Abstract
Background
To investigate the combined enhancing effects of microbubble-contrast SonoVue and oxytocin on high-intensity focused ultrasound (HIFU) ablation of adenomyosis.
Methods
330 patients with adenomyosis were randomly assigned to SonoVue and oxytocin group (group A, n = 82), oxytocin (group B, n = 85), SonoVue (group C, n = 81), or the control (group D, n = 82) for HIFU ablation. In group A, oxytocin was dripped 0.32 IU/min, and HIFU ablation was started one minute after SonoVue injection. In group B, oxytocin was dripped 0.32 IU/min during ablation. In group C, HIFU ablation was started one minute after SonoVue injection. In group D, neither oxytocin nor SonoVue was applied. The clinical data, treatment results, and complications were analyzed.
Results
All participants underwent HIFU treatment safely, and the mean energy efficiency factor (EEF) in the four groups was 4.7 ± 0.9J/mm3, 8.5 ± 0.6J/mm3, 8.9 ± 0.7J/mm3, and 12.6 ± 1.8J/mm3, respectively, with the mean ablation time (AT) of 633.7 ± 55.1 s, 874.2 ± 65.6 s, 936.3 ± 85.2 s, and 1103.2 ± 96.2 s, respectively. The non-perfused volume ratios (NPVR) were 90.4 ± 8.8%, 88.7 ± 9.1%, 89.4 ± 7.2%, 80.5 ± 7.9%, respectively. In addition, EEF and AT were the shortest in group A (p < 0.05). NPVR was significantly higher in group A than in the control group D (p < 0.05). The incidence rates of adverse events were not significantly different in the four groups (p > 0.05).
Conclusions
Compared to the control group, oxytocin combined with SonoVue in HIFU for adenomyosis can significantly decrease the energy and time needed for the ablation and safely enhance the treatment efficiency by improving the cavitation and heating of HIFU ablation and increasing the non-perfused volume ratio.
Introduction
Adenomyosis is a common gynecological disease caused by diffusive or focal interstitial invasion of endometrial glands into the myometrium, which mainly affects premenopausal women [Citation1]. It is characterized by symptoms of menorrhagia and dysmenorrhea accompanied by subfertility [Citation2]. The goal of treatment for adenomyosis is to reduce heavy menstrual bleeding and alleviate associated pain. Routine treatment consists of medication and surgery. Surgery includes hysterectomy, resection of adenomyosis lesion, and uterine artery embolization. Adenomyosis resection is the standard treatment in severe cases with severe anemia and painful shock. However, it is difficult to excise the lesion completely since the boundary of the adenomyotic lesion is obscure. The effective ratio of complete resection of the adenomyosis lesion is approximately 50%, with a high recurrence rate or difficulty maintaining fertility. Further scar tissues after surgery may seriously affect future fertility [Citation3]. Uterine artery embolization (UAE) is minimally invasive, but the long-term follow-up recurrence rate is higher, with a possible consequence of uterine inertia [Citation4]. Conservative treatment is most important in patients who require to preserve fertility. The common medical therapy is mainly hormonal dependent, including gonadotropin releasing hormone analogues (GnRH-a), oral contraceptives, testosterone derivatives, levonorgestrel releasing intrauterine device (IUCD) progestin [Citation5], and nonsteroidal anti-inflammatory drugs (NSAIDs). Hormone therapy is effective in controlling symptoms, but has some adverse effects such as relapse after withdrawal, short course of treatment, and adverse effects on pregnancy [Citation6,Citation7]. Moreover, the effect of medication is only limited to the treatment duration, temporary induction of degeneration of adenomyosis and improvement of symptoms [Citation8]. Therefore, it is important to explore more safe, effective, and noninvasive treatment approaches that can quickly improve symptoms of adenomyosis without adversely affecting the pregnancy outcome.
