Abstract
Objective
To compare the safety, reintervention and pregnancy outcomes between ultrasound-guided high intensity focused ultrasound (USgHIFU) and hysteroscopic myomectomy (HM) for submucosal fibroids.
Materials and methods
A total of 215 patients with a solitary submucosal fibroid treated by USgHIFU or HM at the third Xiangya Hospital were retrospectively reviewed. Among them, 58 treated with USgHIFU, 157 treated with HM.
Results
A significant difference was observed in size, location and type of the fibroids, effective rate, and cumulative reintervention rate between the two groups (p < .05). The size of the fibroids was 57.9 ± 1.9 mm in the USgHIFU group, while it was 32.6 ± 1.2 mm in the HM group. The number of the fibroids at horn or fundus/uterine cavity was 16/42 in the USgHIFU group, while it was 21/136 in the HM group. The number of type I/II/2–5 was 16/17/25 in the USgHIFU group, while it was 133/24/0 in the HM group. In the USgHIFU group, the effective rate was 100% and the cumulative reintervention rate at 50 (17–97) months was 19.0%, while in the HM group, it was 94.3% and 7.6%, respectively. During the follow-up period, the pregnancy rate was 22.4% (13/58) and the reintervention rate due to invalid and recurrence was 15.5% (9/58) in the USgHIFU group, while they were 18.5% (29/157) and 7.0% (11/157) in the HM group. No significant difference was observed between the two groups (p > .05). Furthermore, the reintervention rate was positively correlated with age, treatment methods and parity and fertility requirements. No other significant difference was observed between the two groups.
Conclusions
Both USgHIFU and HM are safe and effective in treating submucosal fibroids. Compared with the HM group, the USgHIFU group had lower postoperative complications, but higher reintervention rate, with similar recurrence rate, pregnancy rate and reintervention rate due to invalid and recurrence. Reintervention was related to age, treatment methods, parity and fertility requirements.
Introduction
Uterine fibroids are the most common benign tumors in gynecology with its incidence rate ranging from 20 to 40% [Citation1]. They are classified into three subtypes according to the locations in the uterus including submucousal, intramural and subserosal fibroids. Generally, nearly 2/3 of patients with uterine fibroids are asymptomatic, and patients with symptomatic fibroids are more likely to be diagnosed with submucous fibroids [Citation2]. Apart from clinical manifestation, treatment principle of submucousal fibroids is also different. The reasons why submucousal fibroid is so distinct from other subtypes are that [Citation3,Citation4]: (1) partly or totally locating in the uterine cavity makes it easy to cause symptoms even if the diameter is very small; (2) the occupation of uterine cavity inevitably affects fertilization; (3) the special location unavoidably induces damage to the endometrium during the treatment especially when the fibroid grows up. Therefore, once submucosal fibroids are diagnosed, it is recommended to treat it as soon as possible, even if no symptoms [Citation5]. At present, submucousal fibroids could be surgically removed by laparotomy, laparoscopic myomectomy, hysteroscopic myomectomy (HM) or noninvasively treated by ultrasound-guided high intensity focused ultrasound (USgHIFU) [Citation6]. In the past, the commonly accepted operative surgery, HM, is reported to have the occurrence of complications such as uterine perforation, hyponatremia and intrauterine adhesion (IUA) [Citation7,Citation8]. What’s more, HM is also limited by the size of the submucosal fibroids. The large size of the submucosal fibroids is associated with not only the increased risk of complications, but also more operations or combination of different surgical approaches which brings about psychological and physiological hurts to patients [Citation9].
As a noninvasive treatment, HIFU has shown its safety, efficiency, quick recovery in the treatment of uterine fibroids [Citation10]. Although many studies have supported the safety and efficacy of HIFU for treatment of uterine fibroids, whether USgHIFU could be applied to treat submucousal fibroids is a debated topic, especially for patients with fertility requirements. As we all known, fibroids could not disappear immediately after the HIFU which may consistently affect fertilization. In previous studies, HIFU was reported to have an overall remission rate of 95.9% at 8 years, with reintervention rate of 5.3% at 4 years without any major complications [Citation11–15]. While patients treated with HM showed the recurrence rate of 22%, with reintervention rate of 31.7% at 6 years, and the pregnancy rate of 33.8–85.8% was achieved [Citation16–18]. Recently, Friedman et al. [Citation8] retrospectively studied 375 women with submucosal fibroids who received USgHIFU treatment and surgical treatment. With the 8-year-follow up, they found USgHIFU possessed statistically higher remission rate, lower recurrence rate and fewer complications. To the best of our knowledge, there remains no comparison between USgHIFU and HM in the treatment of submucousal fibroids in safety, effectiveness and pregnancy outcomes.
