Abstract
Introduction
Unresectable hilar cholangiocarcinoma (UHC) is a malignant tumor and has a poor prognosis. IRE is a novel non-thermal ablative therapy that causes cellular apoptosis via electrical impulses. To compare the curative effect for UHC, chemotherapy plus concurrent IRE and chemotherapy alone were set up.
Materials and methods
From July 2015 to May 2019, 47 patients with UHC were analyzed to chemotherapy + IRE group (n = 23) or chemotherapy alone group (n = 24) in this study. Treatment response was assessed with computed tomography (CT) or magnetic resonance imaging (MRI) 1 month after treatment and every 3 months thereafter. Local tumor progression (LTP), time to LTP, overall survival (OS) and procedure-related complications were compared between the two groups.
Results
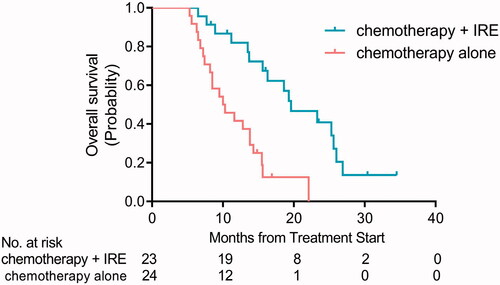
Chemotherapy plus concurrent IRE group showed a tendency toward a decreased rate of LTP (16.7% vs. 39.5%; p = 0.039) and an increased complete response rate (52.2% vs. 12.5%; p = 0.011) compared with chemotherapy alone group. Time to LTP was significantly longer in the chemotherapy plus concurrent IRE group compared to chemotherapy alone group (11.2 months vs. 4.2 months; p = 0.001). Median OS was significantly longer in the chemotherapy plus concurrent IRE group compared to chemotherapy alone group (19.6 months vs. 10.2 months; p = 0.001).
Conclusions
Chemotherapy plus concurrent IRE improved local control and prolonged time to LTP and OS in patients with UHC.
Introduction
Hilar cholangiocarcinoma is a common malignant tumor with a relatively poor prognosis [Citation1]. The overall 5-year survival rate is approximately 13–40% [Citation2–4]. Total 50% of patients are diagnosed during advanced stage of the disease and have missed the opportunity for radical surgical resection because of vascular involvement [Citation5]. Mortality is mainly due to tumor recurrence. Approximately 83% of recurrences are local, suggesting that in such highly invasive tumors, local tumors are not adequately controlled [Citation6].
The gold standard of treatment for patients with unresectable hilar cholangiocarcinoma (UHC) is palliative chemotherapy. There have been large-scale clinical trials with or without combination of gemcitabine with cisplatin, oxaliplatin combined with fluorouracil or capecitabine, and satisfactory results have been achieved [Citation7,Citation8]. S-1 is a new oral fluorouracil prodrug, containing three components which have been well tolerated in previous S-1 studies [Citation9]. Intravenous systemic chemotherapy with gemcitabine combining cisplatin has been proposed as the standard treatment for advanced biliary tract cancer, but most studies have reported toxicities, with grade 3 or higher toxicities occurring in 70% of patients [Citation10,Citation11].
Several ablation techniques have been used to treat UHC, including stereotactic body radiation therapy (SBRT), photodynamic therapy (PDT) and radiofrequency ablation (RFA) [Citation12–15]. These local ablation techniques show some promise, but also have significant limitations. Regarding PDT, due to the use of photosensitizers, photodynamic therapy causes severe skin phototoxicity and damages the normal surrounding tissues [Citation12]. Furthermore, RFA is limited by a heat-sink effect when tumors are located in close proximity to vascular structures [Citation16,Citation17]. In addition, the biggest problem with perihilar ablations is the risk of injury to the central bile ducts in the form of biliary obstruction, biliary strictures, biliary leaks and bilious. Due to the location of the UHC near the bile ducts, hepatic portal vein and hepatic artery, this is particularly challenging.