High-intensity focused ultrasound (HIFU), as a safe and noninvasive promising therapeutic strategy for patients with adenomyosis [Citation9–13], has been applied more frequently in clinical practice over the last ten years. Both focal and diffuse adenomyosis can be ablated by HIFU. Long-term symptom relief is mainly dependent on a high non-perfused volume ratio (NPVR) and is negatively associated with recurrence [Citation14]. Therefore, the key to enhance the effect of HIFU lies in increasing the necrosis volume through strengthening the ultrasonic energy absorbed by the adenomyosis lesion. Recently, there have been many approaches to modify the ultrasonic energy absorbed in the uterine adenomyosis lesion using the ultrasound microbubble contrast agent SonoVue or injection of iodinated oil in the adenomyosis lesion [Citation9,Citation15,Citation16]. Studies have [Citation17,Citation18] demonstrated that the efficacy of HIFU treatment was associated with increased tissue necrosis and could be enhanced by injecting iodized oil or ethanol into the fibroid lesion before HIFU ablation. HIFU combined with the ultrasound microbubble contrast agent SonoVue or ethanol injection needed less treatment time and lower energy dose, but produced larger necrosis volumes than HIFU treatment only [Citation19–21]. Similar enhancement effects have been found in liver and other solid organs [Citation19–21]. These reports [Citation19–21] indicated that the use of the microbuble contrast agent SonoVue could decrease the ablation time and sonication time to massive gray scale changes but increased the NPVR, achieving a better treatment effect of HIFU ablation.
Oxytocin is a hormone secreted by the pituitary gland which is important in the maintenance of uterine contraction. It is usually used to prevent postpartum hemorrhage. When combined with uterine myocyte receptors, it causes uterine smooth muscle to contract and results in a decrease of menstrual blood flow [Citation22–24]. It had been confirmed that oxytocin could decrease the blood loss during laparoscopic myomectomy [Citation25]. It has also been found that oxytocin could decrease the energy for HIFU ablation in adenomyosis and safely and effectively enhance the treatment efficiency [Citation1].
HIFU treatment of adenomyosis is time-consuming, and how to improve the efficacy of HIFU therapy and increase the non-perfused volume while reducing adenomyosis recurrence is an important topic of research. Currently, no studies have been performed on the synergistical effect of the microbubble contrast agent SonoVue and oxytocin on HIFU ablation for adenomyosis. Based on the above studies, it was hypothesized that the use of the microbubble contrast agent SonoVue to enhance ablation effect combined with the use of oxytocin to decrease uterine blood flow would result in a better effect of HIFU ablation on uterine adenomyosis. This study was consequently performed to test the synergistical effect of these two drugs in HIFU ablation for uterine adenomyosis.
Materials and methods
Subjects
This prospective randomized controlled trial was approved by the ethics committees of the First Affiliated Hospital of Kunming Medical University (Kunming, China) (approval no: FEY-BG-39-2.0). Written informed consent was acquired from each patient to participate in the HIFU ablation treatment and for publication of the anonymized clinical data. Inclusion criteria were premenopausal adult women aged greater than 18 years, with dysmenorrhea and/or menorrhagia caused by uterine adenomyosis which was diagnosed by magnetic resonance imaging (MRI) or histopathological examination and treated with HIFU ablation. Exclusion criteria were pregnant or breast-feeding women, menstruation periods, uterine malignancy, endometriosis, lower abdominal surgical scar larger than 10 mm, intestinal tract adhesions to uterus or abdominal wall, failure of kidney, heart, liver, or lung, allergic to fentanyl and midazolam, and acute pelvic inflammation.
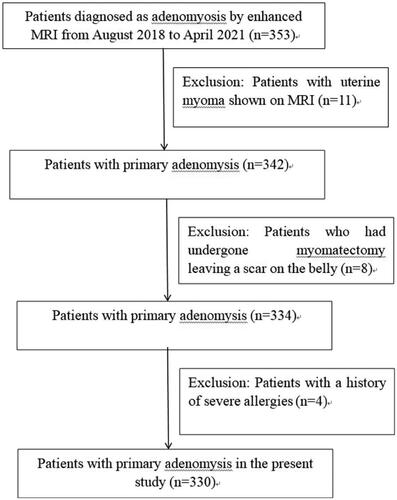
A total of 330 patients with adenomyosis who met the inclusion and exclusion criteria were consecutively recruited from our hospital between 2018 and 2021 for HIFU ablation treatment (). The patients were randomly divided into groups A, B, C, and D with a computer table of random numbers. The microbubble contrast agent SonoVue and oxytocin were used in HIFU ablation in group A (n = 82), only oxytocin in group B (n = 85), only SonoVue in group C (n = 81), and neither SonoVue nor oxytocin in group D (n = 82).