This study aims to compare the safety, effectiveness and pregnancy outcome of patients with submucosal fibroids treated with USgHIFU and HM.
Material and methods
This retrospective study was approved by the ethics committee at the Third Xiangya Hospital of Central South University (NO:quick1 21073).
Patients
A total of 215 patients with submucosal fibroids diagnosed and treated by USgHIFU or HM in the Third Xiangya hospital from 1 January 2012 to 31 December 2018 were enrolled. The median follow-up time was 50 (17–97) months. Among them, 58 cases were treated by USgHIFU and 157 cases were treated by HM.
The inclusion criteria included the following: (1) patients who were diagnosed with single submucosal fibroid (Type I, Type II and Types 2–5 according to FIGO classification) by MRI or color Doppler ultrasound [Citation19]; (2) patients who could easily communicate and voluntarily choose the method of treatment; (3) premenopausal women with uterine fibroids related symptoms.
Exclusion criteria included the following: (1) malignant or suspected malignant tumor; (2) combined with adenomyosis; (3) Type 0 according to FIGO classification; (4) multiple uterine fibroids.
The evaluation criteria of USgHIFU clinical outcome [Citation20]: (1) Cure: the diameter of uterine fibroid reduced by 50% or more, and clinical symptoms disappeared; (2) Effective: the diameter of uterine fibroid reduced by less than 50% or did not increase, and the clinical symptoms reduced; (3) Invalid: the diameter of uterine fibroid increased, and the clinical symptoms did not improve; (4) Recurrence: newly developed uterine fibroids after complete disappearance of initial uterine fibroid or recurred symptoms with initial uterine fibroid did not disappear completely. Effective rate = (cure number + effective number)/total number × 100%. Evaluation criteria of HM clinical outcome [Citation21]: (1) Cure: the uterine fibroid was totally removed, and clinical symptoms disappeared; (2) Effective: a large part of the uterine fibroid was removed, and clinical symptoms improved; (3) Invalid: the uterine fibroid was partially removed, and without clinical symptoms improved; (4) Recurrence: newly developed uterine fibroids indicated by color Doppler ultrasound 6 months after surgery.
USgHIFU procedure
The USgHIFU procedure has been described previously [Citation22]. In short, the treatment time was 3 d after menstruation. Blood routine test and coagulation function test were performed before operation. USgHIFU was performed under intravenous conscious sedation. The device used was a JC200 HIFU tumor therapeutic system (Chongqing Haifu Medical Technology, Co., Ltd., Chongqing, China). Therapeutic ultrasound energy was generated by a transducer with a frequency of 0.8 MHz, a focal length of 15 cm, and a diameter of 20 cm. The patient was prone on the HIFU table, and a Mylab version 70 ultrasound imaging device (Esaote, Genova, Italy) was used to provide real-time imaging to localize and monitor the treatment (). From the deep surface to the superficial surface of the fibroid, the point line plane body mode was used to sonicate the focus layer by layer to cover the target area of the fibroid. Patients should lie prone for 2 h and be given a liquid diet for 3 h after USgHIFU. Normal diet and discharge can be conducted 1 d after the treatment.
Figure 1. Pre- and post-HIFU ultrasound images obtained from a patient with type I uterine fibroid. (A) Before ablation, the diameter of the fibroid prepared for ablation was focused. (B) After ablation, ultrasound image of uterine fibroid with intact psuedocapsule (C) Before ablation, the contrast enhanced ultrasonography image showed homogeneous enhancement within the fibroid. (D) After ablation, no contrast enhancement was observed in the ablation area.