Irreversible electroporation (IRE) is a novel tumor ablation that utilizes high-voltage pulses to construct permanent nanopores within the cellular membrane and results in apoptosis of the targeted cells [Citation18]. The biggest advantage of IRE is that collagen fibers and other connective tissue components don’t degenerate during IRE ablation, therefore, important tissue structures within the ablation zone are not damaged, such as bile ducts, portal veins, and hepatic veins. Thus, IRE ablation may be a more appropriate choice for UHC. Moreover, most research on IRE has focused on delaying bile duct obstruction rather than reducing tumor size [Citation19,Citation20]. Furthermore, no significant improvement in long-term survival has been observed. Hence, it is unknown whether the addition of IRE to chemotherapy could improve local tumor control in UHC patients.
Given the limited therapeutic options for UHC patients, the aim of the present study was to compare the effect of chemotherapy plus concurrent IRE and chemotherapy alone for UHC.
Methods
Patient selection
This retrospective study was approved by the Ethics Committee of the Affiliated Fuda Cancer Hospital, Jinan University [FD-IRB-SL-KY-20210521(02)]. We adhered strictly to the Declaration of Helsinki. We obtained written informed consent from all patients enrolled in the study. From July 2015 to May 2019, the patients were analyzed to either chemotherapy + IRE group or chemotherapy group. Pre-operative biliary drainage was carried out in both groups, five patients had biliary STENT and 19 patients had percutaneous transhepatic biliary drainage (PTBD) in chemotherapy + IRE group, seven patients had biliary STENT and 16 patients had PTBD in chemotherapy group. All patients were routinely evaluated and assigned a treatment plan during our oncological multidisciplinary meeting that that consisted of hepatologists, gastroenterologists, oncologists, and interventional radiologists. The inclusion criteria were as follows: (1) Diagnosed UHC based on clinical manifestations and imaging examinations, including magnetic resonance imaging (MRI) and CT [Citation21]. (2) According to the Bismuth–Corlette classification, all patients were UHC (TNM tumor stage of III and IV) with tumor invasion of blood vessels, without surgical resection and without distant metastases, or suspected metastatic regional lymphadenopathy on imaging studies, tumor size ≤ 5 cm. (3) Patients with adequate bone marrow function (absolute neutrophil count ≥ 1500μL, platelet count ≥ 75,000μL). (4) Patients must have good compliance, undergo regular consultations at the hospital, and be willing to undergo follow-up examinations. Patients were excluded from the study, if (1) Patients with in poor health and could not tolerate general anesthesia and surgery. (2) Patients with a pacemaker or placement of a biliary metal stent. (3) Patients with severe heart rhythm disorders and myocardial injury. (4) Patients with poor compliance that cannot regularly review and receive follow-up.
Chemotherapy plus concurrent IRE procedure
Before IRE ablation, 1000 mg/m2 of gemcitabine was infused intravenously for 30 min. The dose of chemotherapy drugs was calculated based on height and weight. We examined the surrounding invasion of the tumor, involvement of the secondary bile duct and the portal vein and other important ducts without distant metastasis, and no radical surgical resection conditions. For the induction of anesthesia, we used midazolam, etomidate, sufentanil, and vecuronium. Mechanical ventilation with 60% oxygen concentration was used. For maintenance medication, remifentanil and propofol were used in combination with a small amount of isoflurane. Vecuronium was used for muscle relaxation before releasing electrical pulses.
IRE ablation procedure adopted the IRE tissue ablation system (Nanoknife, AngioDynamics, Latham, NY, USA). During the IRE process, ultrasound (IU22, Philips Healthcare, Bothell, WA, USA) combined with computed tomography (CT) were used to guide electrode placement and confirm electrode spacing, depth and parallel axis of all electrodes. The number of electrode used ranges from 2 to 6 basing on the tumor size, with the goal of obtaining at least a 5 mm ablation margin. The electrode spacing was set at 1.5 to 2.5 cm. The effective exposure distance of the electrodes was 2.0–2.5 cm [Citation22]. The IRE was performed with 70–90 pulses per electrode pair and the pulse length was 90 μs with an average voltage of 1500 V/cm. To avoid arrhythmia, pulse energy was released in the ventricular refractory period. If the tumor volume was too large to be completely ablated at one-time, multiple ablation of the tumor zone was performed. Successful completion of the procedure was defined by real-time current changes combined with intraoperative ultrasound and contrast-enhanced CT to identify whether the tumor was completely ablated. After the operation, the patient was transferred to intensive care unit with cardiac monitoring. After the vital signs stabilized, the patient was transferred to the general ward and received symptomatic supportive treatments.
