Abstract
Aims
Type-2 diabetes mellitus (T2DM) is a common health condition which prevalence increases with age. Besides lifestyle modifications, passive heating could be a promising intervention to improve glycemic control. This study aimed to assess the efficacy of passive heat therapy on glycemic and cardiovascular parameters, and body weight among patients with T2DM.
Methods
A systematic review and meta-analysis were reported according to PRISMA Statement. We conducted a systematic search in three databases (MEDLINE, Embase, CENTRAL) from inception to 19 August 2021. We included interventional studies reporting on T2DM patients treated with heat therapy. The main outcomes were the changes in pre-and post-treatment cardiometabolic parameters (fasting plasma glucose, glycated plasma hemoglobin, and triglyceride). For these continuous variables, weighted mean differences (WMD) with 95% confidence intervals (CIs) were calculated. Study protocol number: CRD42020221500.
Results
Five studies were included in the qualitative and quantitative synthesis, respectively. The results showed a not significant difference in the hemoglobin A1c [WMD −0.549%, 95% CI (−1.262, 0.164), p = 0.131], fasting glucose [WMD −0.290 mmol/l, 95% CI (−0.903, 0.324), p = 0.355]. Triglyceride [WMD 0.035 mmol/l, 95% CI (−0.130, 0.200), p = 0.677] levels were comparable regarding the pre-, and post intervention values.
Conclusion
Passive heating can be beneficial for patients with T2DM since the slight improvement in certain cardiometabolic parameters support that. However, further randomized controlled trials with longer intervention and follow-up periods are needed to confirm the beneficial effect of passive heat therapy.
Introduction
The rapidly rising incidence and prevalence of type-2 diabetes mellitus (T2DM) and its associated diseases are global health problems. The diabetes epidemic is related mainly to overnutrition, sedentary lifestyle, and obesity [Citation1].
Individuals with type-2 diabetes mellitus have an increased risk of all-cause mortality due to macrovascular complications like cardiovascular and cerebrovascular diseases [Citation2,Citation3]. Moreover, diabetes is associated with microvascular complications, including nephropathy, neuropathy, retinopathy, and nonvascular consequences, like increased risk of cancer and infection-related reactions [Citation4].
T2DM is characterized by hyperglycemia, resulting from the malfunction of the feedback loops between insulin secretion and insulin action [Citation5]. Defects of insulin action or insulin resistance in the adipose tissue, skeletal muscle, and liver are related to visceral obesity, hyperglycemia-induced oxidative stress, systemic subclinical inflammation, adipokine dysregulation, altered lipid metabolism, and derangements in the insulin-signaling pathway [Citation6]. These factors are deriving from a highly complex network that promotes, maintains, and augments insulin resistance and eventually leads to the macro-micro and nonvascular complications of T2DM [Citation7].
The management of T2DM should focus on hyperglycemia, insulin resistance, prevention or attenuation of late-onset complications, and reduction of cardiovascular and all-cause mortality.
Lifestyle modifications like nutritional changes and physical activity enhancement play an essential role in managing glycemic control in T2DM and reduce cardiovascular risks. Regular physical activity improves insulin sensitivity, glycemic control, lipid profile, and immune function; it simultaneously lowers blood pressure and ultimately decreases cardiovascular and overall mortality [Citation8]. Lifestyle modification and adequate drug therapy would be an ideal and effective comprehensive treatment of T2DM. Unfortunately, compliance to physical activity is often poor, or effective exercise is impossible due to obesity or neuro-musculoskeletal disorders [Citation9]. Further observed issue is the noncompliance of patients with taking the antidiabetic medications regularly [Citation10].
Passive heating in the forms of sauna, hot tub bathing, hot water spa, or balneotherapy is a traditional pursuit in many cultures. Several studies found beneficial effects of frequent passive heating on cardiovascular health, including reducing the risk of cardiovascular mortality [Citation11], hypertension [Citation12], coronary heart disease, and stroke [Citation13].
Numerous studies performed in nondiabetic populations reported the positive impact of frequent passive heating on glucose metabolisms, like improved fasting glucose, insulin concentrations, insulin sensitivity [Citation14–16], and lipid parameters [Citation17]. Studies also reported the amelioration of inflammatory profile and insulin signaling [Citation14].
