Abstract
Background
Chemotherapy is the main treatment strategy for gestational trophoblastic neoplasia (GTN). Surgical resection is crucial to deal with chemoresistance and recurrence following chemotherapy. The aim of this study was to explore if high-intensity focused ultrasound (HIFU) can be used as a complementary technique to surgical procedures in the management of GTN.
Case report
This case report described two females who previously developed chemoresistance or recurrence during chemotherapy and then underwent HIFU as an adjuvant surgical salvage procedure. For high-risk GTN patients with chemoresistance, HIFU treatment decreased the risk of chemoresistance and shortened the course of chemotherapy. It also reduced the dosage of chemotherapeutic agents used for the patient who suffered a recurrence.
Conclusion
For patients with GTN who desire to preserve their uterus, HIFU may be used as a complementary technique to surgical resection in the management of GTN.
Introduction
Gestational trophoblastic disease (GTD), originating from abnormal placentas, encompasses a group of pregnancy-related benign or malignant tumors. Benign GTD is known as a hydatidiform mole (HM) and occurs in what is termed a molar pregnancy; whereas malignant GTD is known as gestational trophoblastic neoplasia (GTN). It includes invasive moles, choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor [Citation1].
Monotherapy is preferred for patients ranked as low-risk GTN; however, high-risk GTN (International Federation of Gynecology and Obstetrics (FIGO) stages II–III disease with a WHO score ≥7, or FIGO stage IV disease) requires multiagent chemotherapy [Citation1,Citation2]. High-risk GTN may necessitate adjuvant surgical procedures for chemotherapy-resistant diseases, such as surgical resection for a solitary lesion in the uterus or lungs [Citation1].
Patients with GTN are often young and have strong fertility intentions [Citation2]. Therefore, conservative management, rather than hysterectomy, is often used in clinical practice. As a non-invasive treatment, high-intensity focused ultrasound (HIFU) has gained substantial attention in the treatment of solid tumors [Citation3]. Its efficacy, safety, and feasibility were confirmed by a series of clinical trials [Citation4]. Moreover, this therapeutic modality was validated by the ablation of malignant solid tumors, including malignant bone tumors and liver cancer [Citation5]. Over the last few years, HIFU has also been used in the treatment of placenta accreta, placenta increta, and cesarean scar pregnancy [Citation6,Citation7], all of which originate from the invasion of cytotrophoblasts into the uterine wall, thereby providing the biological and clinical basis for the use of this non-invasive treatment in the management of GTN. Herein, we describe two patients who underwent HIFU treatment as an adjuvant surgical salvage procedure for chemotherapy-resistant GTN or relapse from remission. The institutional review board of Zunyi Medical University approved this study (protocol number: 2021-021) and waived the requirement for informed consent owing to the retrospective design.
Case presentation
Case one
A 22-year-old G1P1 female whose last menstrual period (LMP) was on 9 May 2020, presented to a local hospital with lower abdominal pain. She experienced abnormal vaginal bleeding for half a month after 3 months of amenorrhea, before visiting the hospital.