The chemotherapy regimen was mainly gemcitabine with cisplatin or oxaliplatin or S1 (only prescribed for Asian patients). A total of 1000 mg/m2 of gemcitabine (at day 1 and day 8) was given and each intravenous infusion time was >30 min. Every 4 weeks was regarded as a course of treatment, and each patient received chemotherapy for 6 courses. Second line chemotherapy regimen such as FOLFOX was treated when the first line regimen failed [Citation23].
Chemotherapy alone
Patients were treated with one of the following regimens: gemcitabine plus cisplatin or S1 or oxaliplatin. Second line chemotherapy regimen such as FOLFOX was treated when the first line regimen failed [Citation23].
Follow-up
The first follow-up was performed 1 month after IRE ablation or chemotherapy, every 2–3 months in the first year, and every 3–6 months subsequently. Abdominal CT or MRI examination was performed at every follow-up. During follow-up, postoperative complications were recorded and classified according to the guidelines of the Interventional Radiology Society [Citation24]. All postoperative and follow-up images were reviewed by a senior radiology resident and a board-certified interventional radiology instructor, with 7 years of oncology imaging and intervention experience, to ensure a consensus.
Evaluation of tumor response
An applied response evaluation criteria in solid tumors (RECIST) was evaluated the efficacy of treatment and defined as follows: complete response (CR) showed no target lesion by arterial phase enhanced imaging [Citation25]. Partial response (PR) showed a ≥30% reduction of the total diameter of the target lesion (arterial phase enhanced imaging); progressive disease (PD) showed an increase by ≥20% of the total diameter of target lesions or new lesions was observed (arterial phase enhanced imaging); stable disease (SD) was attributed to target lesions between PR and PD. Two experienced radiologists independently evaluated the results. If there were disagreements, the radiologists would collectively read the film and reach a consensus. The tumor response rate (RR) was calculated as the sum of the number of tumors that reached CR plus PR and the percentage of total tumors.
Time to local tumor progression and overall survival
The time to local tumor progression (LTP) was described as the duration from the date of the first treatment to an observed new nodule growth along the ablation edge or ablation zone, which was the standard imaging criteria for follow-up imaging examinations [Citation26]. The duration from the date of the first treatment until death or the last follow-up was defined as overall survival (OS). The follow-up time started at the date of initiation of the first treatment and completed at the date of last follow-up or death.
Evaluation of complications
Perioperative complications were defined as any complication that occurred within 90 days of treatment and classified according to the guidelines of the Society of Interventional Radiology [Citation27]. The major complications were those that led to improving levels of care and required hospitalization. All additional complications were considered minor.
Statistical analysis
The endpoint of the present study was LTP, which analyzed by the Kaplan–Meier method. Time to LTP was compared between treatment groups by the log-rank test. The patients’ baseline characteristics and the incidence of complications were compared using the continuity correction and independent sample t and Fisher exact tests. The tumor responses following treatment were evaluated according to the RECIST. Survival curves were analyzed using the Kaplan–Meier method and compared using the log-rank test. Possible factors that influenced LTP and OS were analyzed by a Cox proportional hazards regression model. T statistical test was two-sided, and a p value of <0.05 was considered to be statically significant. All statistical analyses were performed using SPSS software (version 20.0; SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
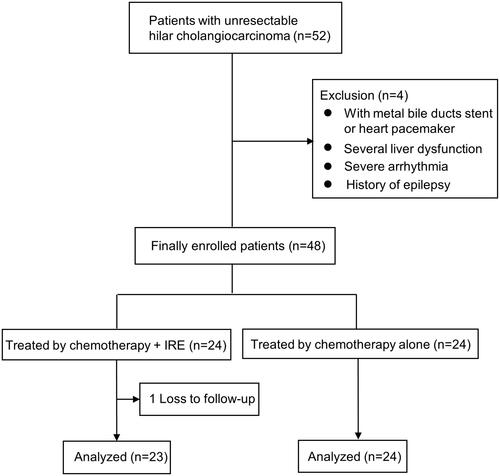
A total of 52 patients that presented with UHC from July 2015 to May 2019. Four patients were found to be ineligible due to a biliary metal stent, heart pacemaker, liver dysfunction, and a history of epilepsy. According to the inclusion/exclusion criteria, 92.3% (48/52) of patients were enrolled. Among them, one patient was lost to follow-up, 23 patients with a median age of 61 years (range of 45–76 years old) were finally analyzed in the chemotherapy + IRE group, 24 patients with a median age of 64 years old (range of 50–78 years old) were analyzed in the chemotherapy alone group (). The baseline characteristics of the 47 patients were presented in , and there was no statistically significant difference between two groups (p > 0.05). provided an example of the typical imaging characteristics of patient with UHC after chemotherapy plus concurrent IRE.
