 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The optimal treatment method for papillary thyroid microcarcinoma (PTMC) is lacking consensus. Here we aimed to compare the efficacy and safety of surgery and microwave ablation (MWA) for PTMC.
Methods
The clinical data of 644 patients with PTMC treated between July 2013 and June 2020 were retrospectively analyzed. A total of 320 and 324 patients underwent MWA and surgery, respectively. We observed lesion changes in the MWA group and compared the recurrence, metastasis, complications, and other health economic indicators between the 2 groups.
Results
The mean follow-up time was 890.7 ± 532.9 (187.9–2679.0) days in the MWA group and 910.9 ± 568.4 (193.8–2821.5) days in the surgery group. In the MWA group, lesion volume increased significantly after ablation and then gradually decreased. The final lesion volume reduction rate was 90.73% ± 7.94%, and 193 lesions (60.3%) disappeared completely. There were no significant intergroup differences in recurrence or metastasis. The incidence of main complications (temporary hypothyroidism, hypoparathyroidism, and temporary hoarseness) was significantly lower in the MWA group than in the surgery group (p < 0.001). The treatment time, intraoperative blood loss, and hospital stay were significantly lower in the MWA group than in the surgery group (p < 0.001).
Conclusions
MWA is effective for treating PTMC, with a low incidence of complications and less trauma. The rates of post-treatment recurrence and metastasis are similar to those of surgery, indicating that MWA is a suitable alternative to surgery.
Introduction
Thyroid cancer is the most common endocrine malignancy worldwide, and papillary thyroid cancer (PTC) is its most common subtype [Citation1]. Papillary thyroid microcarcinoma (PTMC) is defined as PTC involving tumors ≤ 1 cm in diameter [Citation2]. In recent years, with advances in high-frequency ultrasound and fine needle aspiration biopsy (FNAB), the morbidity rate of PTMC has increased significantly [Citation3,Citation4].
PTMC is reportedly a disease with slow progress and low mortality rates [Citation5]. Surgery, the first choice of treatment, is also considered overtreatment because of its disadvantages including high trauma, high risk of complications, and need for long-term medication. The American Thyroid Association (ATA) recommends that active surveillance be adopted for PTMC [Citation6]. However, due to the malignant nature of PTMC, patients often experience significant psychological pressure and find it difficult to accept active surveillance alone. Thus, minimally invasive techniques that can effectively remove these nodules without serious complications would be a welcome treatment option for these patients.
Ultrasound-guided thermal ablation mainly includes laser ablation (LA), radiofrequency ablation (RFA), and microwave ablation (MWA), all of which have been used as alternative treatments for malignant liver, lung, kidney, and adrenal tumors [Citation7–9]. Ultrasound-guided thermal ablation has been used in the treatment of benign thyroid nodules, postoperative PTC recurrence, and lymph node metastasis and has achieved a certain curative effect [Citation10,Citation11]. In recent years, ultrasound-guided MWA has also been used for the treatment of low-risk PTMC [Citation12–17]. However, its clinical value is lacking consensus, and it is unknown whether MWA can replace traditional surgery for PTMC.
This study aimed to compare the local recurrence, lymph node metastasis, and postoperative complication rates of ultrasound-guided MWA and surgical resection for low-risk PTMC cases to further evaluate the efficacy and safety of MWA.
Materials and methods
Patients
This retrospective study was approved by the Institutional Review Board of the Beijing Friendship Hospital of Capital Medical University (2017-P2-192-02), which waived the requirement for informed consent. During the study, the privacy of patients’ personal information was fully guaranteed and the patients’ identities were protected. MWA or surgical resection was selected according to each patient’s situation and preference. All patients provided informed consent for treatment before surgery or MWA.
Between July 2013 and June 2020, 397 patients with PTMC were treated with MWA; of them, 77 were excluded (41 who were lost to follow-up, 36 for whom follow-up information was incomplete). We also included 324 patients with PTMC who underwent surgical treatment during the same period. The inclusion criteria of the 2 groups were as follows: (1) FNAB-confirmed PTMC; (2) presence of a single lesion; (3) maximum lesion diameter, ≤1.0 cm; (4) no invasion of the thyroid capsule; (5) no lymph node or distant metastasis on imaging; and (6) no history of neck radiation. The exclusion criteria were as follows: (1) other type of thyroid cancer; (2) imaging-confirmed cervical lymph node or distant metastasis; (3) severe heart and respiratory disease or liver or kidney failure; and (4) coagulation dysfunction or a bleeding tendency.
