Abstract
Purpose
To assess the absorption rate and factors related to the development of benign thyroid nodules (BTNs) following image-guided microwave ablation (MWA).
Materials and methods
This retrospective study reviewed nodule efficacy in patients who underwent MWA of BTNs between January 2016 and January 2018. The endpoint was a third-year follow-up. Nodules were categorized into those showing complete absorption (volumes with less than 100% volume reduction ratio (VRR) and those showing partial absorption (100% VRR)). Univariable and multivariable regression analyses were carried out to identify variables that were associated with nodule absorption rates.
Results
A total of 173 BTNs (median volume= 4.23 ml; 25–75 percentiles= 2.27–9.00 ml) from 173 patients were evaluated. 49.7% (86/173) of patients had nodules that became completely absorbed. The mean VRRs of all BTNs were 18.0%, 78.7%, 89.0%, 94.5%, and 97.1% at the 1-, 6-,12-, 24- and 36- month follow-ups. At the 3-year follow-up time point, nodule characteristics related to nodule VRR included nodule volume (adjusted odds ratio [AOR], 1.1 [95% CI: 1.0, 1.2]; p = 0.03) and nodule margin (AOR, 5.3 [95% CI: 1.8, 16.0]; p < 0.01). Treatment-related characteristics included energy per ml in nodular volume (AOR, 1.0 [95% CI: 1.0, 1.0]; p < 0.01) and blockage of peripheral flow (AOR, 3.3 [95% CI: 1.3 8.3]; p = 0.01).
Conclusions
US-guided image-guided MWA results in satisfactory long-term outcomes for the patients with BTNs. Factors related to nodule absorption rate were the volume and margin of the nodule, energy per ml in nodular volume and blockage of peripheral flow.
1. Introduction
Thyroid nodules are a common disease in adults. They can be caused by benign and malignant thyroid tumors, hyperplasia, and cysts [Citation1]. The benign or malignant nature of thyroid nodules can be determined prior to clinical treatment using thyroid fine needle aspiration, tissue biopsy, and gene detection, the benign and malignant nature of thyroid nodules can be determined prior to clinical treatment [Citation2]. Current treatment options for benign nodules include surgery, ethanol ablation (EA), laser ablation (LA), microwave ablation (MWA), and radiofrequency ablation (RFA) [Citation3–25].
As a minimally invasive technique, image-guided MWA is used in thermal tissue ablations [Citation26], and has been included in several international guidelines as a first-line treatment for benign thyroid nodules [Citation27–30]. The volume reduction rate of a nodule is an important criterion used to evaluate the success of benign thyroid nodules treatment. However, no systematic study has yet been carried out to evaluate the factors related to volume reduction rates after ablation. In this study, we investigated the long-term effectiveness of MWA treatment on BTNs. In addition, we assessed the usefulness of nodule characteristics and treatment-related findings for predicting nodule volume reduction rates following MWA.
2. Materials and methods
This retrospective study was approved by the institutional review board (approval code: F-KY-0059-20151001001-01), and informed consent for each procedure was obtained from all patients before the procedure. As this was a retrospective study, the requirement for informed consent for the use of the records was waived for this study.
2.1. Patients
Between January 2016 and January 2018, 240 patients with BTNs underwent US- guided MWA in our hospital. We identified 195 consecutive patients for inclusion in this study. The inclusion criteria were: (a) confirmation of the benign nature of the nodule (Bethesda Class II) based on fine-needle aspiration cytology (FNAC) [Citation1,Citation31]; and (b) serum thyroid hormone and thyrotropin levels that were within normal ranges. The exclusion criteria were: (a) confirmation of follicular lesion/atypia of ‘undetermined significance’ and ‘follicularneoplasm’.