There are emerging data about the possible underlying mechanisms of these favorable outcomes. Heat exposure induces microvascular remodeling in muscle tissue, leading to increased glucose uptake, activating heat shock protein expression, inhibiting inflammatory processes, lipogenesis, and oxidative stress [Citation18]. Heat also stimulates adiponectin signaling resulting in improved insulin sensitivity [Citation19].
Passive heating might be an alternative intervention to replicate the beneficial effects of regular physical activity and improve cardiometabolic health in T2DM.
The study aimed to investigate whether passive heat therapy could be an effective strategy in managing T2DM.
Methods
We reported our systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 Statement [Citation19]. We registered the study protocol in advance to the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020221500). We did not deviate from the registered protocol (see https://www.crd.york.ac.uk/prospero).
Search and selection
A systematic search was performed on 1 October 2020, in the following medical databases: MEDLINE (via PubMed), EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). The following search key was used: (hydrotherapy OR balneotherapy OR "heat expos*" OR "passive heat*" OR "heat therap*" OR "water immers*" OR "hot tube" OR "hot bath" OR "warm bath" OR ((increased OR elevated) AND "body temperature")) AND (diabetes OR diabetic OR diabet* OR hyperglyc* OR ((glycemic OR glycaemic) AND control) OR "insulin resistance" OR "insulin sensitivity" OR "hemoglobin a1c" OR "HbA1c" OR "glucose tolerance" OR "metabolic syndrome").
We did not use language or other restrictions. We checked the reference lists of all eligible studies and review articles for additional references.
Two review authors independently (SV, SK) read the titles, abstracts, and full texts of the selected abstracts. A third review author (FD) resolved any disagreement.
Eligibility
We searched for articles reporting on (P) patients with T2DM, (I) treated with chronic passive heat therapy. The control groups were either patients with thermoneutral therapy (C1) or the same patients' baseline status was used as control (C2). The outcomes of interest (O) were pre-and post-treatment cardiometabolic parameters (fasting plasma glucose, glycated plasma hemoglobin, cholesterol, triglyceride, C-reactive protein, blood pressure, weight, waist circumference, body mass index).
Heat therapy was defined as an intervention characterized by whole-body passive heating regardless of the environmental medium. In contrast to active heating, the chronic passive heating consists on several sessions of heat therapy and monitor the long-term changes of physiological parameters [Citation20].
Regarding study design, randomized controlled studies, cohort studies, or case-series reporting on any of the investigated outcomes in T2DM patients before and after heat exposure were eligible. We excluded articles with no available full text.
Data extraction
Data were extracted in a pre-defined Excel (Microsoft Corporation, Redmond, Washington, United States) datasheet by one review author and verified by a second.
The following data were extracted for each eligible article: the first author, year of publication, country of origin, time interval and place of the study, study design, basic demographic characteristic (female percentage, age, number of patients), numerical values for the outcomes pre-and post-treatment (mean, standard deviation, median, interquartile range, or range).
Data synthesis
For the meta-analysis, we used Stata 15 data analysis and statistical software (StataCorp LLC, College Station, TX, USA) if at least three different articles discussed the same outcome. For continuous outcomes (glycated hemoglobin – HbA1c, fasting glucose, triglyceride), we calculated weighted mean differences (WMD) with 95% confidence intervals (CIs) to investigate the differences between baseline and post-treatment. The random effect model was used to calculate the pooled estimates using the DerSimonian-Laird method [Citation21]. A p < 0.05 was considered statistically significant. Heterogeneity was tested with the χ^2 (χ2) test and the I^2 statistic. A p < 0.10 was considered significant.
We did not carry out a meta-analytical calculation for the heat therapy vs. thermoneutral therapy comparison because of the low number of included studies. We discussed these results in our qualitative synthesis.
Risk of bias assessment
We used the Cochrane risk-of-bias tool for randomized trials (RoB 2) [Citation22], and for nonrandomized studies of intervention, the ROBINS-I tool was applied [Citation23]. Because the number of included articles was less than ten, we did not analyze publication bias. Quality assessment was performed by two review authors independently. Disagreements were resolved by consensus.