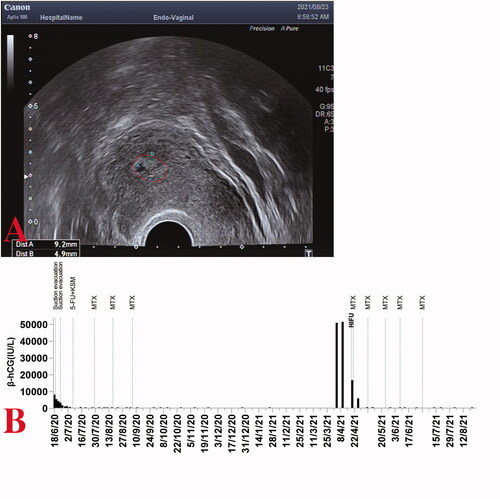
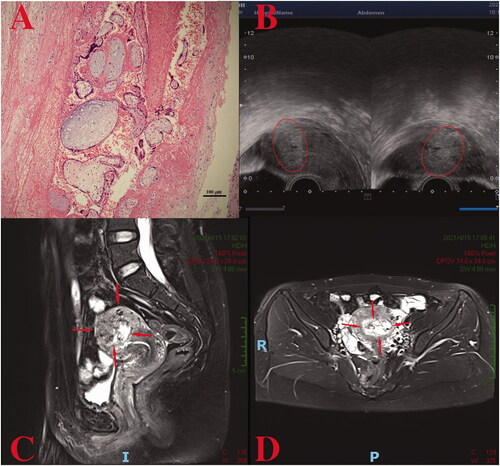
Laboratory examinations at the local hospital revealed a highly elevated serum β-subunit of human chorionic gonadotropin (β-hCG), higher than 100,000 IU/L. With the initial hydatidiform mole diagnosis by ultrasound examination, a suction evacuation procedure was performed on 17 September 2020. The postoperative pathological report of the mass indicated characteristic hydropic changes and mild trophoblastic hyperplasia (). Subsequently, computed tomography (CT) screening showed multiple lung metastases, and the diagnosis of invasive moles was suspected; thus, one course of 5-fluorouracil and dactinomycin (FA) combination chemotherapy was administered.
Figure 1. (A) A hydatidiform mole in the first case: villus edema, mild hyperplasia of trophoblastic tissue; (B) the metastatic tumors in the lung (indicate by the arrows); (C, D) a heterogeneous intramural mass on the right lateral wall of the uterus.
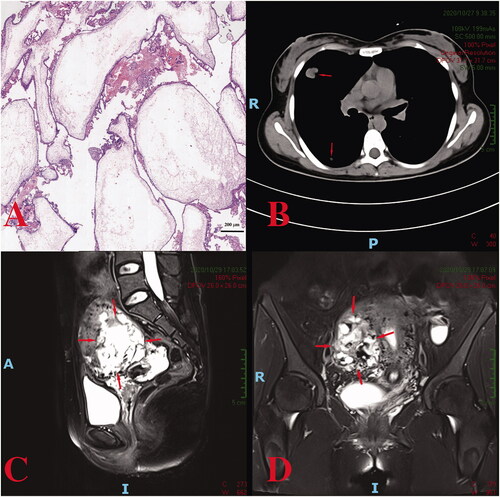
Her β-hCG level decreased to 70,196 IU/L 1 week after chemotherapy but increased to 83,207 IU/L 2 weeks later. The patient was referred to our hospital on 25 October 2020. Blood tests revealed a β-hCG value of 79,959 IU/L. An MRI suggested an invasive mole (space-occupying lesions invading the myometrium of the right lateral wall) (), and CT scans indicated multiple lung metastases (). After comprehensive consideration of the persistently elevated β-hCG value after the evacuation of a molar pregnancy, hydropic changes of chorionic villi (ruling out the diagnosis of choriocarcinoma), an extension of lesions into the myometrium via tissue and multiple lung metastases, a diagnosis of invasive moles (FIGO Stage III: Score 10) was made. One course of the EMA/CO (etoposide, methotrexate, and dactinomycin alternating with cyclophosphamide and vincristine) regimen was administered for 8 days, and a β-hCG level of 53,012.0 IU/L was achieved. Still, it did not decrease logarithmically compared to the pretreatment level.