Figure 2. A 53-year-old male with unresectable hilar cholangiocarcinoma treated with chemotherapy plus concurrent IRE. (A) Preoperative enhanced CT illustrating a hilar cholangiocarcinoma tumor measuring 3.4 cm × 2.8 cm. (B) Two ablation electrodes inserted into the tumor area under the guidance of enhanced CT. (C) 1 months after IRE.
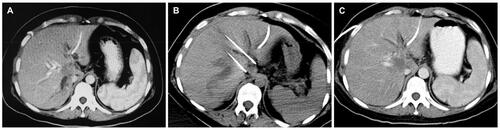
Table 1. Tumor parameters of eligible patients.
Local tumor control and progression
The median patient follow-up time was 13.2 months (range of 1–22.6 months) in the two groups. In the chemotherapy + IRE group, the rates of CR, PR, SD, and PD were 52.2% (12/23), 17.4% (4/23), 13.0% (3/23), and 17.4% (4/23), respectively, but only 12.5% (3/24), 12.5% (3/24), 29.2% (7/24), and 45.8% (11/24) in the chemotherapy alone group correspondingly. Compared with chemotherapy alone group, the tumor RR (CR + PR) was higher in the chemotherapy + IRE group (69.6% vs. 25.0%, p < 0.05; ). Moreover, chemotherapy + IRE group showed a trend toward a decreased rate of LTP (16.7%; 6/36), but 39.5% (15/38) in chemotherapy alone group (p < 0.05). Above all, the median time to LTP of chemotherapy + IRE group was longer than that in the chemotherapy alone group (11.2 months vs. 4.2 months, p < 0.001; ).
Figure 3. Kaplan–Meier curves showing time to local tumor progression (LTP) for patients with UHC in chemotherapy + IRE treatment and chemotherapy alone groups (p < 0.001).
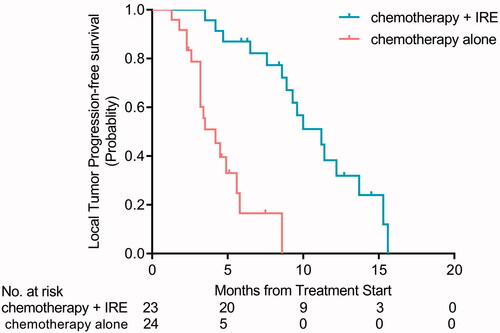
Table 2. Follow-up and local tumor progression.
Overall survival
By comparing to chemotherapy alone, the median OS was also longer in the chemotherapy + IRE group (19.6 months vs. 10.2 months, p < 0.001; ).
Complications
No treatment-related deaths were occurred in all patients. Major complication was cholangitis (n = 1) in the chemotherapy + IRE group. In both groups, minor complications included liver function injury, partial portal thrombosis, abdominal pain, vomiting, myelosuppression, and self-limiting pleural effusion. However, these minor complications were controlled within three days using conservative treatment. No significant differences related to major and minor complications were identified between two groups (p > 0.05; ).
Table 3. Adverse reactions following treatment.
Discussion
UHC is a malignant cancer that generally has a poor prognosis [Citation28]. Systemic chemotherapy is the gold standard for patients who are not eligible for curative resection or liver transplantation. In the past decade, several ablative techniques have been used to treat UHC, such as SBRT, PDT and RFA. These treatments were aimed to relieve the obstruction and improve bile drainage. Furthermore, there was no interventional treatment for the tumor itself. However, little study has been done about chemotherapy plus concurrent IRE for patients with UHC so far.