Results
Search and selection
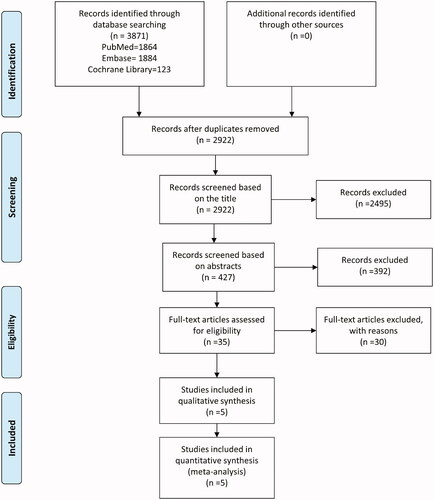
The results of our systematic search and selection detailed on a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart can be seen in . The systematic search yielded 5 [Citation24–28] eligible articles for the qualitative and quantitative analysis, including the data of 193 patients.
Basic characteristics of included studies
The characteristics of the included studies are summarized in . Out of these, five studies reported on the effect of heat-therapy on changes in laboratory parameters included HbA1c, fasting glucose, and triglyceride levels. The proportion of females in these studies changed between 37.5 and 100%, while the age varied between 43 and 75 years.
Table 1. Basic characteristics of included studies.
Quantitative synthesis
Changes in HbA1c levels
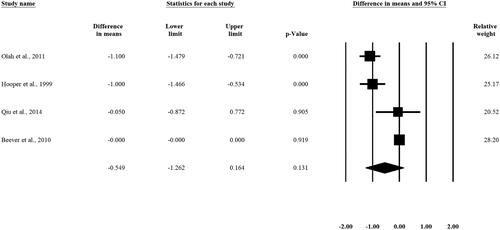
Weighted mean difference (WMD) in HbA1c levels pre-and post-intervention was calculated for 4 studies and did show slight difference what is not statistically significant [WMD −0.549%, 95% CI (-1.262, 0.164), p = 0.131] (). Two out of the four included studies showed a significant difference in the HbA1c pre-and post-interventional levels. [Citation25,Citation27]
Changes in fasting glucose levels
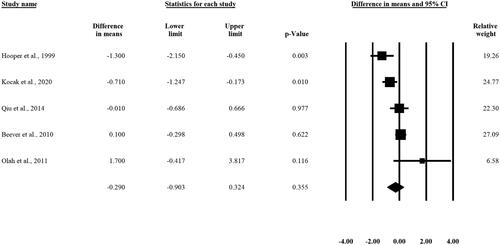
In the case of fasting glucose levels, we analyzed five studies. The result of the analysis did not show any statistically significant difference in the fasting glucose levels pre- and post-intervention [WMD −0.290 mmol/L, 95% CI (-0.903, 0.324), p = 0.355] ().
Changes in lipid levels (cholesterol, HDL, LDL, TG) – supplement figure
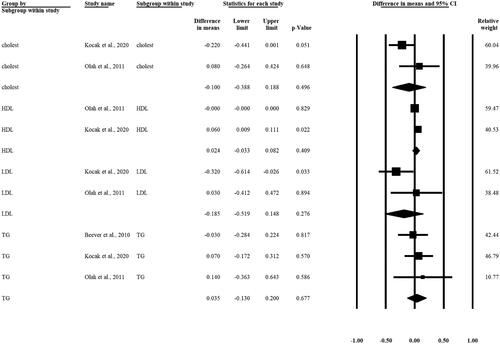
We cannot describe any statistically significant difference regarding different lipid levels (cholesterol, HDL, LDL, TG) in patients pre- and post-intervention. Based on three articles, the WMD in TG levels pre- and post-intervention was calculated [WMD 0.035 mmol/L, 95% CI (−0.130, 0.200), p = 0.677] ().
Qualitative synthesis
Three out of the five included [Citation24–26] articles reported on other laboratory parameters and the changes in the body weight, blood pressure, and heart rate, pre-, and post-intervention. We summarized these parameters in . Based on the paper of Koçak et al. [Citation26] we can observe that HOMA-IR, leptin-, visfatin, and cortisol levels showed a slight decrease after the intervention, while the insulin, CRP, adiponectin levels and ESR increased compared to pre-intervention measures. Beever et al. [Citation24] reported a decreased systolic blood pressure and heart rate. Two articles [Citation24,Citation25] measured changes in body weight in the follow-up period; the mean body weight showed a mild decrease in one of the studies [Citation25] but increased in the other one [Citation24] compared pre- and postinterventions weight and BMI.
Table 2. Summary of findings.