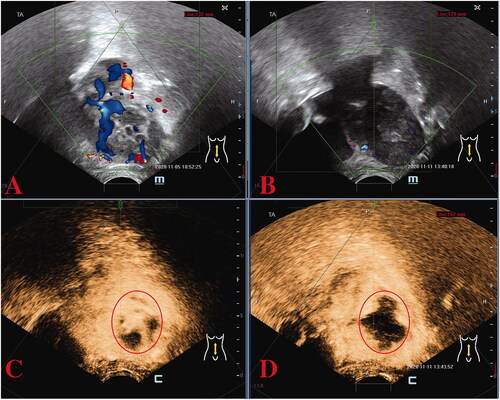
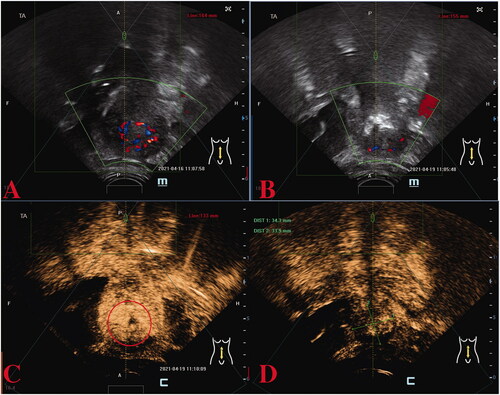
Because the patient had fertility requirements, she was unwilling to have a hysterectomy. Therefore, after a multidisciplinary team (MDT) consultation, HIFU was utilized as an adjuvant surgical salvage procedure for the heterogeneous mass on the right lateral wall of the uterus and was performed under conscious sedation. The device used was a Model JC200 focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). The patients were placed in a prone position on the HIFU table, with the anterior abdominal wall immersed in degassed water. A degassed water balloon was placed between the abdominal wall and the transducer to compress and push the bowel away from the acoustic pathway. The sagittal ultrasound scanning mode was chosen for both pretreatment planning and sonication. Point scan was used, and power was set between 300 and 400 Watts. During the procedure of HIFU, therapeutic power was adjusted based on patient feedback and changes in grayscale on ultrasonographic imaging [Citation6,Citation7]. A total of 399,600 J energy was delivered. Color flow Doppler ultrasonography and contrast-enhanced ultrasound (CEUS) were performed to evaluate the ablation effectiveness (). A level of 21,412.0 IU/L of β-hCG was achieved on postoperative day 2.
Figure 2. Preoperative and post-operative ultrasound assessment of HIFU therapy in the first case. Preoperative ultrasound localization: abnormal echogenicity on the right lateral wall of the uterus with bar-like blood flow signals detected (A), immediate post-operative image (B) showing reduced blood supply in the lesion; the CEUS image before (C) and after (D) HIFU therapy revealing that the non-perfusion region has filled the treatment area of the lesion, indicating a good ablation effect. CEUS, contrast-enhanced ultrasound; HIFU, high-intensity focused ultrasound
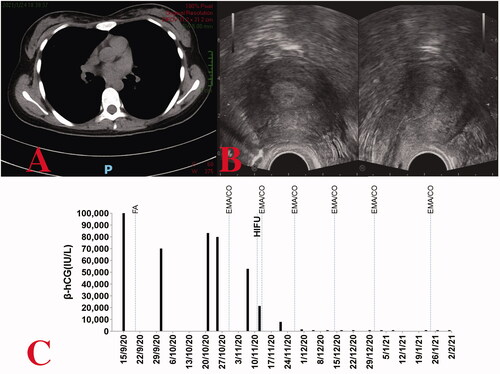
The first post-HIFU EMA/CO chemotherapy was conducted on 13 November 2020, based on considerations of vital signs and the interval between two chemotherapy cycles. Subsequently, the β-hCG value lowered to 7994.0 IU/L at the end of the cycle. After additional three cycles of EMA/CO chemotherapy (on 27 November, 14 December, and 31 December respectively), the β-hCG levels decreased to normal levels (433.4 IU/L, 31.3 IU/L and 3.0 IU/L) (). Another course of chemotherapy followed. No major adverse effects were observed after the post-HIFU chemotherapy. No abnormality was detected on chest CT screening at approximately 3-month post-HIFU therapy (), or on transvaginal ultrasound at the 3-month post-discharge follow-up ().
Figure 3. (A) Chest computed tomography revealing regression of the lung tumor following treatment for case one. (B) On transvaginal ultrasound, no obvious abnormalities are observed (coronal view on the left, sagittal view on the right). (C) Serum β-hCG levels throughout the disease duration for case one.
