Abstract
Background
This study aimed to evaluate whether combined therapy with PD-1 blockade (anti-PD-1) and radiofrequency ablation (RFA) is superior to RFA monotherapy for recurrent hepatocellular carcinoma (HCC).
Methods
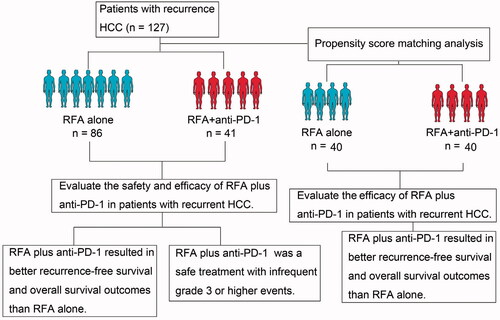
A total of 127 patients who underwent anti-PD-1 plus RFA treatment (n = 41) or RFA alone (n = 86) for recurrent HCC were included in this retrospective study. A matched cohort comprising 40 patients from each group was selected after propensity score matching analysis. Clinical data including post-RFA HCC recurrence (primary endpoint), overall survival (OS) (secondary endpoint), adverse events, and toxic effects were retrospectively analyzed.
Results
The 1-year recurrence-free survival rates for the anti-PD-1 plus RFA and RFA groups were 32.5% and 10.0% after propensity score matching. There were statistically significant differences between the two groups in terms of the recurrence-free survival rate (p = 0.001) and OS rate (p = 0.016). Tumor number, tumor-node metastasis (TNM) stage, antiviral therapy, and anti-PD-1 treatment were demonstrated to be important factors associated with 1-year recurrence-free survival probability by univariate and multivariate analyses. Univariate and multivariate analyses demonstrated that tumor number, TNM stage and anti-PD-1 treatment were significant prognostic factors for OS. RFA treatment-related adverse events included pleural effusions that require drainage and a mild or moderate increase in body temperature. Grade 3 or higher events related to anti-PD-1 treatment occurred in 12.8% (6) of patients and were infrequent.
Conclusions
Combination therapy with anti-PD-1 plus RFA was superior to RFA alone in improving survival in patients with recurrent HCC.
Graphical Abstract
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent malignancy and the fourth most common cause of cancer-related death worldwide [Citation1]. Despite continuous advances in tumor detection, only approximately 10 − 20% of patients are diagnosed with early HCC and qualify for potentially surgical intervention [Citation2]. Even after curative treatment, the recurrence rate is approximately 70% [Citation3]. In addition, due to multifocal intrahepatic or extrahepatic recurrence, impaired liver function, and tumors in unresectable locations, repeated liver resection is feasible in a minority of patients [Citation4]. Radiofrequency ablation (RFA), a novel thermal ablation technique, has become an alternative curative therapeutic option to prevent liver failure and preserve the hepatic parenchyma after surgical resection [Citation5]. In addition, RFA exhibits a lower complication rate and is less invasive in the treatment of solitary and small HCCs than surgical resection [Citation6]. However, the recurrence rate for HCC patients after RFA treatment is high [Citation7], and such recurrent HCC appears to behave more aggressively than before RFA [Citation8,Citation9]. Thus, monotherapy with RFA is still unsatisfactory for patients with HCC.
Recently, a combination therapy strategy has been used to overcome the disadvantages of RFA treatment in the high incidence of recurrence and take full advantage of in HCC management. For example, recent efforts have focused on combining RFA with other anticancer approaches, including transarterial chemoembolization (TACE) and molecular targeted therapy [Citation10]. TACE plus RFA exhibits better overall survival (OS) and recurrence-free survival (RFS) for patients with HCC <7 cm compared to RFA alone [Citation11,Citation12]. However, there were no statistically significant differences in OS rate between TACE plus RFA and RFA alone in the treatment of small HCC nodules (<3 cm) [Citation13]. Sorafenib, a targeted molecular agent, is approved by the United States Food and Drug Administration (FDA) to treat patients with advanced HCC. Recently, some studies found that the 1-, 2-, and 3-year recurrence rates were lower for patients with HCC at different stages of Barcelona Clinic Liver Cancer(BCLC) (0-B1) after sorafenib-RFA combination therapy, compared with RFA alone [Citation14]. Therefore, the combined use of RFA with sorafenib is available for the treatment of HCC, but the reported response rates of sorafenib remain unsatisfactory, ranging from 2.3% to 9.2% [Citation15]. In addition, angiotensin II receptor 1 blockers (sartans) significantly improved the time to recurrence and OS after RFA in patients with HCC [Citation16]. Thus, the RFA-combined therapy strategy for the treatment of HCC may provide promising results.