2.2. Pre-ablation assessment
MWA equipment: We used the KY-2000 2450 MHz microwave system (KY-2000, Kangyou Medical, Nanjing, China) for MWAs in this study. We used a special 17-gauge ablation needle with surface Teflon coating, an effective tip of 3-mm and an axial length of 10 cm. In order to prevent the rod temperature from being too high, we used an internally cooled applicator to reduce shaft temperature. Before the operation, all subjects were evaluated using 2 D-US examinations, color Doppler US, US contrast, laboratory tests, FNA cytology, and clinical examinations. Three orthogonal diameters of the tumors (the maximum upper and lower diameter, maximum left-right diameter, and maximum anteroposterior diameter) were measured using US criteria immediately before MWA. Tumor volumes were was calculated using the following equation: V = πabc/6 (V: volume, a: the maximum upper and lower diameter, b and c: the other perpendicular diameters). The laboratory studies included thyroid function tests [free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH) and antithyroglobulin antibodies (TPOAb], thyroid-stimulating hormone receptor antibody tests (TRAb), a complete blood count, and a blood coagulation test (prothrombin time, activated partial thromboplastin time). Patients on antiplatelet or anticoagulant medicines had them suspended either 72 or 48 h prior to microwave ablation, respectively.
Cosmetic scores were assessed by an experienced physician (nodule component evaluator) and ranged from 1 to 4 [Citation1,Citation32]: (1) no obvious mass was found; (2) a palpable mass was found, but there were no cosmetic problems; (3) cosmetic problems with swallowing only or as detected by an experienced physician; and (4) a readily detected cosmetic problem.
2.3. Ablation procedures
MWAs were completed by the same physician (Shurong Wang) using the same ultrasound instrument. Preoperative evaluation and continuous intraoperative monitoring of vital signs, including electrocardiograms, pulse rates, oxygen partial pressures, and blood pressure, were performed for all patients. The ultrasound images were reevaluated to determine the needle entry path, and venous access was opened for contrast-enhanced ultrasound (CEUS). During the procedure, patients were placed in the supine position with fully exposed necks. The surgeon prepared the surgical area, located the best puncture site, and subcutaneously injected local anesthesia with 1% lidocaine (10 ml). Once the nodule was adjacent to important tissues, such as the carotid artery (CCA) or the recurrent laryngeal nerve (RLN; trachea and/or esophagus), physiological saline or mixed liquid (2 ml 2% lidocaine and 18 ml 0.9% physiological saline) was injected into the surrounding thyroid capsule, which provided a safe thermal barrier for the ablation energy and helped avoid damage [Citation27]. To avoid damaging the nerves and vessels along the approach route, we generally applied a trans-isthmic approach [Citation33]. Using moving-shot techniques, the MWA was carried out in the transverse US view [Citation34]. After all sections were scanned, the echo enhancement area covered the entire nodule and treatment was discontinued. CEUS was performed on each patient immediately after the operation to examine the boundaries of the ablation site and to assess whether the treatment was complete or not. All patients then underwent core-needle biopsies to evaluate the treatment. For nodules with cystic components, MWA was performed on the remaining solid portion after the fluid was aspirated through the needle [Citation35]. Throughout the process, we intermittently asked patients their feelings and assessed their vocalization states. After ablation, all patients were observed for at least 30 min. Additionally, their necks were covered with a small ice bag for 2–4 h to reduce inflammatory exudative bleeding and pain.
2.4. Post-procedural follow-up
Ultrasound examinations, clinical symptom evaluations, and laboratory examinations were carried out on the second day after ablation. Thyroid ultrasonography was performed one month, 6 months, and 12 months after the ablation, and then subsequently every 12 months. VRR was calculated utilizing the following equation and following volume measurements on the conventional US instrument: VRR = [(initial volume – final volume) ×100%]/initial volume. 100% VRR meant that the necrotic tissue was completely removed after ablation therapy, but did not include the removal of calcification that existed before the ablation. Partial absorption was defined as a VRR less than 100%. Complete absorption rate =100%VRR.
2.5. Evaluation of possible associated factors for nodule absorption
Patient data collected included: patient characteristics (age, sex), nodule characteristics (nodule size, composition, margin, calcification, and vascularity) and treatment-related characteristics (delivery energy per ml in nodular volume, blockage of peripheral flow, and number of treated nodules). Two investigators with 8 and 20 years of thyroid imaging experience, respectively, completed image analyses. Nodule composition was classified as either predominantly solid (having a solid portion that was ≥50%) or predominantly cystic (having a solid portion that was <50%). Tumors that could not be discriminated from normal parenchyma were denoted as having ill-defined margins [Citation36]. A four-point scale was used to grade vascularity (grade 0, no vascularity; grade 1, signals in <25% of the nodule; grade 2, signals in 25%–50% of the nodule; and grade 3, intranodular vascularity >50%) [Citation37]. A VRR greater than 50% after 6 months of follow-up time was regarded as a therapeutic success.