Case two
A 34-year-old G9P2 female whose LMP was on 14 April 2020, presented to our hospital on 18 June 2020, for abnormal vaginal bleeding after 65 days of amenorrhea. A suction evacuation was performed since her β-hCG level was 8179.0 IU/L. The initial diagnosis of the hydatidiform mole was made by ultrasound. The postoperative pathological report of the mass indicated a partial hydatidiform mole ().
Figure 4. (A) A partial hydatidiform mole in the second case: decidual-like tissues, villus edema, no obvious hyperplasia of the trophoblastic cells; (B) Ultrasound examination showed an irregular hyperechoic area in the uterine cavity (sagittal view on the left, coronal view on the right); Space-occupying intramural lesions were observed on the left side of the uterine fundus (C, D).
Normally, β-hCG levels drop logarithmically within 24 h following the suction evacuation of a hydatidiform mole. Since this expectation was unmet following the first suction-evacuation procedure, a second attempt was made. Nonetheless, both attempts failed to result in optimum outcomes (β-hCG levels were 4260 IU/L and 1732 IU/L on postoperative day 2 in the first and second attempts, respectively). Since ultrasound examination showed an irregular hyperechoic area in the uterine intracavitary (27 × 33 × 27 mm3), a GTN diagnosis was suspected, then confirmed by MRI findings (a lesion was observed on the left uterine horn). Overall, considering the persistently elevated β-hCG value after the evacuation of a molar pregnancy, hydropic changes of chorionic villi, and extension of the lesions into the myometrium, a diagnosis of invasive moles was made (FIGO Stage I: Score 2).
First, one course of 5-fluorouracil (5-FU) chemotherapy was administered via venous catheterization, and the β-hCG value lowered to 289.6 IU/L. However, owing to a peripherally inserted central catheter (PICC)-related thromboembolic event in the basilic vein, it was then replaced with 5-day methotrexate (MTX) intravenous monotherapy after anticoagulation therapy. After another two cycles of single-agent MTX chemotherapy, the β-hCG levels normalized. The patient was discharged followed by a consolidation course of MTX. Her β-hCG level remains undetected on testing weekly for 3 weeks, and then monthly until 25 January 2021.
On 4 April 2021, she revisited our hospital for self-measured positive urine β-hCG after 1 month of amenorrhea, which was confirmed to be a quantitative β-hCG level of 50,842.0 IU/L by laboratory assessment. An ultrasound showed abnormal echogenicity in the uterine cavity (33 × 34 × 28 mm3) () with abundant blood flow signals, and MRI revealed space-occupying intramural lesions on the left side of the uterine fundus (); GTN (FIGO Stage I: Score 4) was thus diagnosed. HIFU therapy with a total dose of 260,000 J (within 650 s, mean power of 400 W) was performed on 19 April 2021. Doppler ultrasonography and CEUS were performed to evaluate the ablation effectiveness (), and a β-hCG level of 16,681.0 IU/L was achieved on postoperative day 2.
Figure 5. Preoperative and post-operative ultrasound assessment of HIFU therapy in the second case. Preoperative ultrasound localization: abnormal echogenicity on the left side of the uterine fundus with punctate blood flow signal detected (A). Immediate post-operative image with reduced blood supply in the epicenter of the lesion (B); the CEUS image before (C) and after (D) HIFU therapy revealing that the non-perfusion region has filled the treatment area of the lesion. CEUS, contrast-enhanced ultrasound; HIFU, high-intensity focused ultrasound.
The preferred FA combination chemotherapy was substituted with a 5-day MTX monotherapy given her history of previous PICC-related thrombosis. After four cycles of MTX chemotherapy, the β-hCG levels dropped to the normal range (5831.0 IU/L, 146.7 IU/L, 25.9 IU/L, 4.4 IU/L) (). Another consolidation course of MTX followed. While the patient was still under follow-up, the serum β-hCG results were normal. At the time of writing, the irregular hyperechoic area in the uterine intracavitary had gradually shrunken (9 × 5 × 4 mm3 at 1-month post-discharge follow-up) ().
