Abstract
Objectives
Mutations in the human IQSEC2 gene are associated with drug-resistant epilepsy and severe behavioral dysfunction. We have focused on understanding one human IQSEC2 missense mutation (A350V) for which we have created a corresponding A350V IQSEC2 mouse model by CRISPR which demonstrates seizures when the mice are 15–20 days old and impaired social vocalizations in adulthood. We observed that a child with the A350V mutation stops having seizures when experiencing a fever of greater than 38 °C. In this study, we first sought to determine if we could recapitulate this phenomenon in A350V 15–20 day old mice using a previously established protocol to raise body temperature to 39 °C achieved by housing the mice at 37 °C. We then sought to determine if mice in whom seizure activity had been prevented as pups would develop social vocalization activity in adulthood.
Methods
15–20 day old A350V male mice were housed either at 37 °C or 22 °C. Ultrasonic vocalizations of these mice were assessed at 8–10 weeks in response to a female stimulus.
Results
Housing of 15–20 day old A350V mice at 37 °C resulted in a reduction in lethal seizures to 2% (1/41) compared to 45% (48/108) in mice housed at 22 °C, p = 0.0001. Adult A350V mice who had been housed at 37 °C as pups displayed a significant improvement in the production of social vocalizations.
Conclusion
Raising the body temperature by raising the ambient temperature might provide a means to reduce seizures associated with the A350V IQSEC2 mutation and thereby allow for an improved neurodevelopmental trajectory.
Mutations in the X-linked human IQSEC2 gene are associated with drug-resistant epilepsy as well as severe disturbances in cognitive and social behavior [Citation1–4]. Our laboratory has focused on understanding one specific human IQSEC2 missense mutation (A350V) [Citation5] for which we have created a corresponding A350V IQSEC2 mouse model by CRISPR which demonstrates marked abnormalities in social behavior, cognition and seizures [Citation6–8].
Over 20 different seizure medications and the ketogenic diet have failed to reduce seizures in a child with the A350V IQSEC2 mutation. The seizure burden in this child ranges from 5 to 15 a day with both tonic clonic and drop attacks seen [Citation5]. A remarkable finding in this child repeatedly observed beginning at 1 year of age is that when he has a temperature of greater than 38 °C lasting more than 1 day his seizures abruptly stop and then he has a seizure-free window of up to 3 weeks after his temperature has returned to normal.
In this study, we set out to determine if a previously established method to raise the body temperature could result in a cessation of seizures in A350V IQSEC2 mice. Demonstrating that seizures could be reduced in the mice using this approach allowed us to ask whether the heavy seizure burden in A350V mice could play a major role in the abnormal development of their social behavior
Methods
Animals
The generation of A350V IQSEC2 mice in a C57BL6/J background has previously been described [Citation6]. All mice used in this study were generated from a cross between a female heterozygous for the A350V mutation and a wild-type IQSEC2 male resulting in half of the offspring having the mutation (hemizygous in males) and half being wild type for IQSEC2. Only male mice were used for experiments to avoid the confounding effects of X-inactivation in females. Mice were housed in a specific pathogen-free (SPF) facility with free access to water and food at 22 °C. All animals were kept on a 12-h light/12-h dark cycle, light on at 9.00pm, with ad libitum access to food and water. Behavioral experiments took place during the dark phase under dim red light. Mice were genotyped when they were 12–13 days old by polymerase chain reaction (PCR) from extracted tail DNA as previously described [Citation6].
Protocol to generate fever range hyperthermia by raising the ambient temperature to 37 °C
We used a previously published long-term fever range hyperthermia protocol in which mice were housed at a temperature of 37 °C in order to achieve a core body temperature of 39–39.5 °C for 5 days [Citation9,Citation10]. 15-day old pups with their mothers in their regular cages were placed in a neonatal temperature-controlled incubator set at 37 °C for 5 days (Draeger model 8000IC, Lubeck, Germany). A new HydroGel non-wetting water gel pouch (227 g) (Envigo Gel Transit Kit HSD Clear H20 catalog# 70-01-1082) was placed on the floor of the cage every day to help provide adequate hydration to the pups. This protocol was approved by the Institutional Animal Care and Use Committee of the Technion Israel Institute of Technology (protocol #IL-114-09-20). At the completion of the 5-day period in the 37 °C incubators the mice were either sacrificed immediately for harvesting tissues or returned to room temperature housing (22 °C) for subsequent assessment of their vocalization behavior as adults.