Equipment
We used a Hi Vision Ascendus (Hitachi, Tokyo, Japan) ultrasonic diagnostic instrument with a linear array probe (frequency, 5–12 MHz) and a Nanjing Kangyou KY-2000 microwave therapeutic instrument with a frequency of 2450 MHz and a transmitting power of 10–100 W that could be adjusted continuously. The outer diameter of the antenna was 2 mm, total length was 100 mm, microwave transmitting section length was 5 mm, and microwave antenna rod temperature was maintained at 28–32 °C with circulating water cooling.
Surgery group
After general anesthesia was induced, the surgeries were performed by general surgeons with more than 10 years of clinical experience. A 5–6-mm-long incision was made 2 cm above the anterior sternal notch. Radical resection of the PTMC (lobectomy/isthmectomy + lymph node dissection in area VI of the affected side) was performed. Intraoperative nerve monitoring and postoperative negative pressure drainage were performed. The parathyroid tissue was carefully identified within the excised thyroid specimens and lymph node specimens in area VI, subsequently cut out, and implanted into the sternocleidomastoid muscle.
MWA group
The patients were placed in the supine position with the neck fully exposed. The treatment was performed by 2 interventional doctors with more than 5 years of ablation experience. After skin disinfection, 1% lidocaine was used to induce local anesthesia. A mixture of normal saline and lidocaine was injected outside the thyroid capsule according to the nodule’s location to form a ‘liquid isolation zone’ to protect the carotid artery, trachea, esophagus, and other important structures from heat damage. After ultrasound-guided percutaneous puncture, a microwave antenna was inserted into the tumor and the ablation was initiated. The conventional power was 30 W and the duration was 20–120 s. The tumor was ablated from the lower pole to the upper pole. To avoid edge recurrence, the ablated area was expanded, the thyroid tissue 5 mm around the nodules was ablated until the nodules and their surrounding areas were completely covered by a hyperechoic area (). After ablation, the ablation needle was slowly drawn out and the needle channel was burned to prevent tumor cells from spreading along it. During the ablation, the patient spoke intermittently to allow the monitoring for any voice changes. After treatment, the patient’s general state was closely monitored for approximately 60 min, and the pinhole was pressed upon for approximately 10 min to avoid local bleeding. At 60 min after treatment, grey-scale ultrasound was used to evaluate the presence of local bleeding. If bleeding was found, a 50 ml syringe was used to provide suction and the volume of intraoperative blood loss was recorded. Contrast-enhanced ultrasonography was used to evaluate whether the ablation was complete ().
Figure 1. Microwave ablation process. (a) Nodule location was determined before ablation. A hypoechoic nodule, approximately 3 × 3 × 4 mm in size, was found in the left lobe of the thyroid with an unclear boundary and microcalcification inside. (b) The ‘liquid isolation zone’ was injected outside the capsule of the thyroid gland. (c) The ablation started from the lower pole of the nodule. (d) The ablation area was expanded until the nodules and their surrounding areas were completely covered by a hyperechoic area.
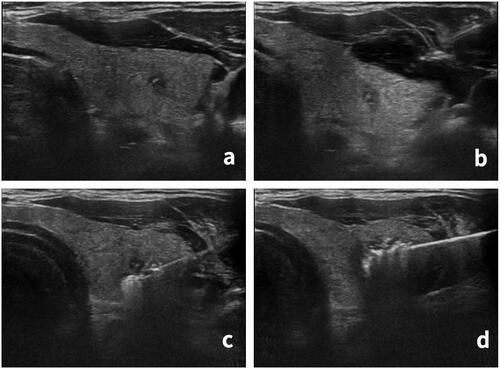
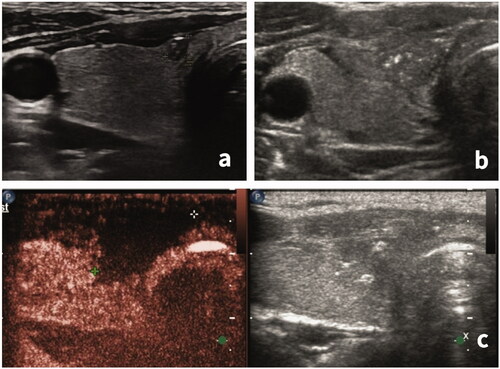
Figure 2. Evaluation of ablation completeness by contrast-enhanced ultrasonography. (a) Before the ablation, a hypoechoic nodule, approximately 3 × 3 × 2 mm in size, was visible in the isthmus of the right thyroid. (b) After the extended ablation, the original nodule was replaced by a patchy and irregularly shaped hypoechoic area. (c) Contrast-enhanced ultrasonography showed no enhancement (complete necrosis) in the ablation area with a range of approximately 18 × 16 × 10 mm.