Two out of the five enrolled studies were randomized controlled trials [Citation27,Citation28]. In these investigations, besides HbA1C, fasting glucose, lipid levels, other laboratory parameters such as 2-h postprandial glucose level, CRP, ferritin, etc., were measured. No statistically significant changes were observed. These laboratory changes are demonstrated in .
Table 3. Summary table with the findings of the randomized controlled studies.
Risk of bias assessment and publication bias
Summary of risk of bias assessment for each outcome is included in Supplementary Figure 1-8. Using the ROBINS-I tool, we found a moderate to high overall risk of bias for all outcomes. Similarly, using the RoB 2 tool, the risk of bias was graded 'some concerns' for HbA1C, fasting plasma glucose, cholesterol, triglyceride, and CRP.
Discussion
This meta-analysis and systematic review aimed to investigate the effect of repetitive or chronic passive heating on glycemic control, insulin resistance, and cardiovascular risk factors in type-2 diabetes mellitus. Our study found no significant reduction in fasting plasma glucose and HbA1c, although there was a trend toward improvement.
Although an increasing number of animal and human studies report positive effects of chronic passive heating on cardiometabolic health, a limited number of studies investigate this intervention in individuals with type-2 diabetes.
With different lengths of interventional periods (12 weeks to 5 months), experimental animal models have proven the positive effect of heat therapy on glucose homeostasis [Citation29–31]. It is hypothesized that a sufficient heat stimulus is required for glycemic control amelioration since a specific core body temperature rise is necessary for an adequate heat shock protein (HSP) response. There is an existing consensus that states the threshold of the heat adaptation of humans is a core body temperature of 38.5 °C and above, this temperature is enough to stimulate and increase the abovementioned HSP response [Citation32]. The induction or restoration of HSPs benefits low-grade inflammation, oxidative stress, insulin signaling, and insulin resistance. Indeed, in murine models, the achieved and investigated body temperature was higher than it would be acceptable and safe in humans [Citation33]. However, Hooper et al. [Citation25] found significant improvements in HbA1c and fasting glucose at a tolerable rise of core body temperature (0,8 °C). Further studies are required to find an effective but safe temperature rise, especially in T2DM patients with impaired thermoregulation and altered thermal sensitivity caused by peripheral neuropathy [Citation34].
Acute heat causes an elevation in fasting plasma glucose in patients with diabetes [Citation35], and especially in individuals with insulin resistance, repetitive passive heating interventions result in improved glycemic control [Citation14]. Another study investigating the effect of passive local heating and mild electric stimulation in patients with T2DM showed improved metabolic parameters and found a positive correlation between the magnitude of the improvements and the lengths of intervention [Citation36]. In a recent cross-sectional study by Kamioka et al., a higher frequency of heat intervention (hot water bathing) associates with decreased risk of poor glycemic control in patients with type-2 diabetes [Citation37].
The beneficial metabolic effects of heat therapy are mainly due to an increase in the expression of intracellular heat shock proteins (HSPs). This response is increased with the frequency of intervention [Citation38]. HSPs have anti-inflammatory effects; they decrease inflammatory cytokines like TNF-α hence ameliorating insulin signaling and improving insulin sensitivity [Citation39,Citation40].
Two of the included studies found a significant decrease in HbA1c level [Citation25,Citation27]. Significant improvement in fasting plasma glucose was measured in two of the enrolled studies as well [Citation25,Citation26].
Hooper et al. included only eight patients with poor glycemic control into his study. In these patients, the effect of any glucose-lowering intervention was more detectable, but the reason of it is unknown (probably according to HbA1c and fasting plasma glucose). Oláh et al. found [Citation27] that in the interventional group decrease of HbA1c level after 12 weeks follow-up was no more observed. The lowering of fasting plasma glucose showed by Koçak et al. [Citation26] can be caused by improved insulin resistance: patients in his study were extremely obese.
These results might suggest that the possible beneficial effects of heating are correlated to the frequency and/or duration of heat intervention. This might also explain the lack of significant changes in glycemic control (HbA1c; and fasting glucose). The included studies have too short interventional period to achieve a significant improvement in glucose homeostasis.
T2DM is also associated with atherogenic dyslipidemia, which is essential in the therapy. In this meta-analysis, there were no significant changes in total cholesterol level, low-density lipoprotein (LDL) level, and high-density lipoprotein (HDL) level. In three of the enrolled studies [Citation24,Citation26,Citation27], lipid levels were measured before and after the interventions, and only Koçak et al. [Citation26] found a significant decrease in LDL cholesterol.
