified through the US, and the enhancement mode was identified using contrast-enhanced US (CEUS). Patients with hyperglycemia and hypertension were required to take medications on time to maintain a stable condition and were forbidden from taking any anticoagulant or antiplatelet medications for at least 7 days before MWA. The process was explained to the patients to calm them emotionally before the procedure. To ensure the smooth progress of MWA, we prepared a bedside bell that the patients can use to alert the doctor if they experienced any discomfort during MWA.
MWA procedure
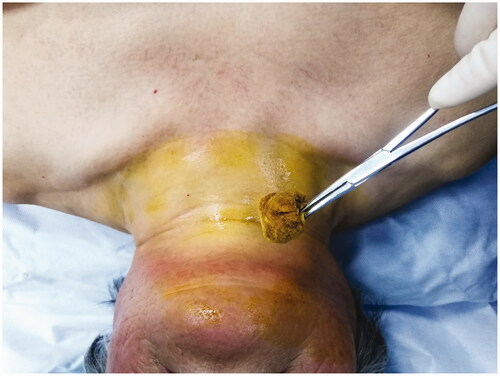
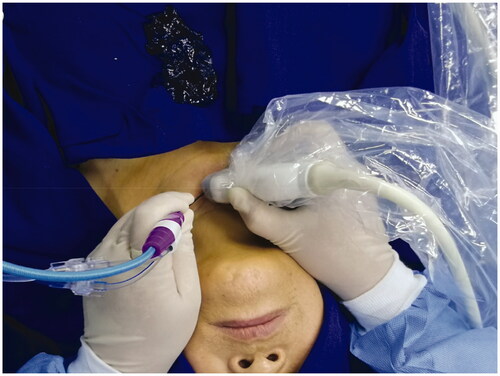
MWA was performed by a chief physician with >10 years of experience, with the cooperation of an assistant physician and a nurse. The patients were placed in a supine position with the shoulder raised and head tilted back, to ensure a hyperextended neck. Continuous electrocardiographic, respiratory, blood pressure and finger oxygen saturation were monitored throughout the whole ablation procedure. Pre-experiments on the ablation instrument and ablation needle were carried out before the actual MWA to ensure the normal functioning of the equipment. Routine sterile skin preparation was performed () with 1% lidocaine as topical anesthesia. Outside of the thyroid capsule, 10 ml of isolation solution was injected using a 10-ml sterile syringe to achieve a hydrodissection technique between the thyroid gland and adjacent tissue; the whole process was guided by color Doppler US to avoid the blood flow (). These steps protected the thyroid gland and the adjacent vital organs [carotid artery, esophagus, trachea, and recurrent laryngeal nerve (RLN)] from thermal injury [Citation26]. A 16G or 17G ablation needle was selected according to the size and location of the nodule (). Routine pre-procedural CEUS () was performed. Under ultrasonic guidance, the ablation needle was slowly passed through the target nodule along the original FNA needle path to the rear, and ablation was performed according to the multidimensional fixed-needle principle () [Citation21]. The needle position was changed when the bubble-like hyperecho was intense, until the strong echo completely covered the focus, continuing to expand over the ablation area, to cover a part of the surrounding thyroid tissue. This was to ensure the completeness of tumor ablation and prevent marginal recurrence of the tumor. During the withdrawal of the needle, the residual heat of the ablation antenna was used to coagulate the needle track to prevent the spread and seeding of tumor cells. Post-procedural CEUS was repeated immediately to assess whether the ablation area completely covered the target nodule (). The power output and ablation time mean range was 29.86 ± 1.27 W and 1.69 ± 0.77 min, respectively.
Figure 2. Injection of isolation fluid to achieve water separation between the thyroid and adjacent tissues.