Post-procedural observation and follow-up
The treatment time, intraoperative blood loss, incision size, length of hospital stay, and treatment costs were recorded. The treatment time in the MWA group began with skin disinfection and ended when the ablation needle was pulled out, while that in the surgery group began with skin disinfection and ended after the skin incision suture. Treatment cost refers to the expenses of treatment itself (MWA or surgery) and relevant examinations before and after treatment, including hospitalization expenses and any adverse events. Pricing was derived from the Beijing Friendship Hospital database.
After 1, 3, 6, and 12 months and 2, 3, 4, 5, 6, and 7 years, local recurrence and cervical lymph node metastasis were observed by ultrasonography. Local recurrence includes the recurrence of PTMC in the original surgical site or original ablation focus as well as the emergence of new PTMC in other parts of the thyroid. Once suspected thyroid nodules or cervical lymph nodes are found, the diagnosis should be confirmed by FNAB. In the MWA group, lesion size and blood flow distribution were recorded 1 h after ablation and at each follow-up. Lesion measurements included 3 diameters (a, b, c), which were used to calculate their volume as follows: v = π abc/6. The lesion volume reduction rate was calculated as follows:
After treatment and follow-up, the patients were carefully observed and questioned regarding the occurrence and recovery of complications, including dysphagia, hoarseness, cough caused by drinking water, hematoma, hyperthyroidism or hypothyroidism, and hypoparathyroidism.
A post-treatment quality of life assessment was performed according to the following criteria: 1) physiological function, measured by life status score after treatment (1–5 points), 2) degree of activity limitation (1–3 points), 3) body pain score (1–6 points), and 4) degree of impact of pain on work and life (1–6 points). A higher overall score indicated a higher quality of life.
Statistical methods
SPSS (version 26; IBM, Armonk, NY, USA) was used to analyze the data. Quantitative data are expressed as mean ± standard deviation and range, and the Mann–Whitney U-test was used to compare groups. Qualitative data are expressed as the number of cases or percentages. The chi-square test or Fisher’s exact test was used to compare the 2 groups. Repeated measures analysis of variance was used to compare the changes in the mean lesion diameter and volume before versus after MWA and at each follow-up as well as any changes in thyroid function before versus after treatment and at each follow-up. Kaplan–Meier survival curves were drawn to compare disease-free survival (DFS) between the groups. Cox regression analysis was used to analyze the influence of various factors on patient prognosis. The threshold for statistically significant differences was set at p < 0.05.
Results
General patient information
The general conditions of the 2 groups are presented in . There were no significant intergroup differences in age, sex, mean diameter, location, boundary, echo, nodule calcification, or follow-up time.
Table 1. Patients’ general situation of two groups.
At the time this article was written, all patients were followed up for at least 6 months. A total of 289 patients (90.3%) were followed up for > 1 year in the MWA group, while 288 patients (88.9%) were followed up for > 1 year in the surgery group. The specific distribution of the follow-up time by group is shown in .
Table 2. Distribution of follow-up time of two groups.
Indicators of clinical and health economics
The treatment time, intraoperative blood loss, incision size, hospital stay, and treatment cost were significantly higher in the surgery group than in the MWA group (p < 0.001). The overall quality of life score of the surgery group was lower than that of the MWA group. There were no significant differences in local recurrence, cervical lymph node metastasis, distant metastasis, or disease-specific mortality rate between the 2 groups ().
Table 3. Clinical and health economic indicators of the two groups (mean ± SD).
Among the 320 patients in the MWA group, 4 (1.3%) had PTMC recurrence (at 2.5–3.7 years) at recurrence sites outside the original lesion, including 1 case of recurrence in the ipsilateral lobe and 3 cases of recurrence in the contralateral lobe. Among the 324 patients in the surgery group, 5 (1.5%) had PTMC recurrence (at 1.8–4.5 years), including one case of recurrence in the surgical area of the original lesion and 4 cases of recurrence in the contralateral lobe.
In the MWA group, there were 6 cases (1.9%) of cervical lymph node metastasis (at 0.5–6.3 years), including 4 of ipsilateral lymph node metastasis and 2 of contralateral lymph node metastasis. In the surgery group, there were 8 cases (2.5%) of cervical lymph node metastasis (at 0.6–5.5 years), including 6 of ipsilateral lymph node metastasis and 2 of contralateral lymph node metastasis.