2.6. Analysis and statistics
Continuous variables are expressed as non-parametric medians with interquartile ranges. Statistical differences in continuous variables were evaluated using the Wilcoxon test for coupled samples. Categorical variables are provided as absolute numbers and percentages. Using univariate and multivariate logistic regression, the following data were analyzed to identify potential factors that may have predicted VRR: patient gender and age; nodule volume, internal nodule component, vascularity, margin, calcification, whether patients had Hashimoto′s thyroiditis; energy per ml in nodular volume of ablation, whether there was blockage of peripheral flow, and the number of treated nodules. Differences were considered statistically significant when the P-value <0.05. Data analysis was performed with SPSS statistical software, version 20.0.
3. Results
A total of 195 patients were initially identified. Twenty-two were excluded due to incomplete preoperative ultrasound data or because they were lost during the follow-up process. Consequently, we ultimately enrolled 173 patients (45 male, 128 female) who participated in a follow-up period of more than 3 years ().
Figure 1. Flowchart illustrates number of patients treated with thyroid nodule percutaneous microwave ablation (MWA), excluded patients, patients included in study, and follow-up.
3.1. Changes in volume and VRR and cosmetic scores
Among the 173 treated nodules, 169 were treated with one MWA session (169/173, 97.7%). The other 4 nodules required a second session which was performed 12 months after the initial procedure. No cases experienced nodule recurrence or regrowth. Of the 173 patients, 86 cases achieved 100% VRR by 36 months after MWA (). Median nodule volumes decreased from 4.23 ml to 0.003 ml (p < 0.01) at the time of final examination (). The mean VRRs of all BTNs were 18.0%, 78.7%, 89.0%, 94.5%, and 97.1% at the 1-, 6-,12-, 24- and 36- month follow-up points, respectively. Therapeutic success was achieved for all BTNs in this series since all had VRRs >50% at the 6-month follow-up. Moreover, the median cosmetic scores were remarkably reduced at the last follow-up compared with the scores at the time of initial evaluation (1.00 vs. 2.00; p < 0.01, ).
Figure 2. MWA treatment of a 59-year old man with a left thyroid predominantly cyst nodule. (A) Two-dimensional ultrasonic image of the thyroid nodule. (B) The contrast enhanced ultrasound image of the thyroid nodule after treatment. (C) Two-dimensional ultrasonic image of the nodule one month after the ablation. (D) Two-dimensional ultrasonic image of the nodule twelve months after the ablation. (E) Two-dimensional ultrasonic image of the nodule twenty-four months after the ablation.
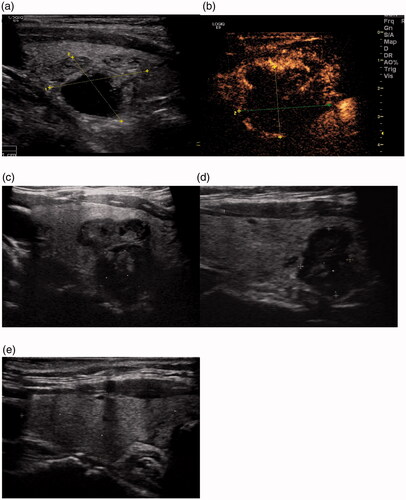
Table 1. The change in nodule volume and cosmetic score before microwave ablation and at each follow-up.
3.2. Factors related to VRR
Two factors were correlated with nodule volume reduction 3 years after ablation (): nodule volume (odds ratio [OR], 1.1 [95% CI: 1.0, 1.2]; p < 0.01) and nodule margin (OR, 0.2 [95% CI: 0.1, 0.4]; p < 0.01). Both of the factors remained significant after applying a multivariable model () (nodule volume: adjusted OR [AOR], 1.1 [95% CI: 1.0, 1.2]; p = 0.03; nodule margin: AOR, 5.3 [95% CI: 1.8, 16.0]; p < 0.01).
Table 2. Univariable related factor analysis for nodule volume reduction rate.
Table 3. Multivariable related factors analysis for nodule volume reduction rate.
