Abstract
Purpose
To evaluate Hyperthermic-Intra-Vesical Chemotherapy (HIVEC) efficacy regarding 1-year disease-free survival (RFS) rate and bladder preservation rate in patients with High-risk Non-Muscle Invasive Bladder Cancer (NMIBC) who fail BCG therapy or are contraindicated to BCG.
Methods
Between June 2016 and October 2019, patients treated with HIVEC for mostly high-risk NMIBC who failed BCG or BCG-naive if BCG contraindicated have been included in our study. These patients had a theoretical indication for cystectomy but were ineligible for surgery or refused it.
Results
Fifty-three patients, median age 72 [39–93] years, were included in this study (n = 29 BCG-failure and n = 24 BCG-naive). The median follow-up was 18 months. The bladder preservation rate was 92.4%. The 12 months-RFS rate was 60.5%. The RFS rates for BCG-naive and BCG-failure groups were respectively 70% and 52.2% at 12 months. Three patients progressed to muscle infiltration, all in the BCG-failure group and all in the very high-risk EORTC group. Two of them developed metastatic disease and died from bladder cancer.
Conclusion
Chemohyperthermia using HIVEC achieved a RFS rate of 60% at 1 year and enabled a bladder preservation rate of 92%. Given the low risk of progression in the BCG-naive group, HIVEC could be a good alternative. Conversely, for patients with very high-risk tumors that fail BCG, cystectomy should remain the standard of care and HIVEC may be discussed cautiously for patients who are not eligible for surgery and well informed of the risk of progression to muscle-invasive disease.
Introduction
The incidence rates of bladder cancer increased in many European countries [Citation1]. Non-muscle invasive bladder cancer (NMIBC) represents 75% of bladder cancer. An adjuvant treatment with intravesical bacillus Calmette Guérin (BCG) is recommended for high-risk (HR) NMIBC. However, up to 50% fail to maintain a response within 5 years [Citation2].
Cystectomy is recommended in BCG-refractory or BCG-unresponsive tumors and should be upfront considered as an immediate option for patients at very-high-risk of progression according to the international guidelines [Citation3]. The management of high-risk muscle invasive bladder cancer (HR-NMIBC) is a current challenge with the aim to enabling bladder preservation while avoiding progression to muscle infiltration, especially for patients suffering from recurrent disease after BCG. On another side, some patients are intolerant and contraindicated to BCG. Although radical cystectomy is recommended, some patients decline to undergo or are ineligible. Efficacious and safe therapeutic options are urgently needed for this challenging patient population.
Radiofrequency induced thermochemotherapeutic effect (RITE) is a microwave bladder heating technique having shown equivalent efficacy to BCG in a randomized trial for patients with intermediate and high-risk tumors [Citation4]. The second technique, Hyperthermic-Intra-Vesical Chemotherapy (HIVEC), a recirculating conductive bladder heater evaluated in our study, has the advantage of being easier to use but is still poorly evaluated in the literature [Citation5,Citation6].
The objective of our study is to evaluate HIVEC regarding the disease-free survival (RFS) rate and bladder preservation in HR-NMIBC patients who fail BCG therapy or are contraindicated to BCG.
Methods
Patients
This is a multicenter, retrospective study, including patients treated with HIVEC between June 2016 and October 2019 in 5 French center (approved protocol number: THERMO-MMC-IPC 2019-019). The requirement for informed consent was waived.
The inclusion criteria were:
Patients with high-risk or very high-risk NMIBC according to the European Organization for Research and Treatment of Cancer (EORTC) classification who have failed treatment with BCG (BCG-failure group) according to the definition of BCG-unresponsive from the International Bladder Cancer Group [Citation7]. Patients with an intermediate risk tumor could be included in this group if the initial tumor was classified as high or very high-risk with early recurrence at the first cystoscopy after BCG therapy as low-grade tumor.
Patients who have a theoretical indication of BCG therapy but who are contraindicated or intolerant to BCG (BCG-naïve group). It could be an absolute contraindication, i.e., grade 3/4 adverse event during previous BCG therapy or it could be a relative contraindication: previous pelvic radiotherapy, immunosuppressive therapy or BCG shortage. Indeed, in 8 cases, the patients could not receive BCG due to national shortage and HIVEC has been proposed as an alternative to BCG.