In the MWA group, 2 patients with cervical lymph node metastasis underwent surgical treatment, while the remaining patients with PTMC recurrence or cervical lymph node metastasis received further MWA treatment. In the surgery group, 2 patients with PTMC recurrence and 2 with cervical lymph node metastasis received further surgical treatment, while the remaining patients with PTMC recurrence and cervical lymph node metastasis received MWA treatment.
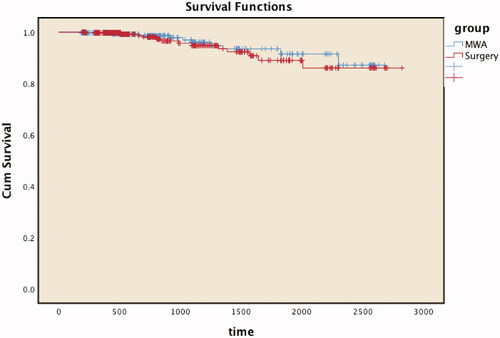
DFS following PTMC was defined as the duration from treatment until a lack of disease and/or recurrence was confirmed postoperatively. The mean DFS was 884 days (range, 188–2679 days [2.4 years]) in the MWA group and 905 days (range, 194–2822 days [2.5 years]) in the surgery group. There was no statistically significant difference in DFS between the MWA group (96.9%) and the surgery group (96.0%) (p = 0.530). The Kaplan–Meier survival curve is shown in .
Figure 3. Disease-free survival curves show no statistically significant differences between the microwave ablation (MWA) and surgery groups during follow-up. Cum: Cumulative.
We conducted Cox regression analysis to explore the effects of various factors (X1, age; X2, sex; X3, mean nodule diameter; X4, nodule location; X5, nodule boundary; X6, nodule echo; X7, calcification; X8, therapeutic method) on patient prognosis. The P-value of the score and change from the previous block were both 0.000. The overall test of the model was statistically significant. The filtered model contained 2 covariates, X3 and X4. The regression coefficient corresponding to covariate X3 (mean nodule diameter) was 0.290 (B), the standard error was 0.112 (SE), the Wald statistic was 6.725, and the P-value was 0.010, indicating that the covariate impacted survival time. The relative risk was 1.336 (Exp [B]), and the corresponding 95% confidence interval (CI) was 1.073–1.663 (95% CI for Exp [B]), the regression coefficient corresponding to covariate X4 (nodule location) was −1.190 (B), the standard error was 0.220 (SE), the Wald statistic was 29.399, and the P-value was 0.000, indicating that the covariate impacted survival time. The relative risk was 0.304 (Exp [B]), and the corresponding 95% CI was 0.198–0.468 (95% CI for Exp [B]).
Complications
MWA was treated under local anesthesia, and 38 patients reported neck pain or a burning sensation during treatment; however, there was no need to suspend the treatment because of discomfort in any cases. The symptoms disappeared at treatment completion, and none of the patients used painkillers during or after the MWA. The incidence of major complications in the MWA group was significantly lower than that in the surgery group (p < 0.001) (). None of the patients in the MWA group had temporary or permanent hypoparathyroidism versus 12 patients in the surgery group with temporary hypoparathyroidism within 1 d to 1 week postoperative, which presented as transient numbness of the hands and feet. However, after removal of the thyroid lobes and lymph nodes in area VI, the parathyroid tissue was located in the specimens and transplanted into the sternocleidomastoid muscle, and no patient had permanent hypoparathyroidism. Although some patients in the 2 groups had diffuse thyroid lesions, the thyroid hormone levels of all patients were generally normal before treatment. The thyroid hormone levels of the MWA group did not change significantly after treatment and remained normal during follow-up. All patients were treated with levothyroxine (Euthyrox or Letrox) for thyroid hormone replacement therapy in the surgery group, and 13 patients had temporary hypothyroidism within 1 d to 2 weeks postoperative. In the MWA group, 12 patients had temporary hoarseness, all of whom recovered after 1–3 months of treatment, while one had a permanent recurrent laryngeal nerve injury, showing mild hoarseness without dysphagia and coughing after drinking water. In the surgery group, 10 patients had temporary hoarseness, all of whom recovered after 3–6 months of treatment, 2 had unilateral recurrent permanent laryngeal nerve paralysis, one had mild hoarseness, and another had moderate hoarseness with mild dysphagia. One patient in the surgery group had hematoma during postoperative observation and was given hemostatic treatment; no cases of obvious bleeding occurred in the MWA group.
Table 4. Comparison of complications between the two groups.