It is well known that exercise can improve diabetes-associated dyslipidemia, but the effect of a theoretically similar intervention like chronic passive heating is controversial in other studies. Kondo et al. could detect positive outcomes in lipids with a local (abdominal) heating method combined with electric stimulation [Citation36]. Another study with a duration of 8-10 weeks planned for effects of heat therapy on insulin resistance (polycystic ovarium syndrome) had beneficial results in glucose homeostasis and lipid profiles [Citation41]. Since insulin resistance plays a pivotal role in developing dyslipidemia associated with diabetes, improvement of insulin sensitivity and glycemic control could also lead to amelioration of lipid profile. Since all of these above-mentioned interventions, such as exercise, diet and passive heating are inexpensive methods for supporting the antidiabetic therapies, the combination of them could be very useful as well [Citation42].
Glucose regulation and is also related to adequate adipokine balance. Adipokines secreted by adipocytes play a crucial role in appetite and satiety balance, fat store regulation, insulin release, and sensitivity [Citation43]. The development and progression of T2DM are associated with adipokine dysregulation related to visceral obesity, as adipokines in this setting alter insulin signaling pathway and affect low-grade inflammation, contributing to insulin resistance [Citation44]. Koçak et al. [Citation26] studied the effect of passive heating on serum adipokines. They found a significant elevation in the level of adiponectin (‘good adipokine’), it has anti-diabetic, anti-inflammatory, and anti-atherogenic effect- [Citation45,Citation46] and a significant reduction in leptin and visfatin levels after 3 weeks of spa therapy, which then may be associated with an improvement of low-grade inflammation and insulin sensitivity [Citation46–48]. Another study reported significant improvement of serum leptin after spa therapy (combined with strict diet) in obese males with or without diabetes, a significant decrease in visfatin in obese, but not in individuals with diabetes. In contrast, no significant change in adiponectin levels were observe after a three-week spa therapy program in obese, diabetic participants [Citation49]. The different results partly could come from the various study populations.
According to the included studies, CRP level as a marker of inflammation is not changed. Former studies showed the beneficial effect of heat therapy on CRP level: among morbidly obese patients with balneotherapy combined with diet and exercise [Citation50], also a trend was observed among patients with a degenerative musculoskeletal disease or with hypertension under balneotherapy [Citation27,Citation51] or patients with fibromyalgia [Citation52]. With a longer intervention period, heat therapy can be an option for ameliorating low-grade inflammation observed in obesity and T2DM. It can be connected with increased expression of HSPs, as mentioned above [Citation53].
The bodyweight lowering effect of heat therapy is unclear: probably combined and longer therapy is needed to achieve the required effect, but that is known that the human body during heat adaptation is lowering its resting metabolic rate (RMR) to aid in the reduction of resting core body temperature [Citation54]. This would result in the reduction of calory expenditure and increase in weight gain in sitting individuals. However hot water bathing results in acute rises of RMR during the period of heating [Citation55]. Passive heating has a beneficial effect on vascular dysfunction and systemic and diastolic blood pressure [Citation56]. Increased skin blood flow through alteration of the shear pattern of arterial blood flow finally enhances vascular remodeling and endothelial function [Citation57]. Due to repeated heat exposure, increased expression of HSP90 results in an increased amount of nitric oxide, consequential vasodilatation, reduce sympathetic activity [Citation58]. It offers an opportunity to improve metabolic and cardiovascular function and reduce cardiovascular events risk [Citation59].
In summary, chronic heat therapy has promising effects on cardiometabolic dysfunction in patients with T2DM. Further randomized controlled study with longer intervention is needed to detect beneficial results for high-risk T2DM patients who cannot exercise regularly.
Strengths and limitation
Our study is the most comprehensive systematic review and meta-analysis in this field. One of the strengths of this study is that the rigorous methodology adhering to the latest international standards was applied. Furthermore, currently, this meta-analysis contains the most outcomes relevant to this topic. This study has several limitations as well. Based on our assessment, the risk of bias was moderate/some concern or high in most studies. These studies contain relatively low numbers of patients, which could contribute to the borderline significant results by decreasing the analyses' statistical power. Some of our results are affected by statistical heterogeneity, probably due to the slight differences in the interventions.