Preoperative preparation
A special bowel preparation was required for all patients, and only liquid diets were allowed two days prior to HIFU. The complex polyethylene glycol electrolyte solution (2000 ml) was administered for every patient before the day of operation. An enema was executed on the morning of HIFU treatment. The skin hair was shaved from the upper margin of the pubic symphysis to the umbilicus, and the area was degreased with alcohol and degassed with a vacuum sucking air before treatment in order to make a safe and clear acoustic pathway. Urinary catheter was inserted in order to inject saline solution to control the bladder volume. A water balloon compressor was applied to push the bowel to prevent acoustic injury. Then, the patient lied on the operation bed in the prone position with the anterior lower abdominal wall contacting cold degassed water. Venous access was opened, and midazole and fentanyl were used according to the body weight. The sedative level was controlled to reach grade 3–4 on the Ramsy scale, and the analgesic effect was graded on the basis of Visual Analogue Scale (VAS) standard to have a pain score of less than 4.
Equipment
The Chongqing JC200 Ultrasound Focused Tumor Treatment System (Chongqing Haifu Technology, Chongqing, China) was applied for HIFU ablation. This system had a transducer (electric power 8.5kVA, frequency 0.5–2MHz, output power ≤400 W, diameter 22 cm, and focal length 14.5 cm), which was accompanied by MyLab70 (Esaote, Genoa, Italy) and a B-mold ultrasound to monitor the HIFU ablation process. The maximal intensity of focus was less than 400 W/cm2, width of focus ≤3mm, and length ≤30 mm. The Philips Achieva 3.0 T MR (Best, the Netherlands) or the GE Sigma HDxt 3.0 T MRI (Waukesha, WI, USA) was applied to examine the patient. Gadolinium-diethylenetetramine pentaacetic acid was used for contrast-enhanced T1 weighted imaging (WI). All patients underwent MRI before and after HIFU. Preoperative clinical examinations, including transvaginal ultrasonography, human chorionic gonadotropin (HCG), CAl25, thinprep cytologic test, chest radiography, and blood test were all performed before ablation.
Treatment
During the HIFU ablation process, the treatment model of point ablation was chosen with a power of 300 to 400 W. Treatment parameters included θ = 90°, and the region of interest (ROI) was selected in the center of adonemosis. The common recommended area was the lower 1/4th area of the lesion to ensure safe performance and avoid intestines or aeration tissue in the acoustic pathway. A well-trained nurse prepared 59 mg SonoVue dissolved in 6 ml sodium chloride or saline. Patients in groups A and C received a bolus injection of 2 ml of SonoVue solution three times in total to observe changes in blood flow and volume of necrotic lesion. The first time of injection was at the start of the HIFU, the second time was when half of lesions had been ablated, and the last time was at the end of the surgery. All participants received 200–500 ml of saline, which was injected into the bladder to ensure that the ultrasound pathway is clear. Preoperative angiography was performed with 2 ml of SonoVue solution to confirm the ROI and the size (length × width × height) of adenomyosis. After angiography, 80 units of liquid oxytocin were injected in 500 ml of 0.9% normal saline dripping at the rate of 0.32 IU/min in groups A and B, whereas 0.9% normal saline was used in group D. HIFU treatment was initiated 1 min after angiography. When ultrasonic gray in the region of interest showed obvious changes, the treatment focus was moved to another layer, and the ablation of the next point was carried out (). The focal length of the treatment focus was 8 mm, width 3 mm, and power increased to 400 W. If the patient had adverse reactions such as lower limb pain, the layers were changed to 100–500 s for each layer until the gray level of the adenomyosis turned white. The distance between each layer was 5 mm. When half layers were ablated, 2 ml of SonoVue solution was injected to monitor the volume of treatment area. Ten minutes after oxytocin withdrawal, angiography was performed. Immediately after the angiography, intravenous drip of oxytocin was administered, and the treatment was performed 1 min after the angiography. Plain and enhancement MRI scans of the pelvic cavity were performed 3 days after HIFU. Then, non-perfused size (length × width × height) of the adenomisis lesion was recorded. The volume formula was V = 0.52 × D1 × D2 × D3, with NPVR = volume of non-perfusion area/volume of adenomyosis × 100%. The vital signs were monitored, including heart and respiration rates, blood pressure, and oxygen saturation levels during the HIFU procedure. The patients were requested to report any discomfort during the procedure. The therapy parameters were recorded, including ablation time (t), output power (P), adverse-reaction (AE) type and frequency. Meanwhile, the three-dimensional diameter of non-perfusion area was measured, and the NPVR was calculated. EEF (Energy efficiency factor) =ηP t/V, where η (η = 0.7) is a constant. EEF was defined as the ultrasound energy needed for the ablation in 1 cubic millimeter of the lesion. The NPVR, defined as ratio of the volume of necrosis lesion to the total adenomyosis lesion, was the key marker to evaluate the treatment effect. Finally, the patients were contacted the following 3 days to record post-procedure adverse events.