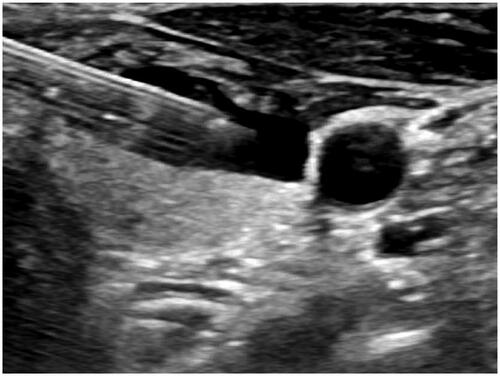
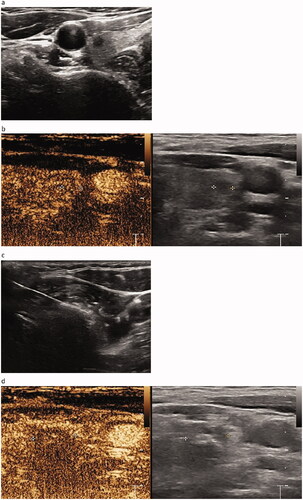
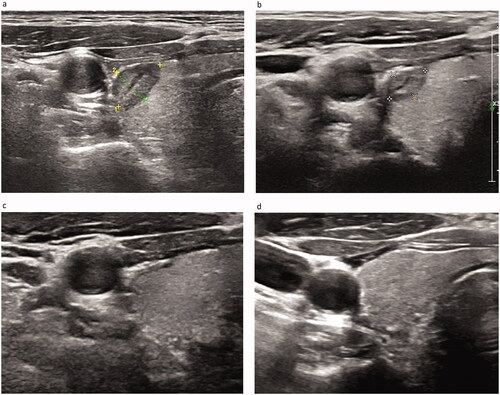
Figure 3. Pre-procedural, procedural, and post-procedural images. (a) Pre-procedural nodule localization. The solid nodule is located in the middle and upper part of the right lobe of the thyroid, with the size of 0.42 × 0.38 × 0.35 cm3. It is hypoechoic, irregular in shape, unclear in boundary, aspect ratio >1, and has no calcification. (b) Pre-procedural contrast-enhanced ultrasound (CEUS). The nodules show low enhancement. (c) Picture of the MWA process. The ablation area is shown as a bubble-like hyperecho. (d) Post-procedural CEUS. The MWA range is significantly larger than that of the tumor.
Throughout MWA, we communicated with the patients intermittently to detect any injuries to the RLN, which could result in a change in the voice. We also asked the patients to score their level of pain according to the NRS. After MWA, an experienced nurse compressed the patients’ incision for 15 min to avoid bleeding, monitored the patients’ post-procedural state in the observation room for at least 30 min, and allowed them to return to the ward if there was no discomfort. Simultaneously, we recorded the three-dimensional diameter of the ablation area (a, b, and c), AAV, operating room (OR) time, and mean hospital stay.
Post-procedural follow-up
Patients were reexamined at 1, 3, and 6 months after MWA and every 6 months thereafter. Changes are shown in . At US examination, we were required to evaluate changes in tumor size, intratumoural vascularity, and development of recurrent and contralateral thyroid and cervical lymph node metastases. Patients were advised to undergo further examination if recurrence and metastasis were suspected. In addition, post-procedural complications, such as reactive hyperplastic lymphadenectasis in the adjacent regions, cervical hematoma, voice changes, and dysphagia, were recorded. Laboratory indices were observed to record the changes in parathyroid and thyroid functions.
Figure 5. Size of the ablation area at (a) 1, (b) 3, (c) 6, and (d) 12 months after MWA. Well-treated tumors exhibiting changes to a hypoechoic nature, marked decrease in size, and loss of internal vascularity on Doppler US. The volume of the nodule is measured from 0.158 cm3 immediately after MWA to completely disappear at 12 months, and the volume reduction rate (VRR) is 100%.
Surgical procedure
Surgery was performed by general surgeons with >10 years of clinical experience. All patients underwent a routine physical examination, and basic laboratory indices were examined 1 day before the surgery. PTMC radical resection (thyroidectomy and isthmus resection + lymph node dissection on the affected side) was performed, and the specific plan was adjusted according to the patient's condition. The tissues excised during the surgery were frozen and sent to the pathology department for diagnosis. Pathological results were obtained within 2 weeks after surgery. Blood loss and incision area were also recorded during the surgery. Post-procedural complications, thyroid tissue loss volume, OR time, mean hospital stay, recurrence, and metastasis were carefully recorded.
Statistical analysis
IBM SPSS Statistics for Windows version 26 (IBM Corp., Armonk, NY, USA) was used for data analysis. Quantitative data are expressed as mean ± standard deviation and ranges. Repeated measures analysis of variance (ANOVA) was used to compare changes in MD and volume of each tumor before MWA, immediately after MWA, and at each follow-up. The Mann–Whitney U-test was used to compare the independent samples between the ablation and surgical groups. Qualitative data from the two groups were compared using either the Chi-square test or Fisher's exact test. The threshold for significant differences was set at p < 0.05.