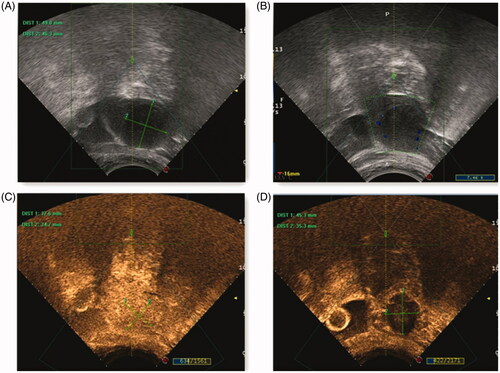
HM procedure
The operation was performed at 3 d after menstruation. Cervical softening preparation was performed 1–2 d before operation. During the operation, intravenous anesthesia was applied, and the patient took the lithotomy position of the bladder. Olympus hysteroscope was used to enter the uterine cavity through the cervix to preliminarily explored uterine cavity and submucousal fibroids. Then, the cervix was dilated to No.10 with the uterine cervix dilator. Finally, the hysteroscopy was replaced and the fibroid was removed step by step with ring electrode. The operation was terminated after no bleeding was observed. The patients were discharged 48 h after operation.
Followed-up observation
Through the electronic case system of the Third Xiangya Hospital of Central South University, the basic clinical characteristics data of 215 patients were collected, including age, body mass index (BMI), hemoglobin, gravidity, parity, miscarriages, position of the uterus, symptoms, history of myomectomy, fertility requirement, size of uterine fibroid, localization of uterine fibroid, type of uterine fibroid (FIGO) and intraoperative complications; The effectiveness and pregnancy outcomes were investigated by telephone interview, including curative effect, postoperative complications, size of uterine fibroids, recurrence, reintervention, interval time of reintervention, number of reintervention, modalities of reintervention, reasons for reintervention, pregnancy, interval time of first pregnancy, conception approach, pregnancy outcome, delivery approaches, pregnancy complications and gestational age.
Statistical analysis
SPSS version 23 (SPSS Inc., Chicago, IL) statistical software was used for data analysis and p < .05 was considered statistically significant. Normally distributed data were depicted using mean ± standard deviation (SD). Skewed data were depicted as the median and interquartile range (IQR). Independent samples T-test, Chi-square test, Fisher exact test and nonparametric test (Mann–Whitney U test) were conducted to analyze baseline characteristics and raw data between the two groups. Binary logistic regression analysis was used for multivariate analysis.
Results
Baseline characteristics
As shown in , there was no significant difference in age, BMI, hemoglobin, gravidity, parity, miscarriages, position of the uterus, symptoms, history of myomectomy and fertility requirements between the two groups (p > .05). The size of submucosal fibroids was larger in the USgHIFU group than that in the HM group (57.9 ± 1.9 vs. 32.6 ± 1.2 mm) (p < .05). In the USgHIFU group, 27.6% (16/58) patients located at horn or fundus and 72.4% (42/58) patients located at uterine cavity; While in the HM group, 13.4% (21/157) patients located at horn or fundus and 86.6% (136/157) patients located at uterine cavity (p < .05). Among the 215 patients, the number of type I, II and 2–5 in the USgHIFU group and the HM group were, respectively, 16, 17, 25 and 133, 24, 0 (p < .05).
Table 1. Comparison of baseline characteristics between patients with submucosal fibroids treated with ultrasound-guided HIFU and HM.
Comparison of safety between USgHIFU and HM
There was no serious intraoperative and postoperative complication in the USgHIFU group. In the HM group, 1 patient (0.6%, 1/157) had intraoperative complication with hyponatremia, 18 patients (11.4%, 18/157) suffered from IUA diagnosed by hysteroscopy after operation and 7 patients (4.5%, 7/157) complained decreased menstrual volume (less than normal menstrual volume) without further diagnosis. There was a significant difference in postoperative complications between the two groups (p = .001) ().
Table 2. Comparison of safety between ultrasound-guided HIFU and HM.
Comparison of effectiveness between USgHIFU and HM
The remission rate of USgHIFU group was 100% (58/58); and in the HM group, the remission rate was 94.3% (148/157), the ineffective rate was 5.7% (9/157). The reintervention rate in the USgHIFU group was higher than that in the HM group (19 vs. 7.6%), and the difference was statistically significant (p = .017). However, the reintervention rate due to ineffective treatment or recurrence was 15.5% (9/58) in the USgHIFU group and 7.0% (11/157) in the HM group, the difference was not statistically significant (p = .057). Compared with the HM group, the USgHIFU group had lower recurrence rate and higher pregnancy rate, but the difference was not statistically significant (p > .05) ().