Changes of lesions in the MWA group
After MWA treatment, the average lesion diameter, volume, and volume reduction ratio at each follow-up time changed continuously ( and ). The mean lesion diameter (13.33 ± 2.88 mm) and volume (1336.95 ± 940.31) at 1 h after ablation were significantly larger than those before ablation (p < 0.001), but each decreased continuously at each follow-up time. Among them, 193 lesions (60.3%) were completely absorbed during follow-up, of which 9 were completely absorbed within 3 months and the rest were completely absorbed within 3–6 months (25 cases), 6 months to 1 year (75 cases), 1–2 years (49 cases), 2–3 years (22 cases), 3–4 years (11 cases), 4–5 years (7 cases), or 5–6 years (4 cases).
Table 5. Changes of mean diameter (mm) of lesions before and after MWA treatment.
Table 6. Changes of lesion volume (mm3) and volume reduction rate (%) before and after MWA treatment.
Discussion
The morbidity of PTMC has increased significantly, but the optimal treatment modality is lacking global consensus. It is worth noting that PTC is an inert disease with good prognosis [Citation18,Citation19]. Therefore, when making treatment decisions, doctors and patients must understand and weigh the risks and benefits of various treatment options. The ATA guidelines emphasize the need to ‘minimise treatment-related morbidity and unnecessary therapy’ [Citation6,Citation20]. Surgery, which has been used routinely to treat PTMC, will result in patients taking thyroid hormone for life and increases the risk of permanent complications, such as hypoparathyroidism and voice changes, which will affect their quality of life [Citation21–25]. Active surveillance has recently emerged as a treatment strategy to avoid overtreatment. Some observational studies have shown that the risk of disease progression caused by active surveillance of PTMC is minimal and will not lead to an increase in disease-specific mortality in appropriately selected patients [Citation18,Citation26,Citation27]. However, D’Agostino [Citation28] and ODA [Citation26] reported that the word ‘cancer’ can cause fear and anxiety in patients and cause great psychological pressure, thus making them seek urgent surgery. At the same time, there is no effective method to identify the progression risk of PTMC; therefore, not all patients find active surveillance easy to accept.
Ultrasound-guided thermal ablation can effectively inactivate the lesion without causing significant trauma to patients, compensating for the shortcomings of the above 2 methods to a certain extent. Increasing evidence has shown that thermal ablation can treat benign thyroid nodules [Citation29–31] and recurrent thyroid cancer [Citation11,Citation32,Citation33] and is a safe and effective method of treating PTMC [Citation14,Citation34–45]. Choi et al. [Citation46] systematically reviewed and analyzed 11 recent studies on thermal ablation for PTMC (including 4 on MWA, 4 on RFA, and 4 on LA) and found that the complete disappearance rate of PTMC was 57.6% (95% CI, 35.4–79.8%); the recurrence rate was 0.4% (95% CI, 0–1.1%); the average VRR was 98.1% (95% CI, 96.7–99.5%); and the incidence of major complications was only 0.7% (95% CI, 0–1.5%), all of which cases were non-life-threatening voice changes. These results demonstrate the efficacy and safety of thermal ablation in the treatment of PTMC.
MWA uses electromagnetic energy to rotate water molecules. Heat is centrifuged around the antenna tip, causing coagulative necrosis in the tumor over a short time through extensive thermal effects. Compared to LA and RFA, MWA has the advantage of providing a larger ablation area in a shorter time and producing more complete tumor killing, which allows the treatment to be completed quickly under local anesthesia [Citation47,Citation48]. These results suggest that MWA is an effective treatment for low-risk PTMC. During treatment, incomplete ablation was avoided by expansion of the ablation confirmed by contrast-enhanced ultrasonography. During follow-up, the lesions were continuously absorbed and the tumor volume decreased. Some lesions (60.3%) were completely absorbed. In the study by Teng DK [Citation49], 40 lesions (97.56%) completely disappeared, which is a better outcome than that in our study. The reason for this may be that the follow-up time was insufficient. In addition, they used low-power (20 W) MWA [Citation42,Citation49], which may explain the high lesion absorption rate. The authors stated that low power can make the process of tumor coagulative necrosis milder, reducing the ‘excessive burn’ in the ablation area, which will eventually be more easily absorbed. The ideal power to effectively inactivate and absorb lesions can be determined through further studies. However, Yue WW [Citation50] used the same 30 W ablation power as ours and reported a 78.1% (89/114) complete disappearance rate, which was also higher than that observed in our study. The complete disappearance rate reported in other studies was also different, ranging from 15.2% to 97.4% [Citation34,Citation38,Citation40,Citation42]. Therefore, in addition to ablation power, we can analyze various factors that affect lesion absorption and absorption speed, such as diffuse thyroid lesion type, thyroid blood supply, original lesion location, size, calcification, ablation time, expansion of ablation range, and post-ablation lesion elasticity. Some studies to date have examined the characteristics of lesions that cannot be completely absorbed. Li J [Citation34] performed ultrasound-guided needle biopsies of unabsorbed lesions and found that the pathological manifestation was high carbonization. Other studies [Citation49,Citation50] found large calcifications in unabsorbed lesions, which did not change significantly during follow-up.