Statistical analysis
The statistical analyses were conducted using the SPSS software (IBM Corporation, Armonk, NY, USA). Data were represented as median ± standard deviation. The paired t test was used to compare the ablation time, EEF, and NPVR between two of the four groups. The SNK-q test was used to make multiple comparisons between the means of four groups. p < 0.05 was considered statistically significant.
Results
A total of 330 consecutive patients with adenomyosis diagnosed by MRI were enrolled, with an age range of 23–50 years (mean 37.7 ± 6.3). Moderate to severe dysmenorrhea was presented in 325 patients, hypermenorrhea was presented in 289, and menostaxis was in 227. Five patients had no obvious symptoms (). Local adenomyosis was presented in 213 patients (64.5%), and diffusive adenomyosis in 117 (35.5%). The lesion was located in the anterior wall in 136 (31.7%) and posterior wall in 194 (58.8%). The maximal diameter of the adenomyosis lesion was 8.1 ± 2.3 cm, the mean body mass index of patients was 22.7 ± 2.8 kg/m2, and the mean distance from the target lesion to the skin surface was 7.5 ± 1.9 cm. The mean abdominal wall thickness was 3.6 ± 0.5 cm, and the mean distance between the target and the sacrococcyx was 3.7 ± 0.8 cm.
Table 1. Basic information and treatment data of patients.
The MRI T2-WI signal intensity was significantly high with a value over 5 in 193 patients (58.5%) and less than 5 in 137 patients (41.4%). The microbubble contrast agent SonoVue was applied in 163 (49.4%) patients, and Oxytocin was used in 167 patients (50.6%). The HIFU ablation was technically successful in all patients (). The ablation time, EEF, and NPVR were 633.7 ± 55.1 s, 4.7 ± 0.9 J/mm3, and 90.4 ± 8.8% in group A, 874.2 ± 65.6 s, 8.5 ± 0.6 J/mm3, and 88.7 ± 9.1% in group B, 936.3 ± 85.2 s, 8.9 ± 0.7 J/mm3, and 89.4 ± 7.2% in group C, and 1303.2 ± 96.2 s, 12.6 ± 1.8J/mm3, and 80.5 ± 7.9% in group D, respectively. Pain was presented in the abdominal region, leg, sciatic, and buttock during HIFU ablation, with the mean pain score in the four areas of 6.1 ± 0.5, 5.3 ± 0.8, 4.4 ± 0.6, and 4.9 ± 0.3, respectively ( and ). The ablation time and EEF in group A were significantly decreased compared with those in each of the other groups (p < 0.05) (). The ablation time and EEF in groups B and C were significantly (p < 0.05) decreased compared with those in group D (). No significant (p > 0.05) difference existed in the ablation time and EEF between groups B and C. A significant (p < 0.05) difference was detected in NPVR between group A and group D (), and no significant (p > 0.05) difference was found in NPVR between the other groups. The incidence rates of abdominal pain in the treated region, leg, sciatic and buttock during HIFU were not significantly different (p > 0.05) () ().
Figure 2. Before HIFU ablation, the lesion of adenomyosis is in the posterior wall of uterus with low echo (A). After HIFU ablation, the echo of adenomyosis turns white(B); Before HIFU, the T2WI MRI shows the adenomyosis in the posterior wall of the uterus with hypointensity signal and hyperintensity pot sac signal (C); On contrast enhanced magnetic resonance imaging after treatment, the non-perfused volume (arrows) in the posterior wall is necrosis of the adenomyosis (D).