Protocol to generate fever using lipopolysaccharide (LPS)
We used a previously published protocol in which intraperitoneal injection of LPS in mice housed at 30 °C was demonstrated to result in a prolonged rise in the core temperature up to 39 °C [Citation11,Citation12]. 15 days old pups were injected intraperitoneally with 2.5 mg/kg of LPS (Sigma lipopolysaccharide E. coli 0111: B4 catalog # 2630). The pups with their mothers in their regular cages were then placed in a Draeger incubator (see above) at 30 °C for 5 days. The injection of LPS for these studies was approved by the Institutional Animal Care and Use Committee of the Technion Israel Institute of Technology (protocol #IL-025-0220).
Assessment of male–female ultrasonic vocalizations
Ultrasonic vocalizations were recorded using a condenser ultrasound microphone (Polaroid/CMPA, Avisoft). The microphone was connected to an ultrasound recording interface (UltrasoundGate 116Hme, Avisoft), which was plugged into a computer equipped with the recording software Avi-soft Recorder USG (sampling frequency: 250 kHz; FFT-length 1024 points; 16-bit format). Vocalizations were recorded in 8–10 week old male wild type or A350V mice during a 5 min interaction with a female C57Bl/6 stimulus, which was preceded by 15 min of habituation to the arena. Ultrasonic vocalizations were analyzed using our TrackUSF custom-made software as described in https://www.biorxiv.org/content/10.1101/575191v1.
RNA isolation and qRT-PCR
Quantitative real-time-polymerase chain reaction (qRT-PCR) was performed on RNA isolated from hippocampal tissue. RNA was isolated using RNAeasy mini kit (Qiagen). RNA was converted to cDNA using the high capacity cDNA reverse transcriptase kit with RNase inhibitor (Applied Biosystems AB4374966) and qRT-PCR was performed with Fast SYBR Green master mix (Applied Biosystems AB-4385612) using the StepOnePlus Real-Time PCR system (ThermoFisher 4376600). Signals for HspA1A, IL-6, TNFα, and IL-17α were normalized to the housekeeping genes HPRT or PGK1 [Citation13].
RTPCR primers
Hspa1a – F: GTGTTTGGACTCTCCCCTGG; R: GGAGAAGCAGCAGAGTCTCC
HPRT – F: CAGTCCAGCGTCGTGATTA; R: TGGCCTCCCATCTCCTTCAT
PGK1 – F: CACCGAGCCCATAGCTCCAT; R: CTGCAACTTTAGCGCCTCCC
IL-6 – F: TAGTCCTTCCTACCCCAATTTCC; R: TTGGTCCTTAGCCACTCCTTC
TNFα – F: CAGGCGGTGCCTATGTCTC; R: CGATCACCCCGAAGTTCAGTAG
IL-17α – F: CCAGGGAGAGCTTCATCTGT; R: AGGAAGTCCTTGGCCTCAGT
Statistical analysis
For the analysis of vocalizations between the four groups (data from WT or A350V mice housed between days 15–20 at 22 °C (RT) or 37 °C (HS)) statistical tests were performed using SPSS 21.0. Data normality was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Two out of the four data sets were distributed normally. For comparison between multiple groups or parameters, a classical analysis of variance (ANOVA) was applied to the data. The ANOVA test was followed by post hoc Student’s t-test with Bonferroni’s correction for prespecified relevant comparisons (WT-RT vs WT-HS and A350V-RT vs A350V-HS). We additionally performed statistical analysis of the vocalizations using a non-parametric test (Mann–Whitney) and obtained a significance of p ≤ 0.05 for both comparisons (WT-RT vs WT-HS and A350V-RT vs A350V-HS).