These patients are in a therapeutic impasse, especially for highest risk tumors and BCG-refractory tumors where these patients may have a theoretical indication for cystectomy but are not eligible or refuse it. Patients included had recent urinary tract imaging (<6 months). Patients were classified into EORTC risk groups according to the recommendations of the EAU [Citation3]. The ethics committees of each institution approved data collection and analysis.
Devices
Mitomycin C (MMC) was heated by the COMBAT BRS (Combat Medical, Wheathampstead, UK) device. This is a recirculating conductive bladder heating device which increases the temperature of the MMC at 43∘C (±1 °C) before instillation [Citation8].
Instillation protocol
Chemohyperthermia was started 4–6 weeks after the first resection or after the second-look resection when recommended. The therapeutic plan consisted of 6 or 8 weekly instillations of 40 mg of heated MMC. The standard protocol was to instill 40 mg of mitomycin in 50 mL of 0.9% sodium chloride solution heated by the HIVEC system during one hour. The number of instillations planned was usually 6. Treatment was discontinued in case of severe (grade 3 or 4) side effects.
Monitoring protocol
Patients had cystoscopy and urinary cytology six weeks after the last instillation, then every three months during the two first years. A bladder resection was considered if a lesion was visualized by cystoscopy or in case of high-grade positivity of the cytology.
Endpoints
Co-primary endpoints were recurrence-free survival time (RFS) and bladder preservation rate (defined as the proportion of patients who did not have a cystectomy among those who did not progress). The secondary endpoints were, time to recurrence, MIBC progression rate, progression-free survival (PFS), time to muscle infiltration, time to a metastatic disease, disease specific survival (DSS), overall survival (OS), and adverse events according to the CTCAE v5.0 classification. The complete response rate at 6 months for BCG-failure with carcinoma in situ was also analyzed. For RFS, a sub-group analysis was performed for BCG-naive and BCG-failure patients separately.
Tolerance was evaluated prospectively using patients reported outcomes questionnaires. At the same time, physician-reported grade 3/4 side effects were collected retrospectively for each patient.
Statistical analysis
Data were collected and analyzed using SAS software version 9.3 (SAS Institute, Cary, NC, USA) and R software version 3.0.3. Clinical and histopathological correlations were tested, using, for continuous (quantitative) variables, the Student’s t-test, and for qualitative variables, the Chi2 test with or without Yates correction depending on the expected numbers. RFS and OS analyses were performed using Kaplan-Meier curves and were compared using the log-rank test with a univariable Cox regression model used to determine unadjusted hazard ratios. The data were considered statistically significant at the 95% confidence interval (p < .05).
Results
Patients characteristics
A total of 53 patients were included in this study, including 24 patients in the BCG-naive group and 29 patients in the BCG-failure group. The median age of the patients was 72 [39–93] years. The characteristics of the population are summarized in .
Table 1. Clinicopathological characteristics of patients.
Oncological outcomes
Median follow-up in our study was 18 months. A total of 22 patients recurred, with a mean time to recurrence of 18 months [5–43], 20 months [5–43] for the BCG-naive group and 15 months [5–31] for the BCG-failure group.
19 patients had a NMIBC recurrence. Treatments in case of NMIBC recurrence included (): radical cystectomy (n = 3), rechallenge intravesical therapy because the recurrence occurred more than one year after the end of HIVEC therapy (BCG n = 6, or HIVEC n = 4), abstention for patients who were not eligible to another treatment (n = 5) and intravenous pembrolizumab (n = 1).
Table 3. Salvage therapy for NMIBC recurrence after HIVEC treatment.
Three patients progressed to muscle infiltration with a mean time to progression of 7 months [Citation5–12]. Two of them developed a metastatic disease and died from bladder cancer whereas one had a cystectomy for an organ-confined disease.
At the end of the follow-up, a total of 5 patients died: 2 from bladder cancer, 1 from a myocardial infarction, 1 from a pulmonary embolism and 1 from head and neck cancer. Overall survival rate was 90.6%.