In recent years, tumor immunotherapy has made significant progress in inhibiting tumor progression [Citation17]. Among them, immune checkpoint inhibitors offer great promise for the approval of nivolumab by the FDA on 23 September 2017, based only on a phase I/II clinical trial for the treatment of patients with HCC [Citation18]. Recently, a previous study showed that nivolumab treatment improved OS and progression-free survival and appears safe in patients with postoperative recurrence of malignant pleural mesothelioma [Citation19]. Thus, it is a strong hint that immune checkpoint inhibitors may achieve good clinical efficacy in patients with recurrent HCC. Meanwhile, several studies have shown that RFA can induce massive necrotic cell death, thus releasing large amounts of tumor antigens that might activate immunity and the presentation of cryptic antigens to induce tumor-specific T cell response [Citation20–22]. Moreover, the combined therapy of RFA and anti-PD-1 antibodies exhibited stronger antitumor immunity and prolonged survival by enhancing T cell immune responses in mouse tumor models [Citation23]. These results suggest that immune checkpoint inhibitors plus RFA may provide promising results in patients with recurrent HCC. However, this has not been reported to date. Therefore, we evaluated the combined safety and efficacy of RFA and PD-1 blockade (anti-PD-1) in patients with recurrent HCC in a retrospective cohort study for the first time.
Methods
Patients
This retrospective study was approved by the Ethics Committee of Southwest Hospital, Army Medical University (Ethical approval number: KY2021046). Written informed consent was obtained from all patients before RFA treatment alone or RFA plus anti-PD-1 treatment. According to the Milan criteria, the inclusion criteria for this study were as follows: (a) patients aged 18 − 75 years; (b) patients were diagnosed with recurrent intrahepatic HCC lesions after hepatic resection or RFA; (c) patients had a solitary intrahepatic tumor or multiple tumors with three or two nodules each ≤3 cm in size; (d) patients had no extrahepatic metastases; (e) patients without invasion of the portal vein, hepatic vein trunk or secondary branches, or the bile duct; (f)no other antitumor therapy received before treatment, including radiotherapy, TACE, and targeted drugs; (g) patients had no residual lesions detected by contrast-enhanced ultrasound after RFA at 24 h, 48 h, and confirmed by contrast-enhanced computed tomography (CT) or gadoxetic acid-enhanced magnetic resonance imaging (EOB-MRI) 1 month after RFA in this study; (h) patients received PD-1 blockade therapy for at least 1 month after RFA in this study. Patients were excluded from our study if they met the following criteria: (a) had severe portal hypertension, a history of esophageal variceal hemorrhage, severe hypersplenism syndrome, or refractory ascites currently or in history; (b) had serious heart, kidney, and other organ dysfunction; (c) had autoimmune disease currently or in history; (d) had other malignant tumors currently or in history; (e) had serious adverse events after PD-1 blockade therapy; (f) received other treatments (stem cell therapy, or immune cell therapy) during the study period.
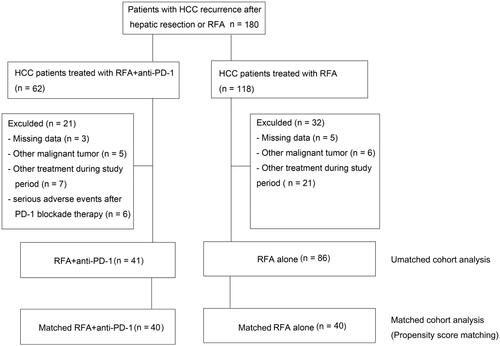
Patients diagnosed with recurrent HCC were recommended for RFA plus anti-PD-1 treatment in principle; however, some patients chose regular examination after RFA foreconomic problems or personal reasons rather than combined treatment. Patients were classified into the RFA + anti-PD-1 group if they received RFA combined with anti-PD-1 treatment or the RFA group if they received only RFA treatment. We reviewed the electronic medical records of 180 consecutive patients with recurrent intrahepatic HCC lesions after RFA or hepatectomy who underwent RFA and received PD-1 blockade therapy or who underwent RFA between November 2013 and December 2019 at Southwest Hospital, Army Medical University. Follow-up data collection was terminated on 30 April 2017. Follow-up data collection was terminated on 31 December 2020.