Results
A350V IQSEC2 mice have lethal seizures between 15 and 20 days after birth
Male A350V IQSEC2 mice have frequent seizures manifested as irregular clonic movements frequently associated with the mouse falling on its side and exhibiting tonic clonic movements of all extremities. Mice viewed to have seizures typically died within a few hours of the onset of seizures. The A350V IQSEC2 mice housed at 22 °C have visible seizures only between 15 and 20 days after birth with approximately 45% of all male mutant mice (48/108) dying during this period with a peak incidence of mortality between post-natal days 17–18 (over 70% of all deaths) (). No seizures or mortality have been observed in A350V IQSEC2 mice after 21 days of age.
Figure 1. Increasing the ambient temperature to 37 °C prevents lethal seizures in A350V mice. A350V IQSEC2 mice were placed in a 37 °C incubator between 15 and 20 days of age and mortality compared to A350V IQSEC2 mice followed in parallel under routine animal facility temperature housing conditions (22 °C). No mortality was observed in wild type IQSEC2 mice at 22 °C or 37 °C under these conditions. Fever range hyperthermia resulted in a dramatic reduction in mortality in the A350V mice (1/41 vs 48/108; p log rank = 0.0001). No deaths occurred after 21 days up to at least 9 months of age.
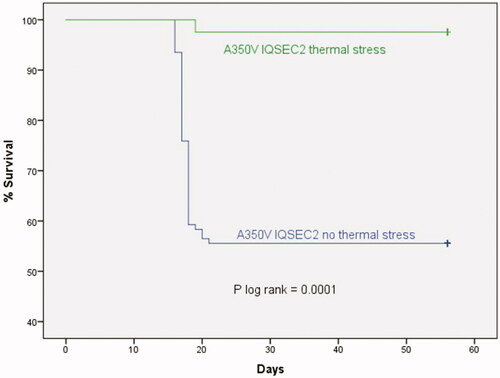
Prevention of lethal seizures in A350V IQSEC2 mice by environmentally induced thermal stress
In order to test whether raising the body temperature in A350V IQSEC2 mice could reduce seizures, we sought an established protocol to sustain an elevated body temperature during the age window when the A350V mice normally have seizures (age 15–20 days as shown in ). Hasday and colleagues [Citation9,Citation10] previously demonstrated that when mice are housed at a temperature of 37 °C, due to a limited capacity to eliminate heat, they develop an increase in core body temperature of approximately 39 °C that is well tolerated for 5 days. In a consecutive series of 41 A350V IQSEC2 mice from 20 different litters housed in a 37 °C chamber starting at age 15 days until age 20 days we observed only one death in the A350V mice (2%) while during this same 15–20 day age window in A350V mice housed at 22 °C the mortality rate was approximately 45% (p < 0.0001 by log-rank) (). No mortality was found in wild-type littermates during this treatment. We also assessed the survival of A350V mice at an intermediate chamber temperature of 30 °C, which is thought to be thermoneutral for mice [Citation14], at which we observed no protection from lethal seizures (mortality rate from days 15–20 of 62%, n = 8). The housing of the mice for only 1 day at 37 °C (starting at day 15) instead of 5 days did not provide significant protection against lethal seizures over the 15–20 day period (mortality rate of 5/16 (31%) with a 1 day 37 °C exposure vs 48/108 (44%) with a 5 day 37 °C exposure, p = 0.31).
Induction of heat shock chaperone Hspa1a in the brain of mice housed for 5 days at 37 °C
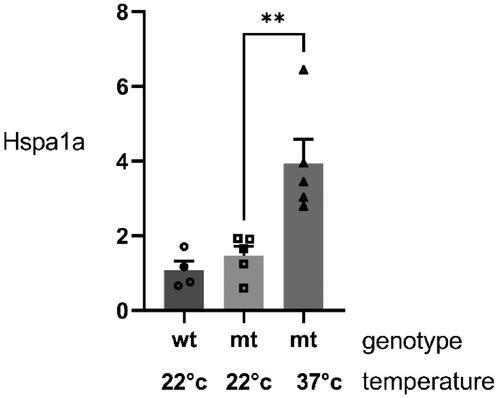
In order to demonstrate that the housing of 15–20 day old mice at an ambient temperature of 37 °C induced a physiologically relevant increase in temperature in brain tissue, we measured the production of heat shock Hspa1a mRNA in the hippocampus after this exposure. Hspa1a is the major Hsp70 in mice and is known to be induced by an increase in temperature in vitro and in vivo [Citation15]. We assessed if the fever range hyperthermia protocol was associated with an increase in Hspa1a in the hippocampus which is the major site of production of IQSEC2 in the brain [Citation16,Citation17] and where IQSEC2 mutation mediated pathology has been shown to be prominent [Citation6,Citation18–20]. Compared to A350V IQSEC2 mice housed at room temperature (22 °C), we observed that A350V mice housed for 5 days at 37 °C demonstrated a significant induction of Hspa1a mRNA by qRT-PCR (2.7-fold increase 3.9 ± 0.6 vs 1.5 ± 0.2 normalized to HPRT, p = 0.007, n = 5 for each group) ().