We published articles similar to this paper in 2018 [Citation40] and 2019 [Citation34]; the patients included in these 2 studies were also included in this study. Our aim was to extend the follow-up time and observe the long-term patient prognosis. At the same time, we also included new patients in this study to further expand the sample size. The 2019 paper reported that 5 cases of lymph node metastasis occurred at 0.5–3.6 years and 2 cases of PTMC recurrence occurred at 3.0–3.7 years in the MWA group. In the surgery group, 5 cases of lymph node metastasis occurred at 1.0–4.6 years versus 1 case of PTMC recurrence at 3.7 years. In our study, 1 new case of cervical lymph node metastasis that occurred at 6.3 years and 2 new cases of PTMC recurrence that occurred at 2.5 years and 3.0 years were added to the MWA group. In the surgery group, 3 cases of cervical lymph node metastasis (0.5 years, 2.3 years, and 5.5 years) and 4 cases of PTMC recurrence (1.8 years, 2.5 years, 3.8 years, and 4.5 years) were added. These results complement those of our previous study. For example, cervical lymph node metastasis in individual patients only occurs 5 or 6 years after treatment, highlighting the importance of long-term follow-up after treatment.
Using Cox regression, we found that the mean diameter (X3) and location (X4) of the nodule affected PTMC and cervical lymph node metastasis recurrence. The incidence of recurrence or metastasis increased 1.336 times for every 1-mm increase in the mean nodule diameter. The PTMC recurrence and metastasis was located in the left or right lobe with no isthmus involvement, which may be due to the low incidence of PTMC in the isthmus and the insufficient number of cases therein.
Teng DK [Citation49] performed MWA in 41 patients with PTMC and reported an incidence of serious complications of 2.4%, similar to our results. However, they completed a 5-year follow-up for all patients, and none showed recurrence or metastasis. In addition to the strict screening of a single thyroid nodule, no lymph node metastasis noted on ultrasonography, and the expansion of the ablation scope during MWA, the difference between this study and the current study is that the thyroid stimulating hormone (TSH) level was controlled at <0.1 mU/L after MWA for 1 month, which may explain why their therapeutic effect was better than ours. Yue WW [Citation41] also used TSH suppression therapy, with an average follow-up of 11 months, and found no cases of recurrence or metastasis. However, most studies on MWA in the treatment of PTMC do not include TSH inhibition therapy after treatment and have a recurrence rate of approximately 0–1.2% and a metastasis rate of approximately 0–3.0% [Citation34,Citation40]. It is worth noting that the different follow-up times of these studies may be the reason for the observed therapeutic effect; therefore, in the future, randomized controlled trials can be used to determine whether TSH inhibition therapy is needed after MWA and whether adverse reactions are observed after TSH inhibition therapy. We should further explore the indications for MWA in the treatment of PTMC. Studies have shown that some clinical features, including sex, age, tumor size, pathological subtype, and BRAF (V600E) gene mutation, can affect PTMC recurrence and metastasis [Citation51–57]. Therefore, before ultrasound-guided ablation is delivered, we should comprehensively analyze various clinical, pathological, and imaging features and make a reasonable risk stratification for patients with PTMC.
With the continuous improvement of thyroidectomy, bleeding, sepsis, and other complications have significantly improved [Citation58]. The main complications of thyroidectomy are currently hypoparathyroidism and vocal cord paralysis. The reported rates of hypocalcemia and vocal cord paralysis are 3.0–7.2% [Citation58,Citation59] and 0.2–5.0% [Citation60,Citation61], respectively, which were similar to those of the surgery group in our study (3.7% and 3.7%, respectively). Temporary hoarseness caused by MWA, the most common complication (3.8%), may be caused by heat injury, bleeding, or other factors. Indeed, the injection of a liquid isolation band and infiltration of a small amount of lidocaine into the recurrent laryngeal nerve can result in temporary recurrent laryngeal nerve paralysis, inflammation, and peripheral nerve fibrosis [Citation62,Citation63]. In our study, there were various degrees of complications in both groups, and the incidence of major complications in the MWA group was significantly lower than that in the surgery group (p < 0.001). Therefore, MWA is a safe alternative for patients with PTMC.