RFA procedure
All the patients included in this study were treated percutaneously with an LDRF-120S radiofrequency ablation device (Lead Electron Corporation, Mianyang, China) following the guidelines of Chinese Expert Consensus Statement issued by the Chinese Society of Liver Cancer and the Chinese Society of Clinical Oncology [Citation23,Citation24]. The RFA procedures were performed by physicians with at least 5-year of experience in ultrasound-guided hepatic RFA. Before RFA, each lesion was confirmed using contrast-enhanced ultrasonography. After local or general anesthesia, the radiofrequency electrode was placed on the tumor lesions under ultrasound guidance. To eliminate residual tumor cells in the treatment area, the open cool-tip electrode was at least 1 cm larger than the maximum diameter of the tumor, and the open cluster electrode was rotated clockwise in situ by 15 °C after closing and reopening for another treatment. Each tumor lesion was treated with a single electrode. For patients with multiple tumors, all lesions were ablated using the same procedure. After RFA for 15 min, 24 h and 48 h, contrast-enhanced ultrasound was used to detect complete destruction of each lesion. Contrast-enhanced CT or gadoxetic acid-EOB-MRI was performed to confirm whether the residual tumor was detected 1 month after RFA. If a residual tumor was still detected, the lesions were retreated using the same procedure.
PD-1 blockade management
Patients in the RFA plus PD-1 blockade (RFA + anti-PD-1) treatment group received 200 mg of camrelizumab intravenously every 2 weeks or 200 mg of sintilimab intravenously every 2 weeks and the initial administration was within 72 h after RFA. Patients received continuous PD-1 blockade treatment until unacceptable toxic effects occurred or there was a loss of clinical benefit. It is possible to observe atypical reactions (e.g., temporary enlargement of the tumor or appearance of new small lesions in the first few months, followed by tumor shrinkage). If the patient's clinical symptoms are stable or continue to decline, even if there is preliminary evidence of disease progression on imaging, based on the judgment of overall clinical benefit, the drug can be considered to continue to be used until disease progression is confirmed.
Determination of complete ablation, tumor recurrence, survival and complications
Complete ablation was defined as the absence of any enhancing lesion at the ablation site on CT or MRI 1 month after RFA. The recurrence of HCC was defined as the appearance of local and distant tumor progression. The local tumor recurrence was defined as the appearance of enhancing lesions at the edge of the ablation site (<2.0 cm from the edge of the ablation site) during follow-up imaging [Citation25]. Distant tumor progression was defined as the appearance of enhancing lesions > 2.0 cm from the edge of the ablation site or new HCC foci during follow-up imaging by CT or MRI. All imaging evaluations were performed by two independent diagnostic radiologists with at least 5-year of experience. The RFS time was defined as the time from complete RFA to recurrence of HCC. The OS time was defined as the time from complete RFA to death or the last follow-up. Patients who remained alive at the last follow-up were considered censored events in the statistical analysis. RFA-related complications were evaluated according to the Dindo–Clavien classification [Citation26].
Follow-up
All patients in this study were followed up 1 month after initial RFA, including CT (or MRI), physical examination, routine blood tests, liver function tests and complications. If complete ablation was attained, follow-up was conducted every 8 weeks. This study was conducted on 31 December 2020. When tumor recurrence was identified during follow-up, patients were treated with RFA, surgery, TACE, and targeted drugs, depending on the clinical presentation such as the characteristics of the recurrent tumor and hepatic function.
Statistical analysis
Statistical analyses were performed using SPSS software (version 25.0; SPSS, Chicago, IL). To reduce the degree of bias or confounding in our results, a propensity score matching analysis was performed [Citation27]. Patients from both groups were paired in a 1:1 ratio, and the caliper was set to 0.100. Ten covariates, including age, sex, chronic hepatitis B, liver cirrhosis, tumor number, tumor size, tumor-node metastasis (TNM), antiviral therapy, hepatic resection, and RFA, were included for propensity score generation. Baseline characteristics between RFA + anti-PD-1 and RFA alone were compared using Fisher’s exact test, Mann − Whitney U test and the x2 test for categorical data. Survival curves were analyzed using the Kaplan − Meier method. The equivalence of the survival curves was evaluated using log-rank statistics. Risk factors of statistical significance in the log-rank statistical analysis were subjected to multivariate survival analysis. All statistical tests were two-tailed, and the statistical significance was set at p < 0.05.