Figure 2. Induction of Hspa1a in the hippocampus of mice housed at an ambient temperature of 37 °C. qRT-PCR for Hspa1a from the hippocampus of 20-day old wild type (wt) mice (n = 4) and A350V (mt) mice (n = 5) housed at 22 °C and 20 day old A350V (mt) mice housed at 37 °C between 15 and 20 days of age (n = 5). There was a significant increase in Hspa1a in the A350V mice housed at 37 °C (one way ANOVA across all groups F(2, 11) = 11.78, p = 0.0018; Student’s t-test comparing A350V mice housed at 37 °C compared to A350V mice housed at 22 °C, p = 0.007).
Failure to prevent lethal seizures in A350V IQSEC2 mice by LPS
Having demonstrated that we could reduce seizures by raising the body core temperature using an environmental stimulus we sought to determine if a protocol reported to raise the body core temperature using a pyrogen (LPS) could also provide protection against lethal seizures in the A350V mice [Citation11,Citation12]. Previous work has established that in rodents endotoxin only induces a rise in body core temperature if the mice are housed at thermoneutrality (30 °C) [Citation11,Citation12]. We injected LPS intraperitoneally at a dose (2.5 mg/kg) at day 15 and housed the mice at 30 °C which has been shown to result in a rise in the body core temperature to 39 °C [Citation12]. We found that LPS had no effect on the incidence of lethal seizures (8/18; 44%) compared to mice that had not been injected with endotoxin (48/108; 44%). Injection with LPS was not associated with a significant change in hippocampal Hspa1a 18 h after the 15-day old mice were injected (1-way ANOVA; F(3, 20)=1.83; p = 0.17) suggesting that in the 15-day old mice LPS does not appear to have produced a rise in body core temperature. In order to verify that the LPS we used was biologically active we measured hippocampal TNFa [Citation21] by qRT-PCR in the LPS injected mice 18 h after they were injected and found an approximately 10- to 20-fold induction of TNFa compared to mice that had not been injected with LPS (1-way ANOVA; F(3, 20) = 3.83; p = 0.026).
Housing mice at 37 °C between 15 and 20 days after birth permits the development of contextually appropriate social vocalizations in adult A350V mice
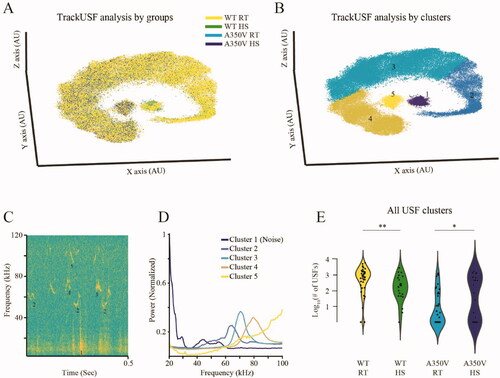
After having demonstrated that we could prevent seizures in A350V mice as pups by housing them at an ambient temperature of 37 °C we sought to determine what consequences this would have on the behavior of these mice when they were adults. We have previously reported that mating calls toward a female mouse are essentially absent in adult male A350V mice [Citation8]. We hypothesized that preventing seizures in A350V mice during a critical period of their development might allow the A350V mice to develop more normal context-appropriate social behavior such as vocalizations in response to a female stimulus. We tested this hypothesis in wild-type and A350V mice which had either been housed at 37 °C or 22 °C between 15 and 20 days of age. Vocalizations and their analysis were performed as described in methods and are shown in . As shown in , we found that the number of vocalizations was significantly increased in adult A350V mice if they had previously been housed at 37 °C (HS) as compared to 22 °C (RT) between 15 and 20 days of age. On the other hand, in WT mice which were housed with HS during this period, there was a significant decrease in the number of vocalizations which they exhibited as adults as compared to WT mice housed at RT (2-way ANOVA, interaction genotype x treatment, F(1, 123) = 15.486, p < 0.001; post hoc t-test with Bonferroni’ s corrections following the main effect, *p = 0.02 comparing A350V ± HS, **p = 0.01 comparing WT ± HS).