Table 3. Comparison of efficacy between ultrasound-guided HIFU and HM.
Among patients received reintervention, 3 patients (3/12, 25%) in the HM group received treatment twice, while no patient in the USgHIFU group received reintervention more than once. No significant difference was observed (p = .082). Compared with the HM group, the USgHIFU group had a significant longer interval of reintervention (38.64 ± 7.4 vs. 12.8 ± 3.8 months) (p < .05). The modalities of reintervention included hysteroscopy or laparoscopy or laparotomy myomectomy, USgHIFU and hysterectomy. The reasons for reintervention included no improvement in symptoms, recurrence, fertility requirements and other gynecological surgery (like cesarean section). There was no significant difference in the modalities of reintervention and the reasons for reintervention between the two groups ().
Table 4. Characteristics of reintervention between ultrasound-guided HIFU and HM.
In addition, the influencing factors of reintervention were analyzed. Univariate analysis showed that reintervention was positively related to age, treatment methods, parity and fertility requirements (p < .05). It was negatively related to BMI, hemoglobin, gravidity, miscarriages, symptoms, history of myomectomy, position of the uterus, size of uterine fibroids, localization of uterine fibroid and type of uterine fibroid (FIGO) (). Further binary logistic regression analysis showed that treatment methods were an independent influencing risk factor for reintervention ().
Table 5. Comparison of baseline characteristics between patients with and without reintervention during follow-up.
Table 6. The binary logistic regression analysis of variance.
Comparison of pregnancy between USgHIFU and HM
A total of 11 patients had fertility requirements when they received USgHIFU treatment; while 48 patients in the HM group had fertility requirements. 7 (7/11, 63.6%) patients in the USgHIFU group and 24 (24/48, 50%) patients in the HM group got pregnant. There was no significant difference in secondary infertility, pregnancy rate, number of pregnancies, interval time of first pregnancy, conception approach, pregnancy outcome, complications of pregnancy, delivery approaches and gestational age was observed between the two groups (). Three (3/7, 42.8%) patients in the USgHIFU group and 7 (7/24, 29.2%) patients in the HM group conceived half a year after treatment. Among these 10 patients, one patient in USgHIFU group and 1 patient in HM group had spontaneous miscarriage after 6 months. The rest patients achieved successful delivery without any pregnancy complications and the babies were born healthy.
Table 7. Comparison of pregnancy in patients with pregnant requirement.
Discussion
HM is a standard operation for submucosal fibroids. Although hysteroscopy remains a relatively safe procedure, complications, such as endometrial damage, air embolism, fluid overload or hyponatremia, uterine perforation limited its utility [Citation8,Citation23]. Meanwhile, the limitation laid in the difficulty for operators to remove the fibroid for one time when the diameter of the fibroid is more than 4 cm or the location of the fibroid is excessive deep [Citation5]. Consequently, the patients may suffer from the increased expenses, extra surgical risks and undue injury to endometrium. The treatment of hysteroscopy combined with laparoscopy might also applied, which not only increases the trauma, but also prolongs the pregnancy interval to one year. With the increasing demand of minimally invasive treatment, USgHIFU emerges as a new noninvasive, feasible, safe and cost-effective alternative in treating uterine fibroids. In the HM, the long-term recurrence rate was 22%, the pregnancy rate ranged 33.8–85.8%, and the incidence of IUA ranged 7.5–21.57% [Citation16,Citation17,Citation24,Citation25]. However, the long-term recurrence rate was 11.9%, the symptom relief rate was 95.9%, the pregnancy rate ranged 7–47% and no postoperative complications were complained in USgHIFU group [Citation13,Citation26]. In our study for median follow-up time was 50 (17–97) months, there were three patients went second hysteroscopic remove of fibroid in HM group. What’s more, compared with the HM group, the USgHIFU group had less postoperative complications (0 vs. 15.9%), higher reintervention rate (19.0 vs. 7.6%). However, no significant difference was observed in recurrence rate (12.1 vs. 17.2%), reintervention rate of ineffective and recurrence (15.5 vs. 7.0%), and pregnancy rate (63.6 vs. 50%).