Results
Participant characteristics
Between 2016 and 2018, a total of 84 patients received MWA, of whom, twelve were lost to follow-up and nine were excluded due to incomplete information. The remaining 63 patients (M:12; F:51) were enrolled in the MWA group, with mean age and nodule diameter of 43.6 ± 14.2 years and 0.5 ± 0.1 cm, respectively. Of the 547 patients with PTMC who underwent surgery, we selected 83 patients according to the entry criteria for the surgery group, with mean age and nodule diameter of 43.3 ± 10.9 years and 0.5 ± 0.1 cm, respectively. No significant differences were noted in the demographic characteristics and pre-procedural ultrasonographic manifestations between the two groups ().
Table 1. Demographic characteristics and ultrasonographic manifestations of the subjects.
Therapeutic effect of MWA
show changes in MD, AAV, and VRR at each follow-up after MWA. All the patients were followed up regularly for 24 months, and the longest follow-up was 42 months. The mean MD of nodules before MWA was 0.45 ± 0.11 cm. The MD of the ablation area 24 months after MWA was 0.02 ± 0.05 cm. The MD of the ablation area (, ) decreased significantly during each follow-up period (p < 0.001). The pre-procedural MWA nodule volume was 0.04 ± 0.03 cm3. The AAV decreased from 1.3413 ± 0.6763 cm3 immediately after MWA to 0.0001 ± 0.0004 cm3 at the 24-month follow-up (p < 0.05), and the VRR was 99.43 ± 1.58% (p < 0.05) (). The downward trend of nodule volume change was significant at each follow-up and slowed gradually over time (). At 12, 18, 24, and 30 months after MWA, the complete absorption rates of the ablation area were 27% (17/63), 40% (25/63), 86% (54/63), and 87% (55/63), respectively. The ablation area was not completely absorbed in the remaining eight patients during the follow-up period. One patient had thyroidectomy shortly after MWA, and complete necrosis of the tumor was confirmed. US-guided biopsy was performed 12 months after MWA in three patients, all of whom demonstrated no viable neoplastic cells.
Figure 6. Maximum diameter of the pre-procedural nodule and ablation area immediately after MWA and at 1, 3, 6, 12, 18, and 24 months follow-up.
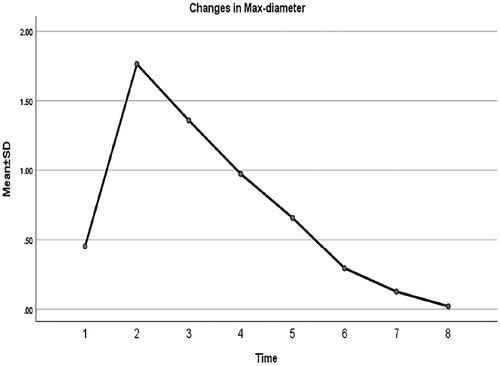
Figure 7. Volume of the pre-procedural nodule and ablation area immediately after MWA and at 1, 3, 6, 12, 18, and 24 months follow-up.
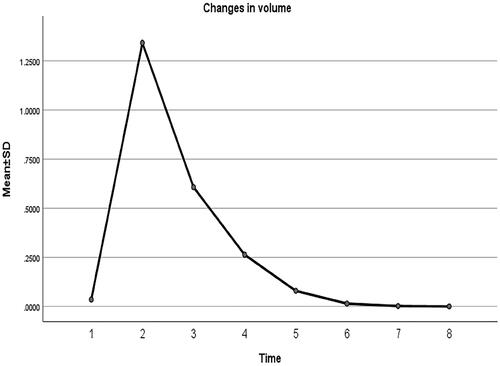
Figure 8. Volume reduction rate of the ablation area immediately after MWA and at 1, 3, 6, 12, 18, and 24 months follow-up.
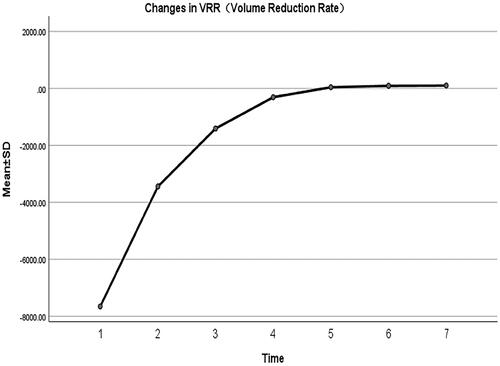
Table 2. Changes in maximum diameter of nodules at each follow-up after MWA.
Table 3. Changes in AAV and VRR at each follow-up after MWA.