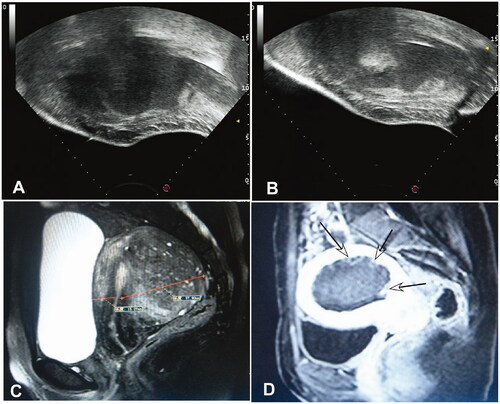
Figure 3. The high-intensity focused ultrasound (HIBU) ablation time, EEF (energy efficiency factor), NPVR (non-perfused volume ratio), and VAS (Visual Analogue Scale) were shown among four groups. (A) A significant (p < 0.05) difference existed in the HIFU ablation time between group A and each of the other three groups (*) or between groups B or C and group D (**). (B) A significant (p < 0.05) difference existed in EEF between group A and each of the other three groups (*) or between groups B or C and group D (**). (C) A significant (p < 0.05) difference existed in NPVR between group A and group D (*). (D) No significant (p > 0.05) difference was found in VAS among four groups. *Indicates a significant (p < 0.05) difference between group A and the other groups in figures A and B or between groups A and D in figure (C). ** indicates a significant (p < 0.05) difference between group B or C and group D.
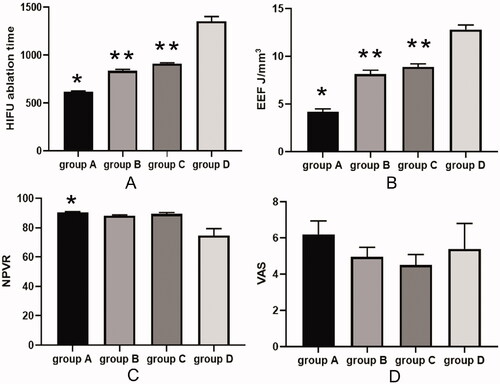
Table 2. Tests of effects between subjects with ablation time as the dependent variable.
Table 3. Complications of HIFU treatment for adenomyosis.
Discussion
As a noninvasive local physical therapy, HIFU has been frequently used for ablating solid tumors in China in recent years, especially for the treatment of uterine fibroids and adenomyosis. It can focus low-energy ultrasonic wave at a point to generate tissue resonance and cause ion friction, thereby generating thermal and cavitation effects to make tumor parenchymal cells undergo coagulative necrosis [Citation26]. Clinical practice reveals that the efficacy of HIFU ablation for different uterine cells is distinct. The reasons for these variabilities include abundant blood supply and water contained in the fibroids [Citation27]. Clinical effects of HIFU in patients with adenomyosis are safe in improving symptoms, which could be considered as the first choice for women with desire to conceive [Citation14].
However, in clinical practice, adenomyosis is difficult to treat and often recurs after incomplete ablation. In our study, it was found that combining oxytocin with SonoVue decreased EEF and shortened the ablation time compared with the use of oxytocin or SonoVue alone. Thus, our study proved that oxytocin and SonoVue had a synergistical effect to reduce EEF and increase the energy concentration. Some studies had indicated that SonoVue or oxytocin alone could reduce EEF [Citation1,Citation15]. In our study, the EEF was more insignificantly reduced in group B than in group C. The EEF and ablation time were shorter in group B or C than in group D. SonoVue is a phospholipid bilayer ultrasound contrast agent containing sulfur hexafluoride and has been widely applied for the diagnosis and evaluation of ablation effect [Citation19,Citation28]. Furthermore, SonoVue could enhance local tissues to absorb the ultrasound energy and provide cavitation nuclei in the acoustic pathway of HIFU. When HIFU power impacts the focus, cavitation effects are produced to augment the ablation volume, thereby immediately and highly elevating the temperature and reducing the total energy required for HIFU ablation [Citation29–31]. In the present study of adenomyosis, HIFU ablation starting at 1 min after SonoVue injection could evidently enhance HIFU ablation, including significantly shortened time to massive gray change, decreased EEF, less total energy, and lower mean power to achieve similar nonperfused volume than HIFU ablation starting at later than 1 min after SonoVue injection [Citation19,Citation32]. After a bolus intravenous injection of SonoVue, the contrast microbubbles travel with blood into the uterus region and are exhaled within 15 min. On the basis of SonoVue specifications, 80% of microbubbles are found in exhaled air within 2 min after SonoVue injection. Therefore, the HIFU treatment was initiated within the shortest time after contrast-enhanced ultrasound to exert the best cavitation effect of microbubbles [Citation15]. It is considered that the contrast-agent microbubble SonoVue causes the energy to accumulate in the target area as the ‘cavitation nuclei’ in a short duration to produce a violent microscopic blast. In the undamaged area, it forms a reflection or scattering ‘wall’ to reflect most of the sound and scattered sound wave back to the focal area and improve the local energy deposition [Citation15]. Some studies had reported higher fractional ablation, lower EEF, and shorter time to massive gray scale change using SonoVue for the treatment of adenomyosis [Citation16]. These findings suggested that cavitation effect is an enhancing factor. In