Compared with the HM, safety seemed to be the most obvious advantage in USgHIFU. In our study, no serious intraoperative and postoperative complication in the USgHIFU group was observed. In the HM group, 1 patient (0.6%, 1/157) had intraoperative complication with hyponatremia, 25 patients (15.9%, 25/157) had postoperative complication with IUA or decreased menstrual volume. IUA formation after myomectomy was reported to have no association with the type of surgery or the type of fibroid, and nearly 20% of the patients suffered from IUA after the treatment of hysteroscopic or laparoscopic myomectomy [Citation27]. IUA is a disease following the basal layer of the endometrium damage due to factors, such as intrauterine operation and infection, resulting in adhesions or atresia of the uterine cavity and cervical canal [Citation28]. The clinical manifestations are decreased menstrual volume, amenorrhea and even infertility, which seriously affect the physical and mental health of patients. In addition, IUA need multiple hysteroscopic surgeries, increasing the life burden of patients and affecting the pregnancy outcome [Citation29]. Furthermore, myomectomy was also reported with safety problems like air embolism and uterine rupture [Citation23,Citation30]. Different from the traditional myomectomy, HIFU ablates uterine fibroids by focused ultrasound without damaging the endometrium and myometrium (). Previous studies have shown that after MR-HIFU ablation of submucosal fibroids, endometrial enhancement was preserved intact or minimally impaired by 95.7%. The impaired endometrium, which is more commonly seen in fibroid protruding into the endometrial cavity, will recover spontaneously [Citation31].
Figure 2. Pre- and post-HIFU MRI images obtained from a patient with type II uterine fibroid. (A) Before HIFU treatment, the endometrium was intact and continuous. (B) After HIFU treatment, the endometrium remained intact and continuous without damage. (C) Before HIFU treatment, the contrast-enhanced MRI image showed heterogeneous enhancement within the fibroid with blood perfusion. (D) After HIFU treatment, the contrast-enhanced MRI image only marginal enhancement of uterine fibroid, indicating that there was no blood perfusion inside the fibroid.
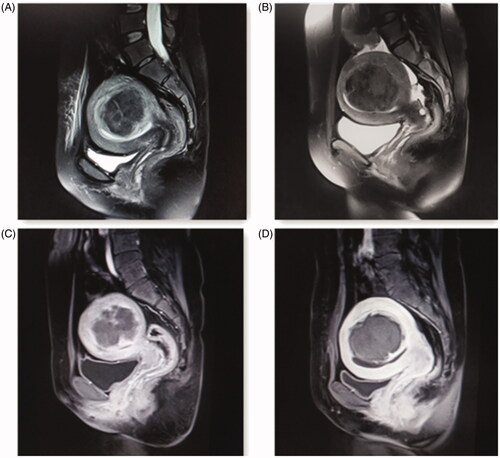
The previous studies have demonstrated that many factors included age, the size of uterine fibroids, distance from fibroid ventral side to skin, position of uterus, location of uterine fibroids, signal intensity on T2WI and enhancement type may affect the treatment results [Citation32–34]. We analyzed the risk factors of reintervention, and found that the reintervention rate was positive correlated with age, treatment methods, parity and fertility requirements. Interestingly, patients who were treated by USgHIFU had larger size of uterine fibroids (57.9 ± 1.9 vs. 32.6 ± 1.2). Moreover, in USgHIFU group, 27.6% of fibroids were located at the corner or fundus of uterus, and 72.4% of fibroids were type II or 2–5. In HM group, 13.4% of fibroids were located at the corner or fundus of uterus, 15.3% of fibroids were type II, and no fibroids were types 2–5. Types 2–5 fibroids were known as difficult type for hysteroscopic treatment, indicating the choice of treatment needs careful consideration. Although USgHIFU group were in such unfavorable initial conditions, in comparation to HM group, USgHIFU still obtained longer interval time of reintervention (38.64 ± 7.4 vs. 12.8 ± 3.8 months), higher symptomatic relief rate (100 vs. 94.3%), similar recurrence rate (12.1% vs. 17.2%) and similar curative reintervention rate (15.5 vs. 7.0%). These results demonstrated that USgHIFU is an effective treatment for submucosal fibroids. USgHIFU also showed higher reintervention rate (19.0 vs. 7.6%), which may be influenced by patients’ demand for pregnancy through the analysis of reintervention reasons. Therefore, for those who were older, or without fertility requirements, or had larger fibroids size, or had fibroids in the fundus or corner of the uterus, or had type II or 2–5 fibroids, USgHIFU might be a good choice owing to less damage, high remission rate of symptoms and similar reintervention rate with hysteroscopy. For younger patients with fertility requirements, the choice may be more difficult. On the one hand, the choice of USgHIFU may increase the probability of reintervention because the fibroid does not shrink immediately. On the other hand, the choice of HM increases the probability of IUA, which brings about multiple extra hysteroscopic surgery.