Complications
The treatment process of all patients was smooth and tolerable. As shown in , during MWA, 19 patients reported burning sensations in the neck while 63 reported transient mild to moderate pain. When the procedure was ended, these symptoms disappeared. Temporary hoarseness returned to normal after a few days. During the post-procedural follow-up, reactive hyperplastic lymphadenectasis lasting 3 months occurred in four patients, but the symptoms disappeared in subsequent reexamination. For the thyroid function test, 32% (20/63) presented with hyperthyroidism (clinical hyperthyroidism, 14% [9/63]; subclinical hyperthyroidism, 17% [11/63]) 1 month after MWA. Within the 12 months of follow-up, thyroid hormone levels returned to normal in all patients in the MWA group. No other complications were observed.
Table 4. Comparison of complications between ablation group and surgery group.
No obvious complications were noted during the surgery procedure. However, during the post-procedural follow-up, the incidence of complications in the surgery group was significantly higher than that in the ablation group. All patients in the surgery group were required to take thyroxine tablets regularly after the surgery. For the thyroid function test, thyroid dysfunction was detected in 87% (72/83) of patients (clinical hyperthyroidism, 49% [41/83]; subclinical hyperthyroidism, 27% [22/83]; clinical hypothyroidism, 4% [3/83]; subclinical hypothyroidism, 7% [6/83]) at 1-month review after surgery. Of 72 patients, 46% had not recovered the thyroid function after more than 12 months, and 6% of these patients still had thyroid dysfunction after 24 months of follow-up, which was considered a permanent thyroid function injury. Other complications, including temporary dysphagia (confirmed by laryngoscopy as laryngeal edema), temporary lymphatic fistula, permanent hoarseness, and parathyroid dysfunction, were noted in the surgery group.
Overall clinical characteristics
shows a significantly smaller thyroid tissue volume loss in the MWA group than in the surgery group (p < 0.001). A significantly longer overall OR time occurred in the surgery group than in the MWA group (p < 0.001). The mean length of hospital stay in the MWA group was significantly shorter than that in the surgery group (p < 0.001). Intraoperative mean blood loss and surgical incision area could not be compared because of the small measured values in patients in the MWA group.
Table 5. Overall comparison between ablation group and surgery group after the procedure.
No evidence of tumor recurrence or metastasis was found in the MWA group during the follow-up. In the surgery group, 24% (20/83) of patients had tumor invasion into the capsule and 21% (17/83) had pre-laryngeal tissue or ipsilateral lymph node metastasis before surgery. One had contralateral thyroid recurrence, and another had lateral cervical lymph node metastasis after surgery. However, no significant difference was noted in recurrence and metastasis between the two groups (p = 1.000) ().
Discussion
The widespread screening and technical improvements in thyroid ultrasonography and US-guided FNA of the neck have led to increased incidental PTMC detection rates [Citation27], which have further increased due to health awareness. In this study, a new treatment, MWA, was applied to treat PTMC. Compared to the possibility of a series of risks in surgery, MWA has the advantages of a small injury, short treatment time, significant efficacy, quick post-procedural recovery, and fewer complications [Citation28]. Furthermore, this approach is consistent with the current international direction of surgical development of minimally invasive therapy [Citation8].
According to the screening inclusion criteria, the nodule diameter was ≤0.6 cm. In a previous study, we analyzed the risk factors of lymph node metastasis in patients with PTMC and found that primary tumors with no extra-thyroid invasion and with <0.65 cm diameter were at greater risk for lymph node metastasis in the central region [Citation29]. Considering this risk, we set the cutoff diameter of the nodule at ≤0.6 cm to reduce the possibility of lymph node metastasis, recurrence rate, and metastasis rate after ablation.
MWA is superior to other thermal ablation methods in terms of volume reduction, rapid heating, ability to ablate larger volumes, higher specificity, temperature for burning and killing of cells, the lesser effect of heat on the nearby blood vessels, and less radiator effect [Citation30–37]. Our treatment efficacy was promising, the AAV significantly increased immediately after MWA and reduced gradually afterward, which was consistent with the results reported by Li et al. [Citation6]. The VRR was better than that reported at the 60-month follow-up by Teng et al. [Citation38] and the 42-month follow-up by Li et al. [Citation6]. Complete disappearance of the nodules in our study was lower than the 97.56% reported by Teng et al. [Citation38], but this may be related to the length of follow-up. Nevertheless, the remaining patients without complete disappearance of the lesions showed a good trend and were followed up regularly.