Figure 3. Prevention of seizures in A350V pups by housing at 37 °C ambient temperature allows for the development of social vocalizations in A350V adults. (A) 3D t-SNE analysis of all ultrasonic fragments (USFs) emitted by 8–10 week old wild type (WT) IQSEC2 and A350V IQSEC2 male mice who had been housed between 15 and 20 days of age either at room temperature (RT) (RT n = 38 for WT and n = 40 for A350V) or 37 °C (HS) (HS n = 24 for WT and n = 25 for A350V), during an encounter with a female C57BL/6J mouse. Each dot represents a USF, color-coded according to genotype and housing temperature. (B) Similar to A, showing the distinct manually-defined clusters color coded and numbered. (C) Examples of USFs, denoted by their cluster numbers, superimposed on the spectrograms of their respective audio signals. Note that cluster-1 represents non-vocal (noise) signals while all other clusters represent genuine vocalizations. (D) Power Spectral Density (PSD) analysis of all USFs recorded from all animals, calculated separately for each cluster, color coded according to B. Note the PSD profile of cluster-1 (noise) which shows a wide range of frequencies, mainly at the lower range. (E) Violin plot of the total number of USFs from all vocalizations clusters (excluding cluster-1) for 8–10 week old wild type or A350V mice housed as pups (day 15–20) at RT or 37 °C. A350V adult mice treated with heat stress as pups exhibited increased social vocalizations while adult WT animals exhibited fewer vocalizations if they had been treated with heat stress as pups (2-way ANOVA, interaction genotype × treatment, F(1, 123) = 15.486, p < 0.001; post hoc t-test with Bonferroni’ s corrections following main effect, *p = 0.02 comparing A350V ± HS, **p = 0.01 comparing WT ± HS).
Interleukin 17a which has been causally linked to the beneficial effect of fever on the social behavioral deficits of autism is induced in the hippocampus of mice housed at 37 °C from days 15 to 20
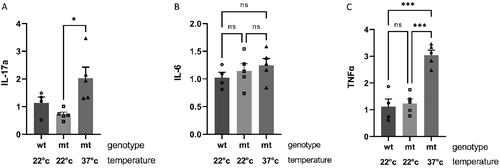
Recently Reed and colleagues have provided evidence that the benefit of fever on social behavioral dysfunction is mediated by the inflammatory cytokine, Interleukin 17-a (IL-17a) [Citation22]. As Il-17a is known to be induced by heat stress [Citation23] we investigated the induction of IL-17a in the hippocampus of A350V mice by qRTPCR and found an approximately 3 fold statistically significant induction in A350V mice housed for 5 days at 37 °C as compared to A350V mice housed at 22 °C (2.0 ± 0.4 vs 0.7 ± 0.1, p = 0.01, n = 5 for both groups) (). We also assessed the induction of two other inflammatory cytokines which have been associated with the heat shock response, IL-6 and TNFa [Citation24], and found that TNFa was significantly induced by the 5-day treatment at 37 °C ().
Figure 4. Induction of inflammatory cytokines in the hippocampus of mice housed at an ambient temperature of 37 °C. qRT-PCR for Il-6, TNFa and Il-17a from the hippocampus of 20-day old wild type (wt) mice (n = 4) and A350V (mt) mice (n = 5) housed at 22 °C and 20 day old A350V (mt) mice housed at 37 °C between 15 and 20 days of age (n = 5). (A) There was a significant change in Il-17a in the A350V mice housed at 37 °C (one way ANOVA across all groups F(2, 11) = 6.53, p = 0.01; *Student’s t-test comparing A350V mice housed at 37 °C compared to A350V mice housed at 22 °C, p = 0.01). (B) There was no significant change in Il-6 in the A350V mice housed at 37 °C (one way ANOVA across all groups F(2, 11) = 0.80, p = 0.47). (C) There was a significant increase in TNFa in the A350V mice housed at 37 °C (one way ANOVA across all groups F(2, 11) = 28.0; p < 0.0001; Student’s t-test comparing A350V mice housed at 37 °C compared to A350V mice housed at 22 °C; ***p < 0.0001).