Pregnancy outcome is the main concern for patients with fertility requirements. Among patients with fertility requirements, USgHIFU group showed similar pregnant rate and interval time (p > .05). Considering that patients in USgHIFU group possessed fibroids which had significantly larger diameter and a great majority of Types II and 2–5, we hold the hypothesis that USgHIFU might be more favorable for those with fertility requirements. As we all know, fibroids with larger diameter or classified as Types II and 2–5 were more likely to be recommended to laparoscopy. Nevertheless, the gestational interval must be postponed at least one year for the risk potential of uterine rupture after laparoscopic treatment performed, which aggravates the time of waiting for a baby. In this study, three patients (3/7, 42.8%) with the mean fibroid size of 61.4 ± 14.9 (69.0, 71.0 and 44.3 mm) were pregnant at 3, 4 and 6 months, respectively, after USgHIFU treatment, while after HM treatment, 7 patients (7/24, 29.2%) with the mean fibroid size of 19.3 ± 8.1 (8–30 mm) were pregnant in half a year (3–6 months). Both HIFU and HM had one patient became spontaneous abortion after 6 months, with 44.3 mm and 25.0 mm size of fibroid, respectively. The rest of the patients achieved successful delivery without any pregnancy complications and the babies were born without any neonatal diseases.
This study is limited because it is a retrospective study and some bias in patients selection may occur. What’s more, the sample size of patients with fertility requirements USgHIFU group was small and all patients did not undergo precise endometrial assessment. Future studies are needed in evaluating endometrial injury by postoperative hysteroscopy. In addition, prospective study is needed in the future to convey more convincing data in comparison of USgHIFU and HM.
In summary, USgHIFU and HM are safe and effective way in the treatment of submucosal fibroids. Compared with the HM group, the USgHIFU group had lower postoperative complications, higher reintervention rate, similar reintervention rate of invalid and recurrence, recurrence rate and pregnancy rate. The reintervention rate was positively correlated with age, treatment methods, parity and fertility requirements. USgHIFU is recommended to patients characterized by older age or no fertility requirements or their fibroids featured by larger size or located in the fundus or corner of the uterus or Type II or 2–5.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512.
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: [email protected]; practice committee of the American society for reproductive medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertil Steril. 2017;108(3):416–425.
- Somigliana E, Reschini M, Bonanni V, et al. Fibroids and natural fertility: a systematic review and Meta-analysis. Reprod Biomed Online. 2021;43:100–110.
- Vitale SG, Riemma G, Ciebiera M, et al. Hysteroscopic treatment of submucosal fibroids in perimenopausal women: when, why, and how? Climacteric. 2020;23(4):355–359.
- Chittawar PB, Kamath MS. Review of nonsurgical/minimally invasive treatments and open myomectomy for uterine fibroids. Curr Opin Obstet Gynecol. 2015;27(6):391–397.
- Smith CC, P R Brown J. A case of cardiac arrhythmia from absorption of normal saline during hysteroscopic myomectomy. J Minim Invasive Gynecol. 2019;26(4):770–773.
- Friedman JA, Wong JMK, Chaudhari A, et al. Hysteroscopic myomectomy: a comparison of techniques and review of current evidence in the management of abnormal uterine bleeding. Curr Opin Obstet Gynecol. 2018;30(4):243–251.
- Rakotomahenina H, Rajaonarison J, Wong L, et al. Myomectomy: technique and current indications. Minerva Ginecol. 2017;69(4):357–369.
- Wang Y, Geng J, Bao H, et al. Comparative effectiveness and safety of High-Intensity focused ultrasound for uterine fibroids: a systematic review and Meta-Analysis. Front Oncol. 2021;11:600800.
- Wang W, Wang Y, Wang T, et al. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol. 2012;22(11):2553–2558.