Comparative studies on MWA and surgical treatment of PTMC worldwide [Citation5,Citation6,Citation28,Citation39,Citation40] showed that a low recurrence rate was an advantage of traditional surgery. However, complications from surgery may exceed the degree of morbidity of the disease itself. In the surgery group, permanent hoarseness suggested an irreversible injury or removal of the RLN during the procedure. And long-term medication increases the anxiety of patients, requiring the extension of follow-up to allow the adjustment of thyroid function, reduces their quality of daily life seriously. However, MWA preserved the integrity of the thyroid tissue and patients did not need to take medications after the procedure; the temporary hyperthyroidism noted following MWA is a manifestation of systemic stress reaction. The proportion of temporary hoarseness (11%) during the MWA was higher than that (4.3%) reported by Li et al. [Citation6] but lower than that (19%) reported by Yue et al. [Citation22]. Hoarseness following MWA may be caused by the effect of local anesthesia on the RLN. In the present study, we used an aqueous solution of lidocaine when the nodules were near the posterior capsule, trachea, or critical arteries but not when the nodules were shallow or near the anterior capsule. Thus, we suggest that the functional state of the RLN should be assessed by communicating with patients during MWA, which would help make timely adjustments as needed and minimize the possibility of injury to adjacent tissues. In addition, the higher incidence of temporary reactive hyperplastic lymphadenectasis after MWA compared to surgery could be attributed to the stimulation of the autoimmune response.
Recurrence is of greater concern than post-procedural complications in patients with thyroid carcinoma. No obvious recurrence or metastasis was found in the MWA group during the follow-up, this could be because (1) we had physicians experienced on benign thyroid nodules with MWA; (2) we only included nodules diagnosed as unifocal PTMC [Citation41], with nodule diameter of ≤0.6 cm [Citation29]; and (3) we ensured that the entry and exit paths of the ablation needle are consistent with the previous FNA needle paths to minimize the spread following FNA and ablation. According to the principle of the multidimensional fixed needle, we ensured the completeness of ablation and prevented tumor edge relapse.
Teng et al. indicated that 20 W of power could ensure a mild process of tumor coagulative necrosis, implying that there would be fewer ‘excessive burns’ in the ablation area, rendering it easily absorbable. However, some surgeons were concerned that thermal ablation for primary thyroid cancer might lead to incomplete tumor necrosis [Citation20]. In this study, we adopted an ablation power range of 29.86 ± 1.27 W because considering the tumor size and location, our goal was to ‘burn’ as tumor much as possible. Large tumors located in deeper parts may not be completely ablated by a lower power, and insufficient residual heat during needle removal may cause tumor spread in the needle tract. Although low power also shows satisfactory results, scarce studies have addressed power and PTMC. Thus, to ensure the completeness and long-term effects of MWA, we insist on using a flexible ablation power and plan to focus on this issue in future studies.
The present study has some limitations. First, MWA as new technology has not been fully implemented in China, and its acceptance by patients is limited, resulting in insufficient sample size, compared to the sample size of 119 in Yue et al.’s study. Although no significant difference was observed between the results of studies with sample sizes of 119 and 21 [Citation21,Citation22], we believe that increasing the sample size in future studies would improve the credibility of the data. Second, the sensitivity of ultrasonography was low, and the nodules may have been missed during the examination. The absence of recurrence and metastasis could not prove lower recurrence and metastasis rates in the MWA group than in the surgery group. Thus, improving the sensitivity of follow-up is essential. However, occult metastasis of low-risk PTMC has little effect on the survival rate [Citation2]. Finally, because PTMC is an inert tumor, several patients had a ‘lazy mentality’ during the follow-up period. Nonetheless, we aspire to extend the follow-up period in subsequent studies.
With a deepening understanding of PTMC, the treatment guidelines are constantly updated. The present study proved that the complication, recurrence, and metastasis rates of MWA are low and the prognosis is good. It retains the normal thyroid tissue and has OR and economic advantages, greatly improving the patients' quality of life. In addition, the cosmetic value could also be a reason for choosing MWA. Therefore, US-guided MWA is a feasible alternative therapy for unifocal PTMC with a diameter of ≤0.6 cm. This finding could play an important role in the future clinical treatment of PTMC.
Author contributions
The study was conceived by C.Z.; the data was collected by X.Y.-N. and S.M.; X.Y.-W. and M.Z.-Z. collated and analyzed the data; X.Y.-W., W.B.-J., and D.N.-J. interpreted the data; X.Y.-W., L.X.-Z., and W.Q.-T searched the literature; and X.Y.-W. wrote the first draft of the paper.