Discussion
GTN is a malignant lesion caused by the aberrant growth of placental trophoblasts. Chemotherapy, supplemented by surgery and radiotherapy, is currently the predominant therapeutic strategy, and chemoresistance and recurrence are now the primary challenges this treatment strategy confronts.
The chemoresistance of GTN is established by the unsatisfactory decrease in β-hCG concentrations (does not decrease logarithmically or plateaus), or abnormal imaging signs (no shrinkage of existing lesions or new lesions developing radiographically), after two combination treatment cycles [Citation2]. Individual risk factors that correlate with FIGO prognostic scoring have been proven to predict the degree of chemoresistance [Citation1]. High FIGO scores have been associated with increased chemotherapy resistance [Citation8,Citation9]. Approximately 25% of patients with high-risk GTN have a poor response to first-line multiagent chemotherapy [Citation10]. The most frequently utilized initial regimen for high-risk GTN is combination chemotherapy with EMA/CO. In his paper on the management of GTN, Newlands ES reported that 14% of high-risk patients with no chemotherapy history developed drug resistance to EMA/CO, and 13 of 272 high-risk individuals died from EMA/CO drug resistance [Citation11]. In the guidelines, adjuvant surgical procedures are considered appropriate therapy for cancers resistant to EMA/CO [Citation1,Citation2]. Surgical resection for chemotherapy-resistant solitary lesions in the uterus or lungs increases chemotherapy sensitivity by diminishing the tumor load, which is an efficient approach to reduce resistance rates. Roughly half of the patients with high-risk GTN undergo surgery during curative chemotherapy [Citation12]. Feng et al. reported that 76% of 42 patients with drug-resistant GTN achieved serologically complete remission after surgical resection followed by chemotherapy [Citation13]. Another study showed that 87.5% of 24 patients with high-risk GTN, who employed the EMA-CO regimen and underwent adjuvant surgical management, survived [Citation14].
In the first case, two cycles of multiagent chemotherapy were delivered before HIFU. Chemoresistance is more likely to develop with an unsatisfactory decrease in β-hCG concentration. After HIFU therapy, no chemoresistance emerged over the subsequent EMA/CO regimes. A total of four courses of chemotherapy were administered until the β-hCG decreased to the normal range (<5 IU/L) (which decreased logarithmically as the number of chemotherapy courses progressed). It has previously been reported that the number of courses for 96 high-risk GTN patients with EMA/CO regimen was 8.5 ± 2.2 until remission [Citation15], and the mean number of chemotherapy courses in China was seven [Citation9]. HIFU therapy shortened the chemotherapy sessions for the high-risk GTN patient. This agrees with previous reports that traditional surgery significantly shortens the treatment duration and lowers the number of chemotherapy cycles administered for GTN patients [Citation16]. HIFU therapy may achieve this effect by significantly diminishing chemotherapy-resistant tumor bulk, as with traditional surgery. If the lesion has been completely removed surgically, the time required for β-hCG to diminish by 50% is about 48 h [Citation17]. Therefore, in this case, HIFU showed a remarkable ‘resection’ effect because the β-hCG level of the patient decreased by half on day 2 post-HIFU. That was also demonstrated by the changes in the grayscale in the tumor tissue area. Noticeable changes in the grayscale were instantly identified on the ultrasound image at the focus during HIFU ablation. Previous investigations have shown that these hyperechoic areas correspond to the extent of coagulative necrosis [Citation18]. Thus, positive oncological outcomes were obtained using HIFU as an adjuvant surgical salvage procedure for chemotherapy-resistant patients, and the adverse effect and toxicity of chemotherapy can be alleviated by the shortened chemotherapy sessions.
Furthermore, most GTN recurrences are caused by resistance to chemotherapy [Citation19]. A previous study showed that the recurrence rate of patients with GTN was 4.7% [Citation20]. Admittedly, the small sample size of patients with recurrence makes it difficult to find an optimal treatment regimen. The patient with low-risk GTN in the second case experienced a relapse within half a year of serologically complete remission.