- Wang T, Wang W, Chen WZ, et al. Efficacy and safety of focused ultrasound ablation in treatment of submucosal uterine fibroids. Zhonghua Fu Chan Ke Za Zhi. 2011;46(6):407–411.
- Wang Y, Liu X, Wang W, et al. Long-term clinical outcomes of US-Guided High-Intensity focused ultrasound ablation for symptomatic submucosal fibroids: a retrospective comparison with Uterus-Sparing surgery. Acad Radiol. 2020;28:1102–1107.
- Xie B, Zhang C, Xiong C, et al. High intensity focused ultrasound ablation for submucosal fibroids: a comparison between type I and type II. Int J Hyperthermia. 2015;31(6):593–599.
- Ren D, Wang W, Wang Y, et al. Ultrasound-guided focused ultrasound ablation of intramural, submucosal and subserosal uterine fibroids: 12-month follow-up results. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(7):978–982.
- Litta P, Conte L, De Marchi F, et al. Pregnancy outcome after hysteroscopic myomectomy. Gynecol Endocrinol. 2014;30(2):149–152.
- Bourdel N, Bonnefoy C, Jardon K, et al. [Hysteroscopic myomectomy: recurrence and satisfaction survey at short- and long-term]. J Gynecol Obstet Biol Reprod. 2011;40(2):116–122.
- Ahdad-Yata N, Fernandez H, Nazac A, et al. [Fertility after hysteroscopic resection of submucosal myoma in infertile women]. J Gynecol Obstet Biol Reprod. 2016;45(6):563–570.
- Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
- Cui Y, Dong Y, Guo B, et al. Effect of HIFU on endometrial receptivity and sex hormone level in uterine fibroid patients and analysis of influencing factors for its treatment rate. Exp Ther Med. 2019;17(3):2291–2297.
- Liu Y, Ran W, Shen Y, et al. High-intensity focused ultrasound and laparoscopic myomectomy in the treatment of uterine fibroids: a comparative study. BJOG: Int J Obstet Gy. 2017;124 (3):36–39.
- Li W, Jiang Z, Deng X, et al. Long-term follow-up outcome and reintervention analysis of ultrasound-guided high intensity focused ultrasound treatment for uterine fibroids. Int J Hyperthermia. 2020;37(1):1046–1051.
- Fukuda T, Fujii T, Saito S, et al. Complications of hysteroscopical myomectomy: a report of two cases. Masui. 2000;49(9):1033–1035.
- Touboul C, Fernandez H, Deffieux X, et al. Uterine synechiae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertil Steril. 2009;92(5):1690–1693.
- Bhandari S, Ganguly I, Agarwal P, et al. Effect of myomectomy on endometrial cavity: a prospective study of 51 cases. J Hum Reprod Sci. 2016;9(2):107–111.
- Anneveldt KJ, van TOH, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Herrmann A, Torres-de LRL, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9(4):190–197.
- Zhang L, Wang M, Zhang Q, et al. Estrogen therapy before hysteroscopic adhesiolysis improves the fertility outcome in patients with intrauterine adhesions. Arch Gynecol Obstet. 2019;300(4):933–939.
- Chen Y, Liu L, Luo Y, et al. Prevalence and impact of chronic endometritis in patients with intrauterine adhesions: a prospective cohort study. J Minim Invasive Gynecol. 2017;24(1):74–79.
- Sentilhes L, Sergent F, Roman H, et al. Late complications of operative hysteroscopy: predicting patients at risk of uterine rupture during subsequent pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005;120(2):134–138.
- Kim YS, Kim TJ, Lim HK, et al. Preservation of the endometrial enhancement after magnetic resonance imaging-guided high-intensity focused ultrasound ablation of submucosal uterine fibroids. Eur Radiol. 2017;27(9):3956–3965.
- Cheng H, Wang C, Tian J. Correlation between uterine fibroids with various magnetic resonance imaging features and therapeutic effects of high-intensity focused ultrasound ablation. Pak J Med Sci. 2015;31(4):869–873.
- Fan HJ, Cun JP, Zhao W, et al. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35(1):534–540.
- Zhao WP, Zhang J, Han ZY, et al. A clinical investigation treating different types of fibroids identified by MRI-T2WI imaging with ultrasound guided high intensity focused ultrasound. Sci Rep. 2017; 7(1):10812.