In addition, in our study, treatment time, intraoperative blood loss, and length of hospital stay were significantly higher in the surgery group than in the MWA group (p < 0.001), indicating that MWA causes less trauma to patients. MWA and surgical trauma as external environmental stimuli can cause noninfectious stress responses. Xu et al. [Citation64] found that the levels of C-reactive protein, interleukin-6, and tumor necrosis factor-α at 24 h after treatment were significantly higher in the surgery group than in the MWA group (p < 0.05). As these 3 indicators reflect the body’s stress level, the stress response of the patients in the surgery group was greater. MWA can significantly reduce physical trauma in patients, promote patient recovery, shorten hospitalization time, and improve quality of life.
Our study has several limitations. First, although the specificity of ultrasonography is high, its sensitivity is low. The main reason for this is that the location of the lymph nodes in the 6 regions is deep, making them difficult to observe clearly; as a result, there was a missed diagnosis of cervical lymph node metastasis at the beginning of the study or during follow-up. Thus, although high-frequency ultrasound technology has progressed greatly, small and micro-cancers that are difficult to recognize will remain ignored. Thyroid cancer often involves one- or two-sided multifocal growth [Citation65], and a new tumor found during follow-up may exist but not be effectively identified when entering the group. Furthermore, the follow-up time in this study was uneven; some patients were followed up for > 7 years, while others were only followed up for a few months. Due to the slow progression of PTMC, the lack of follow-up time will lead to an underestimation of the local recurrence, cervical lymph node metastasis, and the lesion absorption rates in the MWA group. However, as the problem of insufficient follow-up time existed in both groups, the data were comparable between them. Moreover, the follow-up of distant metastasis in this study only included the patient’s complaint and physical examination; it could not completely exclude hidden metastases. Finally, this retrospective study had some selection bias. In the future, prospective research can be designed and performed through a multicentre, large-sample, long-term follow-up randomized controlled trial.
In conclusion, MWA has a reliable effect in the treatment of PTMC, and the nodules can completely disappear in some cases. Compared to surgery, MWA has the advantages of reduced trauma, a low complication rate, low cost, and no need for long-term medication. Therefore, MWA may be an alternative treatment for low-risk T1aN0M0 PTMC.
Acknowledgements
The authors are grateful to Doctor Jianming Li (Department of Interventional Ultrasound, The General Hospital of Chinese People’s Liberation Army, Beijing, China) for helpful discussions and advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
- Lloyd RV, Osamura RY, Kloppei G, et al. Eds. WHO classification of tumors: pathology and fenetics of tumors of endocrine organs. Lyon: France IARC Press; 2017.
- Noone AM, Howlader N, Krapcho M, eds. et al. SEER cancer statistics review, 1975-2015. Bethesda (MD): National Cancer Institute; 2018. Available at http://seer.cancer.gov/csr/1975_2015/. Based on November 2017 SEER data submission, posted to the SEER website.
- Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–791.
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246(3):375–384.
- Bryan RH, Erik KA, Keith CB, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Nori J, Gill MK, Meattini I, et al. The evolving role of ultrasound guided percutaneous laser ablation in elderly unresectable breast cancer patients: a feasibility pilot study. BioMed Res Int. 2018; 2018:9141746.
- Sartori S, Mauri G, Tombesi P, et al. Ultrasound-guided percutaneous laser ablation is safe and effective in the treatment of small renal tumors in patients at increased bleeding risk. International journal of hyperthermia: the official journal of european society for hyperthermic oncology. North American Hyperthermia Group. 2018;35:19–25.
- Pacella CM, Bizzarri G, Magnolfi F, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712–720.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170.
- Nishikawa T, Fukunari N, Nakano M, et al. 15th international thyroid congress program and meeting abstracts. Thyroid. 2015;25 (Suppl 1):P1–A337.
- Jeong SY, Baek JH, Choi YJ, et al. Ethanol and thermal ablation for malignant thyroid tumours. International journal of hyperthermia: the official journal of european society for hyperthermic oncology. North American Hyperthermia Group. 2017;33:938–945.
- Zhou W, Jiang S, Zhan W, et al. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: Preliminary results. Eur Radiol. 2017;27:2934–2940.
- Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21:917–920.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017; 20:11–22.
- Pacella CM, Papini E. Image-guided percutaneous ablation therapies for local recurrences of thyroid tumors. J Endocrinol Invest. 2013;36:61–70.
- Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocrine Pract. 2007; 13(5):521–533.
- Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract. 2007;3(3):240–248.
- Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. 2014;260:601–605. discussion 605–607.
- Wang TS, Goffredo P, Sosa JA, et al. Papillary thyroid microcarcinoma: an over-treated malignancy? World J Surg. 2014;38:2297–2303.