Discussion
We have demonstrated here in a mouse model of the human A350V IQSEC2 mutation that by increasing the ambient temperature to 37 °C between 15 and 20 days of age that we can prevent spontaneous lethal seizures and permit the development of contextually appropriate social vocalizations. As alleviation of symptoms of autism spectrum disorder by fever has been described by several groups [Citation25,Citation26], this finding is potentially of major pathophysiological, mechanistic, and translational importance that may extend far beyond the model used in this study.
An important limitation of this study was our inability to measure the body core temperature in 15–20 day old mice housed at 37 °C due to their small size (5 g) with there being no commercially available means to monitor the core temperature of unrestrained mice of this size [Citation27]. However, the use of a 37 °C ambient temperature for a 5-day duration has previously been shown to result in a rise in body core temperature to 39–39.5 °C in mice [Citation9,Citation10]. Furthermore, our demonstration that hippocampal Hsp70 mRNA is induced by housing the mice at 37 °C ambient temperature provides evidence that these conditions produced a physiologically relevant increase in temperature in brain tissue.
The very high frequency of seizures associated with the A350V IQSEC2 in infancy and early childhood suggests that the cognitive and behavioral abnormalities seen in the child with the mutation are due to an epileptic encephalopathy [Citation28,Citation29]. We have shown here that A350V IQSEC2 mice in whom seizures were prevented showed improved vocalization capacity as adults. This result suggests that aggressive seizure control in the child with the A350V IQSEC2 mutation might allow for an improved neurodevelopmental trajectory. In as much as mouse ultrasonic vocalizations differ from human speech, several genes linked to human speech disorders have been recapitulated in transgenic mice models [Citation30]. Therefore, as the child with the A350V mutation is aphasic with no spoken words, the finding that stopping seizures in A350V mice allows for the development of context-appropriate social vocalizations offers hope that with aggressive learning techniques such as applied behavioral analysis (ABA) [Citation31] it may be possible for the child with the A350V mutation to learn some speech if his seizures are prevented.
We can only speculate at this time as to the mechanism whereby elevated body temperature in the child or housing of 15–20 day old A350V mice at an ambient temperature of 37 °C prevents seizures. One possible mechanism by which elevated temperature or fever may provide protection is via induction of the heat shock protein response. Heat shock proteins function as molecular chaperones for improperly folded proteins [Citation32–34] and promote the restoration of protein folding and normal protein function and may have the capacity to rescue and allow a tolerance of specific missense mutations [Citation34]. We have shown that the heat shock response is induced in mice housed at an ambient temperature of 37 °C which has been shown previously to result in a core body temperature of 39–39.5 °C. We have observed changes in the electrophoretic mobility of the A350V IQSEC2 protein suggesting that the protein is misfolded. The A350V mutation results in the generation of a valine residue which is modeled to be exposed on the surface of the A350V protein representing a potential binding site for heat shock protein chaperones which are thought to recognize hydrophobic epitopes [Citation32,Citation33]. We are currently investigating the effect of heat shock on the folding of the A350V IQSEC2 protein as well as the interaction of A350V with specific heat shock proteins.
We were not able to demonstrate the prevention of seizures in the A350V IQSEC2 mice by LPS which had previously been shown to induce an increase in core temperature in adult mice. One explanation for this may be that the thermoregulatory center mediating the response to pyrogens in older rodents is not mature in 15-day old mouse pups analogous to what is found in many species [Citation35–38] including human newborns less than 2 weeks of age (as well as in the very old) which frequently do not mount a fever response to infection [Citation37]. Indirect evidence for the lack of a fever response in the 15–20 day old pups exposed to LPS is provided by a failure to see a significant increase in Hspa1a with LPS treatment.