Among treatment-related characteristics, energy per ml in nodular volume (OR, 1.0 [95% CI: 1.0, 1.0]; p < 0.01) and blockage of peripheral flow (OR, 3.7 [95% CI: 1.7, 8.2]; p < 0.01) were the two factors showing statistically significant effects on volume reduction in the univariable model (). After applying the multivariable model (), both of these factors remained significant (energy per ml in nodular volume:AOR, 1.0 [95% CI: 1.0, 1.0]; p < 0.01;blocking peripheral flow:AOR, 3.3 [95% CI: 1.3, 8.3]; p = 0.01).
3.3. Complications
No major complications occurred. Additionally, all patients tolerated the treatment well throughout the follow-up period. Five patients (2.9%) had intraoperative or postoperative pain in the neck and/or pain that radiated to the surrounding areas. Reducing the output power of the ablation device or stopping the ablation during the operation was found to alleviate pain. Two patients (1.2%) developed hoarseness, but both recovered after 1–3 months of neurotrophic drug therapy. The median ablation time and median total energy deposition were 3.58 min (25%–75% percentile, 2.00–8.08 min) and 6600 J (25%–75% percentile, 3870–14945 J).
4. Discussion
Therapy for treating benign thyroid nodules mainly focuses on progressive nodular growth, cosmetic problems and symptoms. Compared with other thermal ablations, MWA is more suitable for the treatment of large thyroid nodules, as it has the advantages of fast heating, a larger zone of ablation, and strong coagulation abilities [Citation1,Citation26,Citation38,Citation39]. However, previous examination of VRRs after ablation has been limited to the first few months of follow-up times. To date, there has been no systematic study to evaluate the factors that may affect VRR in the long term [Citation21–23]. This study investigated the long-term effectiveness of MWA treatment for BTNs. The mean VRRs of all BTNs were 18.0%, 78.7%, 89.0%, 94.5%, and 97.1% at the 1-, 6-, 12-, 24- and 36- month follow-up time points. Therapeutic success was achieved for all BTNs in this series since all patients had VRRs >50% at the 6-month follow-up time point. Compared with the initial evaluations, the cosmetic scores were significantly decreased at the time of last follow-up. Our results, similar to prior literature, showed that the volume reduction rapidly increased within the first 6 months and cosmetic problems improved [Citation27,Citation29]. We also found the volume reduction then steadily increased later on. Of the 173 patients, 86 cases achieved a 100% volume absorption rate 3 years after MWA treatment.
Nodule characteristics related to VRR included nodule volume (AOR, 1.1; p = 0.03) and nodule margin (AOR, 5.3; p < 0.01). Treatment-related characteristics associated with VRR included energy per ml in nodular volume (AOR, 1.0; p < 0.01) and blockage of peripheral flow of nodules (AOR, 3.3; p = 0.01).
There is some evidence that supports the theory that initial nodules volume may be related to the VRR of nodules after ablation [Citation40]. Previous studies also showed that small nodules had relatively larger VRR than big nodules, which suggests that initial volume is a predictor of treatment response [Citation16]. If the thyroid nodules are larger, more denatured necrotic tissues might be present after ablation, and the VRR process might be longer [Citation41]. The moving-shot technique was used to improve the efficacy of thyroid ablation and to minimize marginal recurrence [Citation42]. However, boundaries were often not clear for low-position thyroid nodules, which meant they were not completely ablated. After the treated part is absorbed, the untreated part moves up, and necks can be clearly displayed. Among the 173 treated nodules, 4 nodules were treated in two sessions, the second of which was performed 12 months after the initial procedure (). Our study identified that nodule margins were related to nodule VRR. In the present study, individual nodule components were not independent predictive factors of VRR, which was different from findings in short-term studies [Citation18]. This discrepancy may be related to the number of predominantly cystic nodules and/or the length of the follow-up period.
Our study identified energy delivered per ml as a factor related to nodule VRR. The underlying mechanism for this relationship might arise from the process of MWA. If the unit volume energy is too much, the central temperature of the tissue will be too high, often exceeding 150 °C [Citation43,Citation44], which will carbonize the ablated tissue. It is difficult for the body to absorb this carbonization. The fixed electrode technique or high output power was applied to block blood flow in the most richly vascular portions of the nodule. After ablation, we found that the absorption of some nodules with blocked peripheral blood flow was slow. We speculated that blockage of peripheral blood flow was a factor affecting nodule absorption. After ablation, the ablation area enters a period of damage repair, and the necrotic tissue in the ablation area is constantly engulfed and removed by macrophages and other inflammatory response cells [Citation45]. Blocking blood flow around the ablation nodules may break the absorptive balance of necrotic tissue.