In the present study, we compared the treatment effect of chemotherapy plus concurrent IRE and chemotherapy alone in patients with UHC and found that chemotherapy plus concurrent IRE achieved better local tumor control, increased time to LTP, and improved OS. The superiority of chemotherapy plus concurrent IRE compared with chemotherapy alone could be due to several reasons. First, chemotherapy concurrent IRE did not affect the progress of chemotherapy as a first-line treatment strategy for UHC. Second, Martin et al. reported that the concentration of gemcitabine in the reversible electroporation zone of pancreatic tissue treated with IRE plus concurrent gemcitabine was 3.28 times higher than that treated with gemcitabine monotherapy in the tumor-bearing mice [Citation29]. In addition, IRE ablation could maximum destroy tumor cells while achieving recanalization of biliary obstruction. Moreover, Martin et al. [Citation19] also reported 26 patients with advanced hilar cholangiocarcinoma treated with IRE, all of them underwent preoperative PTCD to decompress biliary obstruction, and the results showed that all patients achieved biliary recanalization and removed the PTCD tube, the median time from IRE to PTCD of tube removal was 122 days. In our study, only 34.7% (8/23) patients achieved recanalization of biliary obstruction in chemotherapy + IRE group. The main reason for this difference was due to tumor size, the median size of tumors was 3.4 cm in this study which was bigger than the size in Martin’ report (2.75 cm). Consequently, IRE ablation achieved recanalization of biliary obstruction by killing tumor cells.
Most of all, this study showed a median OS of 19.6 months in the chemotherapy + IRE group. Belfiore et al. [Citation30] reported that 15 patients with unresectable perihilar and intrahepatic cholangiocarcinoma under CT guided IRE were followed up for 90 days, no patients died and the mean survival was 18 months, which indicated that hilar cholangiocarcinoma can benefit from IRE treatment and improve quality of life. Furthermore, the LTP rate of this study in the chemotherapy + IRE group was 16.7%, which lower than the results of previous studies (31.7% and 38%) [Citation31,Citation32]. The chemotherapy + IRE group demonstrated a longer time to LTP than the chemotherapy alone treatment. Our data suggested that IRE ablation can be used as an adjunct to improve the efficacy of chemotherapy and reduce the rate of LTP in patients with UHC.
This study was the first time to explore the role of chemotherapy plus concurrent IRE for the treatment of UHC. In this study, chemotherapy plus concurrent IRE showed promising efficacy related to local control rate, time to LTP, and overall survival compared with chemotherapy alone. Unlike other studies, gemcitabine was intravenously (over approximately 30 min) immediately followed by IRE ablation to benefit from potential concurrence of these treatments. The synergistic effect of IRE and chemotherapy increased the permeability and toxicity of chemotherapeutic agents to the tumor, which in turn improved the local therapeutic effect.
Chemoradiation is a standard treatment option for UHC, which is also recommended by American Hepato-Pancreato-Biliary Association (AHPBA) [Citation33]. Ghafoori et al. retrospectively analyzed the role of definitive chemoradiotherapy for patients with UHC and showed that median survival times were between 13 and 23 months [Citation34]. However, given the burden and toxicity of fractionated radiotherapy administered over several weeks, a one-time session of IRE appeared to be an advantageous approach. These suggested that chemotherapy plus concurrent IRE may be a better choice for patients with UHC.
However, this study had several limitations. First, the results were collected from a small sample. Therefore, the studies of multicenter and larger sample size are required to confirm the present findings in future. Second, some patients received different types of chemotherapy prior to IRE ablation, which may affect the treatment response or local tumor control. In addition, 86.9% of our patients were HBV positive, which greatly differ in studies from Europe and the USA. Hence, the studies of HBV-negative UHC patients are still needed. Finally, heterogeneity in the patients might limit our study.
In conclusion, our results suggested that chemotherapy plus concurrent IRE therapy improved tumor response and local control. Furthermore, compared with chemotherapy alone, chemotherapy plus concurrent IRE prolonged the time to LTP.
Acknowledgements
The authors thank all patients, doctors, nurses for their kindness to support this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Nath MC, Torbenson MS, Erickson LA. Perihilar cholangiocarcinoma. Mayo Clin Proc. 2018;93(3):397–398.
- Chen P, Li B, Zhu Y, et al. Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma. Oncotarget. 2016;7(24):37319–37330.
- Vibert E, Boleslawski E. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg. 2019;269(1):e5–e6.