Results
Baseline characteristics of patients
A total of 127 patients with recurrent HCC underwent RFA plus anti-PD-1 (n = 41) or RFA alone (n = 86) in this study (). Detailed baseline patient characteristics are presented in . There were no significant differences in age, sex, chronic hepatitis B, liver cirrhosis, tumor number, tumor size, TNM, or RFA treatment between the two treatment groups (). Both groups had a male predominance. Patients with chronic hepatitis B and patients with liver cirrhosis accounted for the majority of patients in both groups. A total of 67 patients underwent hepatic resection, with a significant difference between the two treatment groups (36.6% and 60.5% of the RFA + anti-PD-1 group and RFA alone groups, respectively, p = 0.012) (). However, the number of patients with recurrent HCC after monotherapy with hepatic resection in the RFA + anti-PD-1 group was not lower than in the RFA alone group (24.4% versus 30.2%, p = 0.495) (Supplementary Table 1). Patients with recurrent HCC after monotherapy with RFA in the RFA + anti-PD-1 group was more common than in RFA alone group (63.4% versus 39.5%, p = 0.012) (Supplementary Table 1). A total of 31 (24.4%) patients underwent hepatic resection and RFA treatment: six (12.2%) patients in the RFA + anti-PD-1 group, and 26 (30.2%) patients in the RFA alone group. There were significant differences between the two groups (p = 0.027) (Supplementary Table 1). After propensity score matching, there were no significant baseline differences between the two groups.
Table 1. Baseline patient characteristics in the unmatched cohort and the matched cohort.
Complications or adverse events
As shown in , there was no RFA treatment-related in-hospital mortality in either group in this study. There was no grade 3 or higher events in our study, and the overall complication rate was similar between the two groups (p = 0.762). Adverse events in the RFA + anti-PD-1 group were as follows: pleural effusions that require drainage (1/41, 2.4%) and a mild or moderate increase in body temperature (3/41, 7.3%). Adverse events in the RFA alone group were as follows: pleural effusion requiring drainage (2/86, 2.3%) and a mild or moderate increase in body temperature (5/86, 8.1%).
Table 2. Adverse events related to RFA treatment.
The anti-PD-1-related adverse events are shown in . The frequency of treatment-related adverse events (TRAEs) of any grade was 70.2% (33/47). The frequency of grade 3 or higher events was 12.8% (6/47). The most common TRAEs of any grade were fatigue and pruritus. Laboratory TRAEs were infrequent in this study. Approximately <10% of the patients experienced TRAEs of any grade and <3% experienced TRAEs of grade 3 or higher. In addition, the most commonly occurring TRAEs of any grade were skin, gastrointestinal, and hepatic events, and grade 3 or higher sTRAEs occurred in <3% of the patients. Six patients with grade 3 or higher TRAEs were excluded from this study as they met the exclusion criteria.
Table 3. Adverse events related to anti-PD-1 administration.a
Survival
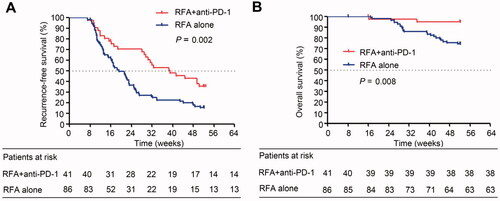
The median RFS (mRFS) was 39.1 weeks (95% confidence interval [CI]: 23.5–54.8) in the RFA + anti-PD-1 group and 19.3 weeks (95% CI: 15.1–23.5) in the RFA alone group. The RFS was significantly longer in the RFA + anti-PD-1 group than in the RFA alone group (p = 0.002) (). Similar results were observed for OS: RFA + anti-PD-1 group, 51.0 weeks versus RFA alone group, 47.6 weeks (p = 0.008) (). After tumor recurrence, 18 patients who met the conditions of RFA were treated with RFA plus regorafenib, and eight patients who were not suitable for RFA were treated with regorafenib plus TACE in the RFA + anti-PD-1 group. In addition, patients after tumor recurrence in the RFA + anti-PD-1 group continued camrelizumab or sintilimab treatment. In the RFA alone group, 20 patients who met the RFA treatment after tumor recurrence were treated with RFA plus sorafenib, six patients who met the conditions of RFA after tumor recurrence were treated with RFA plus regorafenib, four patients after tumor recurrence were treated with hepatic resection plus regorafenib, and six who were not suitable for RFA were treated with regorafenib plus TACE.