For nearly all low-risk GTN patients, first-line single-agent MTX, actinomycin D, or fluorouracil chemotherapy are ideal therapeutic options [Citation1]. However, many patients with low-risk GTN require second-line chemotherapy. It has been reported that a change in therapy regimens due to methotrexate resistance was necessary for 30.9% of 485 individuals [Citation21]. Another study reported that multiagent chemotherapy is required to achieve remission in 10–15% of patients with low-risk disease [Citation10]. Therefore, for recurrent low-risk GTN, multiagent chemotherapy regimens have become the most common therapeutic option [Citation1]. Several studies have been conducted worldwide, but the most effective chemotherapy regimen remains unclear [Citation8,Citation19,Citation22]. 5-FU combination regimens appear to be favored in China [Citation22], though time-dependent 5-FU anti-tumor action requires ongoing central venous catheterization and infusion, which may cause inflammation, infection, and venous thrombosis [Citation23]. The patient in the second case suffered the latter during her first hospitalization. Therefore, treatment regimens without PICC, such as MTX, are necessary. However, MTX regimens are no longer recommended as monotherapy due to less efficacy, especially for patients with recurrence [Citation1], and current evidence suggests that patients in this category with a pretreatment β-hCG concentration of >50,000 IU/L could be more prone to MTX resistance [Citation24]; thus, adjuvant therapy is warranted.
Surgery also plays an important role in the treatment of recurrent GTN [Citation25]. Resection of tumor lesions in the liver, uterus, and lung are effective adjunct treatments with curative potential. The incidence of remission in patients with recurrence who underwent surgery was substantially higher than that in non-surgical patients [Citation19]. In our study, HIFU therapy, as an emerging noninvasive and accurate treatment option to substitute traditional surgical procedures, was opted as an adjuvant surgical salvage procedure in the second patient, and the direct surgical effects were validated by a substantial reduction in β-hCG following surgery. Furthermore, the treatment effect was excellent in the second patient, considering that the β-hCG decreased from above 50,000 IU/L to the normal range through four courses of MTX monotherapy after HIFU treatment.
HIFU treatment for GTN management lies in tissue degradation and tumor vascular damage produced by coagulation necrosis. This is generated by energy-gathered ultrasound on malignant sites buried deep inside tissues, with millimeter precision. This, followed by tumor vascular disruption [Citation5] and endocytosis of the necrotic tissue by the body’s immune system, achieves the goal of an entirely noninvasive tumor ‘resection’. Because metastatic lesions are commonly hemorrhagic given the fragile vessels of trophoblast tumors [Citation1], HIFU therapy also reduces the risk of metastatic disease through minimal surgical extrusion and bleeding.
HIFU, in addition to the direct use of energy-based therapies to destroy tumor cells, has been shown to boost the anti-tumor activity of the immune system [Citation26]. After HIFU treatment, the expression of numerous tumor antigens in debris provides a possible source for the activation of the immune system [Citation27]. CD3, CD4, CD8, B lymphocytes, and NK cells were shown to infiltrate the treated lesions after HIFU ablation, accompanied by FasL+, granzyme+, and perforin+ tumor-infiltrating lymphocytes in considerable numbers [Citation28], which convert a nonimmunogenic tumor to an immunogenic tumor [Citation29]. Immuno-enhancement effects might potentially be the essential component of the therapeutic effects of HIFU therapy for GTN.
The two patients described here underwent HIFU therapy as a uterine-sparing adjuvant surgical salvage procedure. The uterine-sparing ultrasound ablation therapy attenuated chemoresistance and shortened the chemotherapy sessions for high-risk patients, as well as reduced dosages and classes of chemotherapeutic agents for patients with recurrence. Therefore, HIFU therapy may replace traditional surgery as the adjuvant therapy modality of GTN in patients who desire to preserve their uterus. However, there were a limited number of patients and no control group, among the limitations of our study. This innovative combination of treatments for GTN management must be validated by further research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abu-Rustum NR, Yashar CM, Bean S, et al. Gestational trophoblastic neoplasia, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(11):1374–1391.