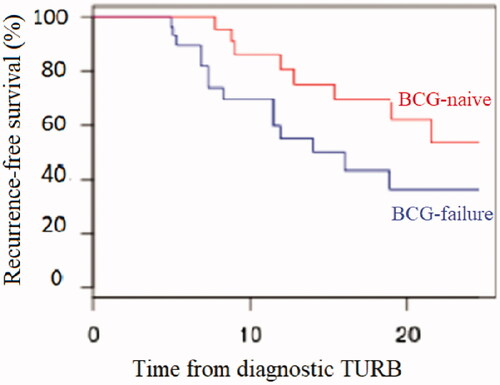
The RFS rates for the whole population at 3-, 6-, 12- and 18 months were respectively 94.3%, 80.4%, 60.5% and 46.2%. For the BCG-naive group of patients, the RFS rates at 3-, 6-, 12- and 18 months were respectively 100%, 95.7%, 70% and 61.1%. For the BCG-failure group, the RFS rates at 3-, 6-, 12- and 18 months were respectively 89.7%, 67.9%, 52.2% and 33.3% ().
Figure 1. Kaplan-Meier curves for recurrence-free survival: BCG-failure versus BCG-naive sub-group analysis.
In the BCG-failure group, in case of presence of Cis, complete response rates at 6 months were 75%.
The bladder preservation rate was 92.4%.
Predictive factors of recurrence and progression after HIVEC
In univariate analysis, only the tumor grade was associated with RFS (), 0% (0/6) low grade recurred and 46.8% (22/47) recurred (p = .028) while the presence of Cis was not a predictive factor of recurrence in our series. Pure Cis (without papillary tumor associated) recurred in 66.7% of cases versus 36.4% for papillary tumors, but this difference was not significant (p = .09).
Table 2. Predictive factors of recurrence after HIVEC.
None of the different clinicopathological factors was predictive of progression to muscle-invasive disease in univariate analysis. The three patients who progressed had a very high-risk tumor according to the EORTC classification. However, the EORTC classification was not significantly associated with the risk of progression (p = .058). It is also noteworthy that the three patients who progressed were in the BCG-failure group (10.3% of patients in the BCG-failure group progressed during follow-up whereas none of the BCG-naive patients have progressed).
Safety
No severe side effect (grade 3 or 4) has occurred. Grade 1 or 2 side effects consisted of: 29.4% positive urine culture, 38.2% pain, 14.7% hematuria, 52.9% urgency, 8.8% myalgia, 26.5% bladder spasm, 2.9% skin allergy. The percentage of delivered/theoretical instillations was 93.7%. Five patients received less than 5 instillations with early treatment discontinuation due to minor side effects (grade 1 or 2).
Discussion
Management of HR-NMIBC is a challenge to allow bladder preservation while avoiding progression toward a muscle-invasive disease. Second-look resection and adjuvant BCG therapy have been shown to decrease the risk of recurrence and progression [Citation9,Citation10]. For patients who failed BCG, radical cystectomy remains the standard [Citation3]. However, cystectomy is associated with a high morbidity and some patients are ineligible for surgery, due to their comorbidities. For these reasons, some patients will attempt bladder preservation therapies.
One of them is chemohyperthermia. By increasing the intracellular concentration of mitomycin and thereby its effectiveness, hyperthermia seems to have a synergistic effect with chemotherapy [Citation11]. In terms of safety, it has already been shown that heated MMC is at least equivalent to BCG whether with RITE [Citation12] or with HIVEC [Citation13]. Our study confirms these data since we did not have any major side effects.
In terms of efficacy, thermo-chemotherapy with HIVEC is still poorly evaluated. Regarding RITE (Synergo® device), the first results are encouraging suggesting a superior efficacy of RITE compared to passive MMC and an efficacy at least equivalent to that of the BCG in terms of RFS for intermediate or high-risk NIMBC [Citation4]. There is only one randomized phase III study assessing RITE efficacy in patients who fail BCG [Citation13]. This study included 104 patients treated with either RITE or BCG. At 1 year, RFS in the RITE group was 49%, which is close to our results (52.2% in the BCG-failure group). RFS at 2 years was close to BCG in the overall cohort (35% versus 41%, p = .49), with a non-significant improvement in the subgroup of patients without cis (53% versus 24%, p = .11). Conversely, in the group of patients with Cis, BCG was significantly better in terms of recurrence-free survival at 2 years (49% versus 26%, p = .01).