Figure 2. Kaplan − Meier curves show recurrence-free survival and overall survival in the RFA + anti-PD-1 and RFA alone groups in the unmatched cohort. (A) Recurrence-free survival analysis in patients receiving RFA + anti-PD-1 treatment and in patients receiving RFA alone treatment (log-rank test, x2 = 9.434, p = 0.002). (B) Overall survival analysis in patients receiving RFA + anti-PD-1 treatment and in patients receiving RFA alone treatment (log-rank test, x2 = 7.114; p = 0.008).
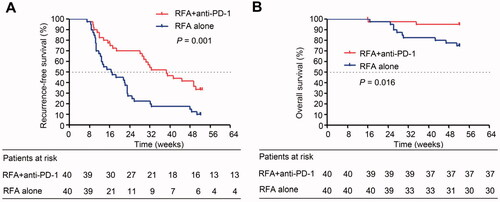
When only the propensity score-matched patients were considered, the mRFS was 38.6 weeks (95% CI, 26.6–50.5) in the RFA + anti-PD-1 group and 16.7 weeks (95% CI, 9.0–24.5) in the RFA alone group. RFS was significantly longer in the RFA + anti-PD-1 group than in the RFA alone group (35.2 weeks versus 22.2 weeks, p = 0.001), and the OS was significantly longer in the RFA + anti-PD-1 group than in the RFA alone group (50.9 weeks versus 47.3 weeks, p = 0.016) ().
Figure 3. Kaplan − Meier curves show recurrence-free survival and overall survival in the RFA + anti-PD-1 and RFA alone groups in the matched cohort. (A) Recurrence-free survival analysis in patients receivingRFA + anti-PD-1 treatment and in patients receiving RFA alone treatment (log-rank test, x2 = 11.142; p = 0.001). (B) Overall survival analysis in patients receivingRFA + anti-PD-1 treatment and in patients receiving RFA alone treatment (log-rank test, x2 = 5.857; p = 0.016).
Subgroup analysis
The HR and 95% CI of each subgroup were calculated using a stratified Cox regression model. As shown in Supplementary Table 2, the RFA + anti-PD-1 treatment provided significant clinical benefit for RFS and OS in subgroups analyzed, although some patients had characteristics associated with poor prognosis such as chronic hepatitis B, liver cirrhosis, two or three tumors, larger tumor size (maximum diameter > 1.8 cm), and higher stage of TNM. Similar results were observed in the propensity score-matched patients ().
Table 4. Subgroup analysis of recurrence and overall survival by the stratified Cox regression model in the matched cohort.
Uni- and multivariate analyses
Univariate Cox regression analysis and multivariate Cox regression analysis were used to evaluate the predictors of RFS and OS in propensity score-matched patients. As shown in and Supplementary Table 5, tumor number (HR, 4.027; 95% CI, 1.956 − 8.292; p = 0.000), TNM stage (HR, 4.027; 95% CI, 1.956 − 8.292; p = 0.000), antiviral therapy (HR, 0.373; 95% CI, 0.167 − 0.836; p = 0.017), and anti-PD-1 treatment (HR, 0.345; 95% CI, 0.196 − 0.608; p = 0.000) were significantly correlated with RFS in the univariate Cox regression analysis. Multivariate Cox regression analysis showed that tumor number (HR, 3.060; 95% CI, 1.606 − 5.830; p = 0.001), TNM stage (HR, 3.060; 95% CI, 1.606 − 5.830; p = 0.001), antiviral therapy (HR, 0.418; 95% CI, 0.235 − 0.745; p = 0.003), and anti-PD-1 treatment (HR, 0.416; 95% CI, 0.248 − 0.698; p = 0.001) were independent predictors of RFS ( and Supplementary Table 5). Univariate Cox regression analysis showed that tumor number, TNM stage, and anti-PD-1 treatment were associated with OS and were significant prognostic factors for OS ( and Supplementary Table 7). In the unmatched cohort, tumor number, TNM stage, and anti-PD-1 treatment were also significant prognostic factors for RFS and OS (Supplementary Tables 4 and 6).
Table 5. Univariate and multivariate analysis of the relative risk of recurrence and overall survival in the matched cohort.