- Hauch A, Al-Qurayshi Z, Randolph G, et al. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. 2014;21:3844–3852.
- Rosato L, Pacini F, Panier SL, et al. Post-thyroidectomy chronic asthenia: self-deception or disease? Endocrine. 2015;48:615–620.
- Hoang JK, Nguyen XV, Davies L. Overdiagnosis of thyroid cancer: answers to five key questions. Acad Radiol. 2015;22:1024–1029.
- Ukrainski MB, Pribitkin EA, Miller JL. Increasing incidence of thyroid nodules and thyroid cancer: does increased detection of a subclinical reservoir justify the associated anxiety and treatment? Clin Ther. 2016;38:976–985.
- Oda H, Miyauchi A, Ito Y, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26(1):150–155.
- Brito JP, Ito Y, Miyauchi A, et al. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26(1):144–149.
- D’Agostino TA, Shuk E, Maloney EK, et al. Treatment decision making in early-stage papillary thyroid cancer. Psychooncology. 2018;27(1):61–68.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and Meta-analysis. Endocrine. 2020;67(1):35–43.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session–prospective randomized study. Radiology. 2012;263(3):909–916.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897–4903.
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36(1):359–367.
- Li J, Liu Y, Liu J, et al. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia. 2019;36(1):640–646.
- Ji L, Wu Q, Gu J, et al. Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging. 2019;19(1):16.
- Zhang Y, Zhang MB, Luo YK, et al. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8(12):5450–5458.
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64(1):109–117.
- Jeong SY, Baek JH, Choi YJ, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34(5):611–616.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018;34(5):653–659.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30(2):150–157.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Zhang L, Zhou W, Zhan W, et al. Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg. 2018;42(8):2476–2484.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an italian experience. Front Endocrinol (Lausanne). 2021;11:575152.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and Meta-analysis. Thyroid. 2020;30(5):720–731.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22(9):1983–1990.
- Dodd GD, 3rd, Dodd NA, Lanctot AC, et al. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267(1):129–136.
- Teng DK, Li WH, Du JR, et al. Effects of microwave ablation on papillary thyroid microcarcinoma: a five-year follow-up report. Thyroid. 2020;30(12):1752–1758.
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab. 2020;105(6):dgaa128.
- Wang WH, Xu SY, Zhan WW. Clinicopathologic factors and thyroid nodule sonographic features for predicting Central lymph node metastasis in papillary thyroid microcarcinoma: a retrospective study of 1204 patients. J Ultrasound Med. 2016;35(11):2475–2481.
- Takaki H, Cornelis F, Kako Y, et al. Thermal ablation and immuno- modulation: from preclinical experiments to clinical trials. Diagn Interv Imaging. 2017;98(9):651–659.
- Chen Y, Sadow PM, Suh H, et al. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and Meta-analysis. Thyroid. 2016;26:248–255.
- Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016;26:807–815.
- Oh HS, Park S, Kim M, et al. Young age and male sex are predictors of large-volume Central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid. 2017;27:1285–1290.
- Liu J, Singh B, Tallini G, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–1264.
- Bernstein J, Virk RK, Hui P, et al. Tall cell variant of papillary thyroid microcarcinoma: clinicopathologic features with BRAF(V600E) mutational analysis. Thyroid. 2013; 23:1525–1531.
- Gupta S, Vasu Reddy C, Chettri ST, et al. Clinicopathological features and complications of thyroid operations: a single Centre experience. Indian J Otolaryngol Head Neck Surg. 2013;65:140–145.
- Son HJ, Kim JK, Jung YD, et al. Comparison of outcomes between hemithyroidectomy alone and hemithyroidectomy with elective unilateral Central neck dissection in patients with papillary thyroid microcarcinoma. Head Neck. 2018;40:2449–2454.
- Friguglietti CU, Lin CS, Kulcsar MA. Total thyroidectomy for benign thyroid disease. Laryngoscope. 2003;113:1820–1826.
- Ernandes-Neto M, Tagliarini JV, Lopez BE, et al. Factors influencing thyroidectomy complications. Braz J Otorhinolaryngol. 2012;78:63–69.
- Baek JH, Lee JH, Sung JY, et al.; Korean Society of Thyroid Radiology. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multi- center study. Radiology. 2012;262(1):335–342.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Xu B, Zhou NM, Cao WT, et al. Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. World J Clin Cases. 2018;6(15):936–943.
- Zheng W, Wang K, Wu J, et al. Multifocality is associated with Central neck lymph node metastases in papillary thyroid micro-carcinoma. CMAR. 2018;10:1527–1533.