- Groot Koerkamp B, Wiggers JK, Gonen M, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2016;27(4):753.
- Matsuo K, Rocha FG, Ito K, et al. The blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215(3):343–355.
- Lunsford KE, Javle M, Heyne K, Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC), et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3(5):337–348.
- Watanabe A, Kida M, Miyazawa S, et al. Phase I trial of combination chemotherapy with gemcitabine, cisplatin, and S-1 in patients with advanced biliary tract cancer. World J Gastroenterol. 2015;21(19):5979–5984.
- Uwagawa T, Sakamoto T, Abe K, et al. Phase I trial of S-1 every other day in combination with gemcitabine/cisplatin for inoperable biliary tract cancer. Cancer Chemother Pharmacol. 2015;75(1):191–196.
- Shoji H, Morizane C, Sakamoto Y, et al. Phase I clinical trial of oral administration of S-1 in combination with intravenous gemcitabine and cisplatin in patients with advanced biliary tract cancer. Jpn J Clin Oncol. 2016;46(2):132–137.
- Morizane C, Okusaka T, Mizusawa J, members of the Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG), et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30(12):1950–1958.
- Tsukiyama I, Ejiri M, Yamamoto Y, et al. A Cost-Effectiveness analysis of gemcitabine plus cisplatin versus gemcitabine alone for treatment of advanced biliary tract cancer in Japan. J Gastrointest Cancer. 2017;48(4):326–332.
- Wagner A, Denzer UW, Neureiter D, et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: a multicenter prospective phase II study. Hepatology. 2015;62(5):1456–1465.
- Dolak W, Schreiber F, Schwaighofer H, Austrian Biliary RFA Study Group, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28(3):854–860.
- Laquiere A, Boustiere C, Leblanc S, et al. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc. 2016;30(3):1242–1248.
- Mahadevan A, Dagoglu N, Mancias J, et al. Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma. J Cancer. 2015;6(11):1099–1104.
- Chen R, Lu F, Wu F, et al. An analytical solution for temperature distributions in hepatic radiofrequency ablation incorporating the heat-sink effect of large vessels. Phys Med Biol. 2018;63(23):235026
- Kodama H, Ueshima E, Gao S, et al. High power microwave ablation of normal swine lung: impact of duration of energy delivery on adverse event and heat sink effects. Int J Hyperthermia. 2018;34(8):1186–1193.
- Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality-clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48.
- Martin EK, Neal B, Egger ME, et al. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. Hpb (Oxford). 2018;20(11):1092–1097.
- Li M, Li K, Qi X, et al. Percutaneous transhepatic biliary stent implantation for obstructive jaundice of perihilar cholangiocarcinoma: a prospective study on predictors of stent patency and survival in 92 patients. J Vasc Interv Radiol. 2016;27(7):1047–1055e2.
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517.
- Appelbaum L, Ben-David E, Faroja M, et al. Irreversible electroporation ablation: creation of large-volume ablation zones in in vivo porcine liver with four-electrode arrays. Radiology. 2014;270(2):416–424.
- Lamarca A, Palmer DH, Wasan HS, Advanced Biliary Cancer Working Group, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701.
- Cardella JF, Kundu S, Miller DL, Society of Interventional Radiology, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20(7 Suppl):S189–S91.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
- Ahmed M, Technology Assessment Committee of the Society of Interventional Radiology Image Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11–17.
- Esnaola NF, Meyer JE, Karachristos A, et al. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–1369.
- Bhutiani N, Agle S, Li Y, et al. Irreversible electroporation enhances delivery of gemcitabine to pancreatic adenocarcinoma. J Surg Oncol. 2016;114(2):181–186.
- Belfiore MP, Reginelli A, Maggialetti N, et al. Preliminary results in unresectable cholangiocarcinoma treated by CT percutaneous irreversible electroporation: feasibility, safety and efficacy. Med Oncol. 2020;37(5):45.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275e1.
- Niessen C, Thumann S, Beyer L, et al. Percutaneous irreversible electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci Rep. 2017;7:43687.
- Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB. 2015;17(8):691–699.
- Ghafoori AP, Nelson JW, Willett CG, et al. Radiotherapy in the treatment of patients with unresectable extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2011;81(3):654–659.