Discussion
In this retrospective study, we first reported the efficacy of anti-PD-1 therapy on RFS and OS outcomes in recurrent HCC after curative RFA treatment before and after the propensity score matching analysis. Our results showed that patients with recurrent HCC had significantly better RFS and OS outcomes in the RFA + anti-PD-1 group than in the RFA alone group before and after propensity score matching analysis. In addition, multivariate analysis showed that anti-PD-1 therapy was an independent prognostic factor for PFS and OS in recurrent HCC after curative RFA treatment before and after propensity score matching analysis.
Previous studies have shown that single-agent checkpoint inhibitors do not show a better survival outcome in patients with HCC [Citation28,Citation29]. In this study, there were two important differences between our study and previous studies. First, all patients included in this study underwent curative RFA before anti-PD-1 therapy. RFA has been shown to induce T cell immune responses, as well as PD-L1 expression, in synchronous colorectal cancer liver metastases and tumor-bearing mice [Citation23,Citation30]. In addition, T cell immune responses induced by RFA led to a detectable antitumor reactivity in mouse models [Citation23,Citation31]. Previous studies have shown that PD-L1, PD-1 and CTLA-4 contribute to the inhibition of thermal ablation-induced antitumor activity [Citation23,Citation32]. Positive PD-L1 expression in patients is associated with an objective response to anti-PD-1 therapy [Citation33,Citation34]. PD-L1 immunohistochemistry tests have been approved by the US FDA to calculate the efficacy of anti-PD-(L)1 therapy in NSCLC and several other cancers [Citation35].Thus, RFA can be a useful antigen source for the induction of antitumor immunity. Meanwhile, some preclinical studies have shown that the combination of radiotherapy and PD-L1/PD-1 blockade synergistically enhances antitumor immunity [Citation36,Citation37]. These results suggest that local antitumor treatment by radiotherapy can elicit an immune response, providing a better opportunity for PD-1/PD-L1 blockade therapy. Second, patients with no extrahepatic metastases and no invasion of the portal vein, hepatic vein trunk, or secondary branches were included in this study. However, all studies on single-agent checkpoint inhibitor treatment have evaluated patients with advanced HCC [Citation28,Citation29]. Therefore, all patients in our study had better physical conditions than those of the previous studies.
In this study, the stratified Cox regression model before and after the propensity score matching analysis showed that RFA + anti-PD-1 treatment provided significant clinical benefits for RFS and OS in all analyzed subgroups. A previous report showed that liver metastases in patients with advanced melanoma were associated with reduced ORR and PFS during anti-PD-1 therapy [Citation38]. In addition, a recent report showed that patients with an ECOG performance status of one or more, bone metastases, and liver metastases had shorter 5-year OS in advanced melanoma, renal cell carcinoma, and non-small cell lung cancer [Citation39]. Therefore, these results suggest that anti-PD-(L)1 therapy will provide a better clinical benefit for patients with early HCC. Our results support this concept. The findings of this study showed that patients with one tumor experienced a better clinical benefit in RFS and OS than those with two or three tumors (RFS: HR = 0.400 versus HR = 0.518; OS: HR = 0.014 versus HR = 0.609) during anti-PD-1 therapy in the matched cohort. Similar results were found in patients with TNM stage I tumors and smaller tumor size (maximum diameter ≤ 1.8 cm). Although some studies have not shown a better survival outcome in patients with HCC during single-agent checkpoint inhibitor therapy, more studies, as well as our results, suggest that anti-PD-1 treatment could provide a better clinical benefit for patients with early HCC pretreated with RFA. In addition, multivariate analysis showed that tumor number and anti-PD-1 treatment were independent prognostic factors for RFS and OS before and after the propensity score matching analysis. A possible reason is that neovascular invasion and extrahepatic dissemination occur more easily in patients with HCC with multiple lesions [Citation40].
In this study, the RFA treatment-related adverse events were pleural effusion requiring drainage and a mild or moderate increase in body temperature, which is consistent with historical data in other trials [Citation14,Citation25]. The spectrum of adverse events observed with anti-PD1 therapy was similar to that reported in previous studies [Citation28,Citation41,Citation42]. Six patients with grade 3 or higher TRAEs were excluded from this study as they met the exclusion criteria. In the analysis of complications with the anti-PD1 therapy, the above six patients were reincluded. The frequencies of any grade TRAEs and grade 3 or higher events in this study were 70.2% [Citation35] and 12.8% (6), respectively, which is not consistent with historical data in other trials of pembrolizumab (any grade TRAEs, 98.2%; grade 3 or higher events, 61.1%) or atezolizumab (any grade TRAEs, 96.4%; grade 3 or higher events, 52.0%) for hepatocellular carcinoma [Citation41,Citation42]. The lower incidence of grade 3 or higher events in our study may be due to patients enrolled without intrahepatic and extrahepatic metastases and without invasion of the portal vein, hepatic vein trunk or secondary branches. The population of patients included in this study with better physical condition well tolerated the toxicity caused by anti-PD-1 therapy.