addition, no major complications or adverse events were found in the SonoVue groups. A retrospective study evaluating 941 patients with adenomyosis had confirmed the cavitation enhancement effect of SonoVue in HIFU ablation [Citation9], with a similar lower EEF for treating large lesion volumes in the SonoVue group compared to the control group. These results indicated that the higher the number of microbubbles remaining in the target region, the better the ablation effect. Peng et al. also believed that the potential explanation for the SonoVue effect is cavitation and heating by the microbubbles [Citation19]. Despite the differences between uterine fibroids in other studies and adenomyosis in our study, our results indicated that SonoVue improved the ablation of HIFU by cavitation and heating. Similarly, there was a strong reflection of therapeutic sonography during HIFU ablation with massive gray scale change. Isern et al. reported that the EEF was the lowest when HIFU ablation was started 1 min after injection of the microbubble contrast [Citation33], and the EEF of 5.4 J/mm3 at 1 min in our study was similar to previously reported values. Because of short effective time, the EEF was more reduced in group B than in group C. The efficacy of sulfur hexafluoride microbubbles in reducing the EEF is weaker than that of oxytocin.
Oxytocin is a uterotonic agent used most commonly in obstetric practice. It decreases the risk of postpartum hemorrhage and improves uterine smooth muscle contraction to reduce blood flow and decrease the lumen size of the artery [Citation34,Citation35]. Some studies showed that oxytocin receptors were enriched in the pregnant uterus, fibroids, and adenomyosis [Citation22,Citation36–38]. Oxytocin used in patients with adenomyosis had significantly decreased blood flow compared with that in the control group [Citation1]. In our study, the oxytocin was administered at the speed of 0.32 IU/min in groups A and B during HIFU ablation. By using similar ablation power, oxytocin had significantly augmented NPVR, decreased EEF, and shortened ablation time than in the control group. This is because oxytocin can shrink the uterine artery and decrease blood perfusion of the adenomyotic lesion, leading to significant reduction of ultrasound energy and ablation time required for necrotizing the adenomyotic lesions [Citation36]. Because of less blood flow to take away the acoustic energy, more energy is focused on the target lesion. On the contrary, if the blood supply is affluent with larger arteries, the acoustic energy may be fastly dissipated, thus requiring longer surgery time and more energy for ablation. In our study, the oxytocin was applied during the whole ablation procedure, and the EEF was more reduced in group B than in group C. When oxytocin was combined to oxytocin receptors in the adenomyosis, the uterine arteries would contract, and sulfur hexafluoride microbubbles would stay in the lesion of adenomyosis for a longer period of time, resulting in the cavitation effect for a longer time. Therefore, SonoVue and oxytocin had a synergistic effect to increase the deposition of ultrasonic energy and shorten the ablation time, and our study had confirmed that the EEF and ablation time were the shortest in group A than in the other groups (p < 0.05).
Oxytocin has some adverse effects, including nausea, vomiting, tachycardia, hypotension, anaphylaxis, and edema. It may be related to the dose and rate of dripping. Therefore, a moderate dose of oxytocin of 80 units was used in 500 ml of 0.9% saline at the speed of 0.32 IU/min, which caused no serious adverse events in our study. Pain caused by uterine contractions appeared in some of our patients because of the application of oxytocin.
Other symptoms included pain in the sciatic area, treated region, leg or buttock, which were not caused by oxytocin (). Leg pain was due to ultrasound energy radiation to the sciatic nerve, and intravenous injection of dexamethasone 10 mg per day for three days could completely resolve the leg pain. There was no significant difference in adverse effects observed among the four groups. Some patients had vaginal discharge a few days after the HIFU procedure and even lasted 1 month, with no significant difference between the four groups. The complications in most participants disappeared 7 days later without any specific treatment. Only two patients had vaginal discharge that lasted for one month. No severe complications such as bowel injury or nerve injury occurred in our study. Therefore, this dose of oxytocin was safe and effective in improving the effects of HIFU ablation on adenomyosis.