The ability of environmentally induced hyperthermia to reduce seizures in the A350V IQSEC2 mouse model has important potential translational applications in man. Inspired by the current study showing protection by environmentally induced hyperthermia in A350V IQSEC2 mice we have recently assessed the effect of raising the body temperature of a child with the A350V IQSEC2 mutation using a Jacuzzi set at 40 °C for periods of 15 min twice a day and have found a profound 90% reduction in seizure activity ([Citation39]. We hope that this will inspire others to investigate the possible beneficial role of moderate environmentally induced hyperthermia to other protein folding disorders.
While the curative power of fever and raising body temperature dates back to antiquity with Hippocrates describing the beneficial effect of fever on epilepsy [Citation40] we are unable to find any reference in the modern literature of the use of fever therapy in any patient to prevent seizures. We are not suggesting that raising the body temperature should be tried for the vast majority of children with seizures, and our finding regarding A350V is contrary to current clinical experience showing that an elevated body temperature is a frequent cause of seizures in children [Citation41]. We are proposing here that raising the body core temperature may be beneficial in some settings, particularly those due to a protein folding abnormality, and is worthy of additional investigation. Unraveling the mechanism responsible for the protection by environmentally induced hyperthermia in the A350V model may provide new treatments for many neurodevelopmental and neurodegenerative disorders [Citation42,Citation43].
Acknowledgments
We wish to thank Mr. Paul Zino for his excellent help in the care of the animals used in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Shoubridge C, Tarpey PS, Abidi F, et al. Mutations in the guanine nucleotide exchange factor gene IQSEC2 cause nonsyndromic intellectual disability. Nat Genet. 2010;42(6):486–488.
- Zerem A, Haginoya K, Lev D, et al. The molecular and phenotypic spectrum of IQSEC2-related epilepsy. Epilepsia. 2016;57(11):1858–1869.
- Shoubridge C, Harvey RJ, Dudding-Byth T. IQSEC2 mutation update and review of the female-specific phenotype spectrum including intellectual disability and epilepsy. Hum Mutat. 2019;40(1):5–24.
- Mignot C, McMahon AC, Bar C, et al. IQSEC2-related encephalopathy in males and females: a comparative study including 37 novel patients. Genet Med. 2019;21(4):837–849.
- Zipper R, Baine SD, Genizi J, et al. Developmental progression of intellectual disability, autism and epilepsy in a child with an IQSEC2 gene mutation. Clin Case Rep. 2017;5(10):1639–1643.
- Rogers EJ, Jada R, Schragenheim-Rozales K, et al. An IQSEC2 mutation associated with intellectual disability and autism results in decreased surface AMPA receptors. Front Mol Neurosci. 2019;12:43.
- Levy NS, Umanah GKE, Rogers EJ, et al. IQSEC2 associated intellectual disability and autism. Int J Mol Sci. 2019;20(12):3038.
- Jabarin R, Levy N, Abergel Y, et al. Pharmacological modulation of AMPA receptors rescues specific impairments in social behavior associated with the A350V IQSec2 mutation. Transl Psych. 2021;11:234.
- Hasday JD, Garrison A, Singh IS, et al. Febrile range hyperthermia augments pulmonary neutrophil recruitment and amplified pulmonary oxygen toxicity. Am J Path. 2003;162(6):2005–2017.
- Sareh H, Tulapurkar ME, Shah NG, et al. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperones. 2011;16(3):297–307.
- Rudaya AY, Steiner AA, Robbins JR, et al. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1244–R1252.
- Schneiders J, Fuchs F, Damm J, et al. The transcription factor nuclear factor interleukin 6 mediates pro- and anti-inflammatory responses during LPS-induced systemic inflammation in mice. Brain Behav Immun. 2015;48:147–164.
- Panina Y, Germond A, Masui S, et al. Validation of common housekeeping genes as reference for qPCR gene expression analysis during iPS reprogramming process. Sci Rep. 2018;8(1):8716.
- Gordon CJ. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol Behav. 2017;179:55–66.
- Faulkner SH, Jackson S, Fatania G, et al. The effect of passive heating on heat shock protein 70 and interleukin-6. Temperature. 2017;4(3):292–304.