This retrospective, non-randomized study had several limitations. The baseline demographics were well matched after the propensity score analysis between the two groups; however, prospective randomized controlled trials are needed for further research. In addition, the number of patients included in this study was limited (41 patients in the RFA + anti-PD-1 group versus 86 patients in the RFA alone group). Therefore, studies with larger sample sizes are needed to confirm our findings. In addition, the follow-up time was only 1 year. Therefore, patients should be followed up for assessment of RFS and OS for at least 3 − 5 years in future studies.
In conclusion, RFA + anti-PD-1 is a safe and effective therapy for treating patients with recurrent HCC with tumor diameter <3 cm and no more than three tumors, no extrahepatic metastases, and no invasion of the portal vein, hepatic vein trunk, or secondary branches.
Supplemental Material
Download PDF (325.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
- Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361(15):1437–1447.
- Erridge S, Pucher PH, Markar SR, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104(11):1433–1442.
- Ding H, Su M, Zhu C, et al. CT-guided versus laparoscopic radiofrequency ablation in recurrent small hepatocellular carcinoma against the diaphragmatic dome. Sci Rep. 2017;7:44583.
- Na BG, Kim JM, Oh DK, et al. Clinical outcomes of laparoscopic radiofrequency ablation of single primary or recurrent hepatocellular carcinoma (≤3 cm). Ann Surg Treat Res. 2017;92(5):355–360.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
- Lee HY, Rhim H, Lee MW, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23(1):190–197.
- Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58(5):1667–1680.
- Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–3527.
- Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: current status. Cancer Lett. 2016;370(1):78–84.
- Xu Z, Xie H, Zhou L, et al. The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432.
- Iezzi R, Pompili M, Posa A, et al. Combined locoregional treatment of patients with hepatocellular carcinoma: state of the art. World J Gastroenterol. 2016;22(6):1935–1942.
- Feng X, Xu R, Du X, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol. 2014;109(12):1891–1899.
- Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24(Suppl 1):S3–S10.
- Facciorusso A, Del Prete V, Crucinio N, et al. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J Gastroenterol Hepatol. 2015;30(11):1643–1650.
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337.
- Tella SH, Mahipal A, Kommalapati A, et al. Evaluating the safety and efficacy of nivolumab in patients with advanced hepatocellular carcinoma: evidence to date. Onco Targets Ther. 2019;12:10335–10342.
- Nakamura A, Kondo N, Nakamichi T, et al. Initial evaluation of nivolumab in patients with post-operative recurrence of malignant pleural mesothelioma. Jpn J Clin Oncol. 2020;50(8):920–925.
- Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29(9):5045–5051.
- Lin WX, Fifis T, Malcontenti-Wilson C, et al. Induction of Th1Immune responses following laser ablation in a murine model of colorectal liver metastases. J Transl Med. 2011;9:83.
- Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251(1):58–66.
- Shi L, Chen L, Wu C, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res. 2016;22(5):1173–1184.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802.
- Khan KN, Yatsuhashi H, Yamasaki K, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32(2):269–278.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Hu H, Chi JC, Liu R, et al. Microwave ablation for peribiliary hepatocellular carcinoma: propensity score analyses of long-term outcomes. Int J Hyperthermia. 2021;38(1):191–201.
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202.
- Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase 3 study of nivolumab (NIVO) vssorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30(Suppl 5):v874–v875.
- Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457.
- den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64(11):4024–4029.
- Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72(2):430–439.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454.
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074.
- Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA. 2017;318(17):1647–1648.
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695.
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377.
- Nosrati A, Tsai KK, Goldinger SM, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–1147.
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5(10):1411–1420.
- Yuan H, Lan Y, Li X, et al. Large hepatocellular carcinoma with local remnants after transarterial chemoembolization: treatment by sorafenib combined with radiofrequency ablation or sorafenib alone. Am J Cancer Res. 2019;9(4):791–799.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905.
- Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820.