- Xiang Y, Zhou Q, Wu XH, et al. Guidelines for the diagnosis and treatment of gestational trophoblastic diseases. Chin J Pract Gynecol Obstet. 2018;34(9):994–1001.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327.
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93(8):890–895.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11(3–4):149–154.
- Lin Z, Gong C, Huang Q, et al. A comparison of results following the treatment of placenta accreta and placenta increta using high-intensity focused ultrasound followed by hysteroscopic resection. Int J Hyperthermia. 2021;38(1):576–581.
- Zhu X, Deng X, Wan Y, et al. High-intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine. 2015;94(18):e854.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynecol Obstet. 2018;143:79–85.
- Liu W, Zhao W, Zhang YQ, et al. Curative effects and influenced factors of EMA-CO as an initial regimen for the treatment of high-risk gestational trophoblastic neoplasia. Zhonghua yi Xue za Zhi. 2018;98(47):3896–3899.
- Lurain JR, Nejad B. Secondary chemotherapy for high-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2005;97(2):618–623.
- Newlands ES. The management of recurrent and drug-resistant gestational trophoblastic neoplasia (GTN). Best Pract Res Clin Obstet Gynaecol. 2003;17(6):905–923.
- Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204(1):11–18.
- Feng FZ, Xiang Y, Cao Y, et al. Efficacy of surgical management combined with chemotherapy in the treatment of drug-resistant gestational trophoblastic neoplasm. Zhonghua Fu Chan Ke Za Zhi. 2008;43(10):728–731.
- Lurain JR, Singh DK, Schink JC. Role of surgery in the management of high-risk gestational trophoblastic neoplasia. J Reprod Med. 2006;51(10):773–776.
- Kim SJ, Bae SN, Kim JH, et al. Effects of multiagent chemotherapy and independent risk factors in the treatment of high‐risk GTT-25 years experiences of KRI‐TRD. Int J Gynecol Obstet. 1998;60:S85–S96.
- Eysbouts YK, Massuger LFAG, IntHout J, et al. The added value of hysterectomy in the management of gestational trophoblastic neoplasia. Gynecol Oncol. 2017;145(3):536–542.
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717–729.
- Wang ZB, Wu F, Wang ZL, et al. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou province miniswine. Ultrason Sonochem. 1997;4(2):181–182.
- Kong Y, Zong L, Cheng H, et al. Management and risk factors of recurrent gestational trophoblastic neoplasia: an update from 2004 to 2017. Cancer Med. 2020;9(7):2590–2599.
- Balachandran K, Salawu A, Ghorani E, et al. When to stop human chorionic gonadotrophin (hCG) surveillance after treatment with chemotherapy for gestational trophoblastic neoplasia (GTN): a national analysis on over 4,000 patients. Gynecol Oncol. 2019;155(1):8–12.
- McNeish IA, Strickland S, Holden L, et al. Low-risk persistent gestational trophoblastic disease: outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol. 2002;20(7):1838–1844.
- Alazzam M, Tidy J, Osborne R, et al. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2012;12:CD008891.
- Peng M, Ding Y, Yu L, et al. Tegafur substitution for 5-Fu in combination with actinomycin D to treat gestational trophoblastic neoplasm. PLoS One. 2015;10(11):e0143531.
- McGrath S, Short D, Harvey R, et al. The management and outcome of women with post-hydatidiform mole ‘low-risk’ gestational trophoblastic neoplasia, but hCG levels in excess of 100 000 IU L(-1). Br J Cancer. 2010;102(5):810–814.
- Cao Y, Xiang Y, Feng F, et al. Surgical resection in the management of pulmonary metastatic disease of gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2009;19(4):798–801.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245–W252.
- Wu F, Wang ZB, Cao YD, et al. Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation. Ann Surg Oncol. 2007;14(3):1237–1242.
- Lu P, Zhu XQ, Xu ZL, et al. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145(3):286–293.
- Eranki A, Srinivasan P, Ries M, et al. High-intensity focused ultrasound (HIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin Cancer Res. 2020;26(5):1152–1161.