- Murphy JA, Jense ON, Walikonis RS. BRAG1, a Sec7 domain-containing protein, is a component of the postsynaptic density of excitatory synapses. Brain Res. 2006;1120(1):35–45.
- Sakagami H, Sanda M, Fukaya M, et al. IQ-ArfGEF/BRAG1 is a guanine nucleotide exchange factor for ARF6 that interacts with PSD-95 at postsynaptic density of excitatory synapses. Neurosci Res. 2008;60(2):199–212.
- Sah M, Shore AN, Petri S, et al. Altered excitatory transmission onto hippocampal interneurons in the IQSEC2 mouse model of X-linked neurodevelopmental disease. Neurobiol Dis. 2020;137:104758.
- Jackson MR, Loring KE, Homan CC, et al. Heterozygous loss of function of IQSEC2/Iasec2 leads to increased activated Arf6 and severe neurocognitive seizure phenotype in females. Life Sci Alliance. 2019;2(4):e201900386.
- Brown JC, Petersen A, Zhong L, et al. Bidirectional regulation of synaptic transmission by BRAG1/IQSEC2 and its requirement in long-term depression. Nat Commun. 2016;7:11080.
- Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58(1):61–77.
- Reed MD, Yim YS, Wimmer RD, et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577(7789):249–253.
- Wang X, Ni L, Wan S, et al. Febrile temperature critically controls the differentiation and pathogenicity of T helper 17 cells. Immunity. 2020;52(2):328–341.
- Dukay B, Csoboz B, Toth ME. Heat shock proteins in neuroinflammation. Front Pharm. 2019;10:920.
- Curran LK, Newschaffer CJ, Lee LC, et al. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120(6):e1386–e1392.
- Grzadzinski R, Lord C, Sanders SJ, et al. Children with autism spectrum disorder who improve with fever: insights from the simons simplex collection. Autism Res. 2018;11(1):175–184.
- Meyer CW, Ootsuka Y, Romanovsky AA. Body temperature measurements for metabolic phenotyping in mice. Front Phys. 2017; 8:520.
- Jain P, Sharma S, Tripathi M. Diagnosis and management of epileptic encephalopathies in children. Epilepsy Res Treat. 2013;2013:501981.
- Lado FA, Rubboli G, Capovilla G, et al. Pathophysiology of epileptic encephalopathies. Epilepsia. 2013;54:6–13.
- Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25(4):166–177.
- Foxx RM. Applied behavior analysis treatment of autism: the state of the art. Child Adolesc Psychiatr Clin N Am. 2008;17(4):821–834.
- Valastyan JS, Lindquist S. Mechanisms of protein-folding diseases at a glance. Dis Model Mech. 2014;7(1):9–14.
- Luengo TM, Mayer MP, Rudiger SGD. The Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell Biol. 2019;29(2):164–177.
- Karras GI, Yi S, Sahni N, et al. HSP90 shapes the consequences of human genetic variation. Cell. 2017;168(5):856–866.
- Conklin P, Heggeness FW. Maturation of tempeature homeostasis in the rat. Am J Physiol. 1971;220(2):333–336.
- Cooper KE, Veale WL, Kasting N, et al. Ontogeny of fever. Fed Proc. 1979;38(1):35–38.
- Haahr S, Mogensen S. Function of fever. Lancet. 1977;310(8038):613.
- Satinoff E, McEwen GN, Williams BA. Behavioral fever in newborn rabbits. Science. 1976;193(4258):1139–1140.
- Levy AP, Levy NS, Heyman E, et al. Reduction in seizure burden in a child with a A350V IQSEC2 mutation using heat therapy with a jacuzzi. Clinical Case Rep. 2021;9(9):e04734.
- Duffell E. Curative power of fever. Lancet. 2001;358(9289):1276.
- El-Rahdi AS. Fever management: evidence vs current practice. World J Clin Ped. 2012;1:29–33.
- Hunt AP, Minett GM, Gibson OR, et al. Could heat therapy be an effective treatment for Alzheimer’s and Parkinson’s diseases? A narrative review. Front Physiol. 2020;10:1556.
- Paul S, Mahanta S. Association of heat-shock proteins in various neurodegenerative disorders: is it a master key to open the therapeutic door? Mol Cell Biochem. 2014;386(1–2):45–61.