Acknowledgments
We would like to thank Taylor & Francis Editing Services for providing language guidance for this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Haddad RI, Nasr C, Bischoff L, et al. NCCN guidelines insights: thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw. 2018;16(12):1429–1440.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid NODULES-2016 UPDATE. Endocr Pract. 2016;22(5):622–639.
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1856–1883.
- Li J, Liu Y, Liu J, et al. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia. 2019;36(1):640–646.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018;34(5):653–659.
- Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet. 2017;390(10092):415–423.
- Ha EJ, Baek JH, Sun MB. Minimally invasive treatment for benign parathyroid lesions: treatment efficacy and safety based on nodule characteristics. Korean J Radiol. 2020;21(12):1383–1392.
- Li X, Fan WJ, Zhang L, et al. CT-guided percutaneous microwave ablation of liver metastases from nasopharyngeal carcinoma. J Vasc Interv Radiol. 2013;24(5):680–684.
- Liang P, Wang Y, Zhang D, et al. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol. 2008;180(3):844–848; discussion 848.
- Wang Y, Liang P, Yu X, et al. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009;25(6):455–461.
- Belfiore G, Ronza F, Belfiore MP, et al. Patients' survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82(1):177–181.
- Dong P, Wu XL, Sui GQ, et al. The efficacy and safety of microwave ablation versus lobectomy for the treatment of benign thyroid nodules greater than 4 cm. Endocrine. 2021;71(1):113–121.
- Quang KH, Quoc HN, Huu VV, Van KN, et al. Efficacy of microwave ablation in the treatment of large (≥3 cm) benign thyroid nodules. World J Surg. 2020;44(7):2272–2279.
- Wu W, Gong X, Zhou Q, et al. US-guided percutaneous microwave ablation for the treatment of benign thyroid nodules. Endocr J. 2017;64(11):1079–1085.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–e16.
- Pacella CM. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20(4):347–349.
- Zhi X, Zhao N, Liu Y, et al. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia. 2018;34(5):644–652.
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166(6):1031–1037.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab. 2020;105:dgaa128.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30(2):150–157.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol. 2021;11:575152.
- Thong ISK, Jensen MP, Miró J, et al. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;18(1):99–107.
- Morelli F, Sacrini A, Pompili G, et al. Microwave ablation for thyroid nodules: a new string to the bow for percutaneous treatments? Gland Surg. 2016;5(6):553–558.
- Huang MK, Liu JB. Application of ultrasonography in the diagnosis and management of papillary thyroid microcarcinoma. Adv Ultrasound Diagn Ther. 2020;4:284.
- Xu B, Zhou NM, Cao WT, et al. Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. WJCC. 2018;6(15):936–943.
- Zhao C, Jiang W, Gao Y, et al. Risk factors for lymph node metastasis (LNM) in patients with papillary thyroid microcarcinoma (PTMC): role of preoperative ultrasound. J Int Med Res. 2017;45(3):1221–1230.
- Wang L, Xu D, Yang Y, et al. Safety and efficacy of ultrasound-guided percutaneous thermal ablation in treating low-risk papillary thyroid microcarcinoma: a pilot and feasibility study. J Can Res Ther. 2019;15(7):1522–1529.
- Min Y, Wang X, Chen H, et al. Thermal ablation for papillary thyroid microcarcinoma: how far we have come? Cancer Manag Res. 2020;12:13369–13379.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–S203.
- Chen J, Cao J, Qiu F, et al. The efficacy and the safety of ultrasound-guided ablation therapy for treating papillary thyroid microcarcinoma. J Cancer. 2019;10(21):5272–5282.
- Cui T, Jin C, Jiao D, et al. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: a systematic review and meta-analysis. Eur J Radiol. 2019;118:58–64.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22(9):1983–1990.
- Dodd GD III, Dodd NA, Lanctot AC, et al. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267(1):129–136.
- Teng DK, Li WH, Du JR, et al. Effects of microwave ablation on papillary thyroid microcarcinoma: a five-year follow-up report. Thyroid. 2020;30(12):1752–1758.
- Shen Y, Liu M, He J, et al. Differences in treatment outcomes between ultrasound-guided percutaneous microwave ablation and endoscopic thyroidectomy for patients with papillary thyroid microcarcinoma: protocol for a systematic review and Meta-analysis. Medicine. 2019;98(1):e13953.
- Shen K, Xue S, Xie Y, et al. Comparison of thermal ablation and routine surgery for the treatment of papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):913–924.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64(1):109–117.