Some limitations existed in our study, including one center study, small cohorts of patients, Chinese patients enrolled only, differences in the way of HIFU ablation in different physicians performing the ablation, dosage of oxytocin and microbubble contrast agent SonoVue, volume and location of the adenomyosis in the uterus, which may all affect the outcomes of the study and its generalization. Future studies will have to resolve all these issues for a better outcome.
In conclusion, compared to the control group, oxytocin combined with SonoVue in the treatment of adenomyosis with HIFU ablation can significantly and synergistically decrease the energy and time needed for the ablation and enhance the treatment efficiency by improving the cavitation and heating of HIFU ablation and increasing the non-perfused volume.
Ethical approval
Informed consent was acquired from all patients or their legal guardians. The study was approved by the Institutional Review Board of the first affiliated hospital of Kunming Medical University (Kunming, China) (approval no: FEY-BG-39-2.0), where the study was conducted.
Author contributions
Ruihong Yao was responsible for all the conceptualization, methodology, statistical analysis,and composition of the original draft. Bulang Gao was responsible for reviewing and editing the manuscript.Jihong Hu collected and analyzed the data.Tao Wang enrolled participants and collected data. WeiZhao was responsible for designing the study and performing data analyses. All the authors contributed to the interpretation of the data and the critical revision and approval of the article. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data and materials analyzed in this study are included in the manuscript. The data used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Zhang X, Zou M, Zhang C, et al. Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomysis: a prospective study. Eur J Radiol. 2014;83(9):1607–1611.
- Garavaglia E, Audrey S, Annalisa I, et al. Adenomyosis and its impact on women fertility. Int J Reprod Biomed. 2015;13:327–336.
- Zhu L, Chen S, Che X, et al. Comparisons of the efficacy and recurrence of adenomyomectomy for severe uterine diffuse adenomyosisvia laparotomy versus laparoscopy: a long-term result in a single institution. JPR. 2019;12:1917–1924.
- Meridith J. Englander.Uterine artery embolization for the treatment of adenomyosis. Semin Intervent Radiol. 2008;25(4):387–393.
- Levgur M. Therapeutic options for adenomyosis: a review. Arch Gynecol Obstet. 2007;276(1):1–15.
- Nothnick W, Alali Z. Recent advances in the understanding of endometriosis: the role of inflammatory mediators in disease pathogenesis and treatment. F1000Res. 2016;5(2):186–129.
- Vercellini P, Vigano P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275.
- Pontis A, D'Alterio MN, Pirarba S, et al. C. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol. 2016;32(9):696–700.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32(5):496–503.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124(3):207–211.
- Zhou CY, Xu XJ, He J. Pregnancy outcomes and symptom improvement of patients with adenomyosis treated with high intensity focused ultrasound ablation. Zhonghua Fu Chan Ke Za Zhi. 2016;51:845–849.
- Jaeyoon P, Seong LJ, Jae-Hwan C, et al. Effects of highintensity-focused ultrasound treatment on benign uterine tumor. J Korean Med Sci. 2016;31:1279–1283.
- Feng Y, Hu L, Chen W, et al. Safety of ultrasoundguided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem. 2017;36:139–145.
- Zhang L, RaoF, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96(6):707–714.
- Jingqi W, Lu Z, Jun Z, et al. Clinical usefulness of the microbubble contrast agent SonoVue in enhancing the effects of High-Intensity focused ultrasound for the treatment of adenomyosis. J Ultrasound Med. 2018;37(12):2811–2819.
- Cheng C-Q, Zhang R-T, Xiong Y, et al. Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases: retrospective analysis of contrast safety. Medicine. 2015;94(16):e729.
- Li Q, Xiao YB, Liang ZG, et al. Ablation of leiomyomas using a combination of HIFU and iodized oil in vitro. Ultrasound Med Biol. 2012;38(9):1576–1581.
- Yang Z, Zhang Y, Zhang R, et al. A case-control study of high-intensity focused ultrasound combined with sonographically guided intratumoral ethanol injection in the treatment of uterine fibroids. J Ultrasound Med. 2014;33(4):657–665.