There were several limitations to our study. First, because we retrospectively reviewed the data, our results should are only correlational, not causational. Another drawback may be that about 11.3% of patients originally enrolled were not ultimately included because of incomplete follow-up data. However, it is worth noting that patients with incomplete data were excluded in order to improve the relatability of the results. Another strength of our study was that we were able to identify some sonographic features and treatment-related findings that could predict the final outcome.
5. Conclusion
Our study demonstrated that MWA is effective for treating BTNs, as it induced a significant volume reduction in nodules without severe complications. In addition, nodule volume and margin, energy per ml in nodular volume and blockage of peripheral flow were factors that we found to be related to nodule absorption rate.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
Reference
- Gharib H, Papini E, Paschke R, et al. American association of clinical endocrinologists, associazione medici endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2010;33(5):250–287.
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313(9):926–935.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Sung JY, Kim YS, Choi H, et al. Optimum firstline treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? Am J Roentgenol. 2011;196:210–214.
- Yue WW, Wang SR, Lu F, et al. Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: a propensity score matching study. Endocrine. 2017;55(2):485–495.
- Lee JH, Kim YS, Lee D, et al. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010;34(7):1488–1493.
- Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33(9):1971–1977.
- Baek JH, Jeong HJ, Kim YS, et al. Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid. 2008;18(6):675–676.
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34(5):784–791.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81(5):905–910.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19(3):219–225.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194(4):1137–1142.
- Døssing H, Bennedbaek FN, Hegedüs L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules-a prospective randomized trial. J Clin Endocrinol Metab. 2013;98(7):E1213–7.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33(8):911–919.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Døssing H, Bennedbaek FN, Hegedus L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165(1):123–128.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian Minimally Invasive Treatments of the Thyroid Group. Thyroid. 2020;30(12):1759–1770.
- Trimboli P, Bini F, Marinozzi F, et al. High-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules without anesthesia or sedation. Endocrine. 2018;61(2):210–215.
- Lang BHH, Woo YC, Chiu KW. Two-year efficacy of single-session high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol. 2019;29(1):93–101.
- Feng B, Liang P, Cheng ZG, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166(6):1031–1037.
- Yue WW, Wang SR, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–e16.
- Korkusuz H, Nimsdorf F, Happel C, et al. Percutaneous microwave ablation of thyroid nodules. Functional imaging in comparison to nodular volume reduction at 3 months of followup. Nuklearmedizin. 2015;54(01):13–19.
- Heck K, Happel C, Grünwald F, et al. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia. 2015;31(5):560–567.
- Liu YJ, Qian LX, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med. 2017;242(15):1515–1523.
- Lee HY, Baek JH, Ha EJ, et al. Malignant-looking thyroid nodules with size reduction: core needle biopsy results. Ultrasonography. 2016;35(4):327–334.
- Moon WJ, Baek JH, Jung SL, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12(1):1–14.
- Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012;2012(919650):919650.
- Ji Hong M, Baek JH, Choi YJ, et al. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. J Vasc Interv Radiol. 2015;26(1):55–61.
- Baek JH, Ha EJ, Choi YJ, et al. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. 2015;16(6):1332–1340.
- Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation: multicenter retrospective study. Radiology. 2008;247(3):762–770.
- Zhao CK, Xu HX, Lu F, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. CH. 2017;65(4):393–405.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Na DG, Baek JH, Jung SL, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017;18(1):217–237.
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session–prospective randomized study. Radiology. 2012;263(3):909–991.
- Park HS, Baek JH, Choi YJ, et al. Innovative techniques for image-guided ablation of benign thyroid nodules: combined ethanol and radiofrequency ablation. Korean J Radiol. 2017;18(3):461–469.
- Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014;15(6):836–843.
- Ahmed M, Brace CL, Lee FT, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369.
- Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16(4):192–200.
- Vanagas T, Gulbinas A, Pundzius J, et al. Radiofrequency ablation of liver tumors (I):biological background. Medicina. 2010;46(1):13–17.