With a median follow-up of 18 months, we were able to show a RFS rate at 1 year of 60% with HIVEC with a bladder preservation rate was 92.4%. However, it is noteworthy that the RFS rate differs between BCG-naive and BCG-failure sub-groups.
For BCG-naive patients, it is established that the risk of recurrence after a first line of BCG therapy is 20% at 6 months and 25% at 12 months, while the risk of progression is 3% at 6 months and 5% at 12 months [Citation7]. In our series, RFS rate in the BCG-naive group was 70% at 12 months signifying that less than a third of the patients recurred at 1 year. These results are favorable since HIVEC seems to have an efficacy similar to that of BCG, suggesting that HIVEC could be a good alternative, especially for patients contraindicated to BCG.
The BCG-failure group had a worse prognosis in terms of recurrence and progression risk. Indeed, in our series, a half of patients recurred within the first year. According to the recommendations for clinical trials in BCG unresponsive NMIBC [Citation7], and taking in account the fact a placebo-controlled arm is not ethical, single arm trials with new agents are now being conducted with the objective of achieving a RFS rate of at least 30% at 12 months. For BCG unresponsive Cis, the objective of at least 50% of complete response at 6 months is considered as clinically meaningful. With a RFS rate of 52.2% at 12 months in our BCG-failure whole population and with a complete response rate of 75% at 6 months (and 60% at 12 months) for BCG unresponsive Cis in our study, chemohyperthermia could therefore be considered as a valid option for patients who fail BCG.
In our study, CIS was not a predictive factor of recurrence after HIVEC, even if pure CIS seem to have a distinct profile with two third of patients who recur early after HIVEC. Conversely, the outcomes seem favorable in the group of patients with papillary tumors without associated CIS, with less than a third of patients having recurred. Our results are in agreement with the literature. Jong and al [Citation6], in a cohort of 55 BCG-refractory patients treated with HIVEC, found a 50% recurrence-free rate after a median follow-up of 14 months. However, the tumor characteristics of this cohort differed from ours as 49% of patients had pure CIS, with an early recurrence rate of 30% at 3 months in this subpopulation.
Risk of progression is also an important endpoint in this bladder preservation approach. The progression rate is also very different between the two sub-groups in our study. Indeed, 3 patients (10.3%) had a disease progression to muscle infiltration in the BCG-failure group, all of very high-risk according to the EORTC, while no progression was observed in the BCG-naive group after a median follow-up of 20 months. It should be noted that among the 3 patients who progressed to muscle infiltration, 2 patients presented upfront distant metastases and one patient was operated on with N + status on final pathological specimen, underlying the poor prognosis of these progressive patients. Therefore, patients with very high-risk tumors who fail BCG should be well informed of the risk of undertreatment and putative progression to muscle-invasive disease despite treatment with HIVEC, with the possibility of rapid lymph node involvement and/or metastatic spread due to the aggressive profile of this type of tumors.
Other drugs are being studied for BCG-unresponsive population. Recently, pembrolizumab (monotherapy intravenous) has been evaluated in this indication [Citation14]. De Wit et al. reported the results of the CIS cohort and showed a complete response rate at 3 months of 38.8%, with a recurrence within one year for almost half of the responder patients (54% of responders remain free from recurrence at 1 year, i.e., 21% of the whole population). In a recent meta-analysis on bladder sparing therapies, Kamat et al. reported a pooled 12-mo response rate of 36% for trials with one or more prior BCG courses, with a lower response in case of inclusion of ≥50% of patients with CIS [Citation15]. However, there is a great heterogeneity and variability regarding the treatment strategies. Emerging treatments currently in development, such as IFN-based gene therapy [Citation16] or reverse thermal gel [Citation17], show promising efficacy.
Our study has several limitations. First, this is single-arm study, with two different populations (BCG-naive and BCG-failure) with different prognosis and profile. No comparative design is acceptable for patients who fail BCG (since randomization versus cystectomy is not ethically conceivable) but this could be considered a weakness for the BCG-naive patients’ sub-population. Second, even if the data were collected prospectively, the outcomes were assessed retrospectively with some patients for whom follow-up was carried out in another center. Then, there are two distinct sub-populations with different prognosis and therefore with therapeutic objectives, ultimately justifying being studied separately in larger cohorts. Finally, there is no maintenance after the induction courses; although monthly maintenance MMC decreases the recurrence rate with an indication of 1 year of maintenance for intermediate risk NMIBC [Citation18], the role of MMC maintenance in a chemohyperthermia protocol has not been investigated.