- Peng S, Xiong Y, Li K, et al. Clinical utility of a microbubble-enhancing contrast ("SonoVue") in treatment of uterine fibroids with high intensity focused ultrasound: a retrospective study. Eur J Radiol. 2012;81(12):3832–3838.
- Chung DJ, Cho SH, Lee JM, et al. Effect of microbubble contrast agent during high intensity focused ultrasound ablation on rabbit liver in vivo. Eur J Radiol. 2012;81(4):e519–e523.
- Luo W, Zhou X, Ren X, et al. Enhancing effects of SonoVue, a microbubble sonographic contrast agent, on high intensity focused ultrasound ablation in rabbit livers in vivo. J Ultrasound Med. 2007;26(4):469–476.
- Otonkoski S, Sainio T, Komar G, et al. Oxytocin selectively reduces blood flow in uterine fibroids without an effect on myometrial blood flow: a dynamic contrast enhanced MRI evaluation. Int J Hyperthermia. 2020;37(1):1293–1300.
- Yu SC, Cheung EC, Leung VY, et al. Oxytocin-augmented and non-sedating high-intensity-focused ultrasound (HIFU) for uterine fibroids showed promising outcome as compared To HIFU alone or uterine artery embolization. Ultrasound Med Biol. 2019;45(12):3207–3213.
- Lozinski T, Filipowska J, Krol P, et al. Oxytocin administration in High-Intensity focused ultrasound treatment of myomata. Biomed Res Int. 2018;2018:7518026.
- Wang CJ, Lee CL, Yuen LT, et al. Oxytocin infusion in laparoscopic myomectomymay decrease operative blood loss. J Minim Invasive Gynecol. 2007;14(2):184–188.
- Zhao WP, Han ZY, Zhang J, et al. A retrospective comparison of microwave ablation and high intensity focused ultrasound for treating symptomatic uterine fibroids. Eur J Radiol. 2015;84(3):413–417.
- Fan H-J, Cun J-P, Zhao W, et al. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35(1):534–540.
- Peng S, Hu L, Chen W, et al. Intraprocedure contrast enhanced ultrasound: the value in assessing the effect of ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids. Ultrasonics. 2015;58:123–128.
- Moyer LC, Timbie KF, Sheeran PS, et al. High-intensity focused ultrasound ablation enhancement in vivo via phase-shift nanodroplets compared to microbubbles. J Ther Ultrasound. 2015;3:7.
- Hamano N, Negishi Y, Takatori K, et al. Combination of bubble liposomes and high-intensity focused ultrasound (HIFU) enhanced antitumor effect by tumor ablation. Biol Pharm Bull. 2014;37(1):174–177.
- Zhou D, Wang Z, Sun Y, et al. Superparamagnetic PLGA-iron oxide microspheres as contrast agents for dual-imaging and enhance the effects of high intensity focused ultrasound ablation on liver tissue. RSC Adv. 2015;5(45):35693–35703.
- Jiang N, Xie B, Zhang X, et al. Enhancing ablation effects of a microbubble-enhancing contrast agent (“SonoVue”) in the treat ment of uterine fibroids with high-intensity focused ultrasound: a randomized controlled trial. Cardiovasc Intervent Radiol. 2014;37(5):1321–1328.
- Isern J, Pessarrodona A, Rodriguez J, et al. Using microbubble sonographic contrast agent to enhance the effect of high intensity focused ultrasound for the treatment of uterine fibroids. Ultrason Sonochem. 2015;27:688–693.
- Roach MK, Abramovici A, Tita AT. Dose and duration of oxytocin to prevent postpartum hemorrhage: a review. Am J Perinatol. 2013;30(7):523–528.
- Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anaesthesiol. 2011;24(3):255–261.
- Richter ON, Kubler K, Schmolling J, et al. Oxytocin receptor gene expression of estrogen-stimulated human myometrium in extracorporeally perfused non-pregnant uteri. Mol Hum Reprod. 2004;10(5):339–346.
- Loddenkemper C, Mechsner S, Foss H-D, et al. Use of oxytocin receptor expression in distinguishing between uterine smooth muscle tumors and endometrial stromal sarcoma. Am J Surg Pathol. 2003;27(11):1458–1462.
- Mechsner S, Bartley J, Loddenkemper C, et al. Oxytocin receptor expression in smooth muscle cells of perineal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril. 2005;83(4):1220–1231.