Despite these limitations, our cohort is one of the largest retrospective series of patients treated with HIVEC, and the preliminary results are encouraging. Prospective and comparative studies with homogeneous populations are awaited. These studies will have to answer two different questions: the efficacy of HIVEC in patients with high-risk NMIBC (compared to BCG therapy) and the place of HIVEC among bladder preservation strategies in the population of BCG-refractory or BCG-Unresponsive tumors.
In conclusion, chemohyperthermia using HIVEC achieved a RFS rate of 60% at 1 year and enabled a bladder preservation rate of 92%. Given the low-risk of progression in the BCG-naive group of patients, chemohyperthermia could be a good alternative to early cystectomy, in particular for patients contraindicated to BCG. Conversely, for patients with very high-risk tumors that fail BCG, cystectomy should remain the standard of care and HIVEC may be discussed cautiously for patients not eligible for surgery and well informed of the risk of progression to muscle-invasive disease. The results of prospective trials are needed to confirm this.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8(1):1129.
- Witjes JA. Management of BCG failures in superficial bladder cancer: a review. Eur Urol. 2006;49(5):790–797.
- Babjuk M, Burger M, Compérat EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ). Eur Urol. 2019;76(5):639–657.
- Arends TJH, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette-Guérin for adjuvant treatment of patients with intermediate- and high-risk non–muscle-invasive bladder cancer. Eur Urol. 2016;69(6):1046–1052.
- Sousa A, Piñeiro I, Rodríguez S, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32(4):374–380.
- de Jong JJ, Hendricksen K, Rosier M, et al. Hyperthermic intravesical chemotherapy for BCG unresponsive non-muscle invasive bladder cancer patients. Bladder Cancer. 2018;4(4):395–401.
- Kamat AM, Sylvester RJ, Böhle A, et al. Definitions, end points, and clinical trial designs for Non-Muscle-Invasive Bladder Cancer: recommendations from the international bladder cancer group. J Clin Oncol. 2016;34(16):1935–1944.
- Irani J. Intravesical instillations in non-muscle-invasive bladder cancer (NMIBC): prospects for thermochemotherapy. Prog En Urol. 2016;26(14):860–864.
- Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2018;73(6):925–933.
- Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer: intravesical BCG plus TUR vs TUR alone in Ta and T1 bladder cancer. BJU Int. 2001;88(3):209–216.
- van der Heijden AG, Verhaegh G, Jansen CFJ, et al. 2005; Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol. 173(4):1375–1380.
- Gofrit ON, Shapiro A, Pode D, et al. 2004; Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 63(3):466–471.
- Marquette T, Walz J, Rybikowski S, et al. 2020 ; [Safety of Hyperthermic IntraVEsical Chemotherapy (HIVEC) for BCG unresponsive non-muscle invasive bladder cancer patients]. Prog Urol. 30(1):35–40.
- Balar AV, Kulkarni GS, Uchio EM, et al. 2019; Keynote 057: Phase II trial of pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus calmette-guérin (BCG). J Clin Oncol. 37(7_suppl):350–350..
- Kamat AM, Lerner SP, O’Donnell M, et al. 2020; Evidence-based assessment of current and emerging bladder-sparing therapies for non–muscle-invasive bladder cancer after Bacillus Calmette-Guerin therapy: a systematic review and meta-analysis. Eur Urol Oncol. 2020:32201133.
- Duplisea JJ, Mokkapati S, Plote D, et al. 2019; The development of interferon-based gene therapy for BCG unresponsive bladder cancer: from bench to bedside. World J Urol. 37(10):2041–2049.
- Kleinmann N, Matin SF, Pierorazio PM, et al. 2020; Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. S1470-2045(20):30147–30149.
- Friedrich MG, Pichlmeier U, Schwaibold H, et al. Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with Bacillus Calmette-Guérin (BCG) in patients with non-muscle-invasive bladder carcinoma. Eur Urol. 2007;52(4):1123–1129.