Implication for practice and research
Heat therapy seems to be a good candidate to complement the standard treatment of these patients. Good quality randomized controlled trials are required to elucidate the potential of heat therapy in T2DM and further specify the target population of this treatment who benefit most from heat therapy.
Our findings support the potential role of heat therapy in the treatment of T2DM. Despite the non-significant results, a clear tendency toward improved glycemic control was observed. To enhance the role of these effects, further animal studies could be helpful as well.
Previous reports
Hereby, we state that there is no previous submission or report that might be regarded as redundant publication of this work.
Ethical approval
No ethical approval was required for this review, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduct or interpretation of our study.
Supplemental Material
Download PDF (597.2 KB)Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
The sponsor or the funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Additional information
Funding
References
- IDF Diabetes Atlas. 7th ed. Brussels, Belgium: IDF Executive Office; 2015.
- Ling W, Huang Y, Huang Y-M, et al. Global trend of diabetes mortality attributed to vascular complications, 2000-2016. Cardiovasc Diabetol. 2020;19(1):182.
- Tancredi M, Rosengren A, Svensson A-M, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732.
- Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335.
- Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. IJMS. 2020;21(17):6275.
- Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223.
- Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275.
- Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the american diabetes association. Diabetes Care. 2016;39(11):2065–2079.
- Pei L, Wang Y, Sun CY, et al. Individual, social and environmental predictors of regular exercise among adults with type 2 diabetes and peripheral neuropathy in China. Int J Nurs Pract. 2016;22(5):451–460.
- Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604–609.
- Laukkanen T, Kunutsor SK, Khan H, et al. Sauna bathing is associated with reduced cardiovascular mortality and improves risk prediction in men and women: a prospective cohort study. BMC Med. 2018;16(1):219.
- Zaccardi F, Laukkanen T, Willeit P, et al. Sauna bathing and incident hypertension: a prospective cohort study. Am J Hypertens. 2017;30(11):1120–1125.
- Ukai T, Iso H, Yamagishi K, et al. Habitual tub bathing and risks of incident coronary heart disease and stroke. Heart. 2020;106(10):732–737.
- Ely BR, Clayton ZS, McCurdy CE, et al. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2019;317(1):E172–E182.
- Hesketh K, Shepherd SO, Strauss JA, et al. Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am J Physiol Heart Circ Physiol. 2019;317(1):H114–H123.
- Hoekstra SP, Bishop NC, Faulkner SH, et al. Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J Appl Physiol (1985). 2018;125(6):2008–2018.
- Pallubinsky H, Phielix E, Dautzenberg B, et al. Passive exposure to heat improves glucose metabolism in overweight humans. Acta Physiol (Oxf). 2020;229(4):e13488.
- Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab. 2014;3(8):781–793.
- Oster H. Getting hot about diabetes-Repeated heat exposure improves glucose regulation and insulin sensitivity. Acta Physiol (Oxf). 2020;229(4):e13524.
- Cullen T, Clarke ND, Hill M, et al. The health benefits of passive heating and aerobic exercise: to what extent do the mechanisms overlap? J Appl Physiol (1985). 2020;129(6):1304–1309.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177–188.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- Beever R. Do far-infrared saunas have cardiovascular benefits in people with type 2 diabetes? Can J Diabetes. 2010;34(2):113–118.
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924–925.
- Koçak FA, Kurt EE, Milletli Sezgin F, et al. The effect of balneotherapy on body mass index, adipokine levels, sleep disturbances, and quality of life of women with morbid obesity. Int J Biometeorol. 2020;64(9):1463–1472.
- Oláh M, Koncz Á, Fehér J, et al. The effect of balneotherapy on antioxidant, inflammatory, and metabolic indices in patients with cardiovascular risk factors (hypertension and obesity)-a randomised, controlled, follow-up study. Contemp Clin Trials. 2011;32(6):793–801.
- Qiu Y, Zhu Y, Jia W, et al. Spa adjuvant therapy improves diabetic lower extremity arterial disease. Complement Ther Med. 2014;22(4):655–661.
- Bathaie SZ, Jafarnejad A, Hosseinkhani S, et al. The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26(6):577–585.
- Chung J, Nguyen A-K, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739–1744.
- Kondo T, Sasaki K, Matsuyama R, et al. Hyperthermia with mild electrical stimulation protects pancreatic β-cells from cell stresses and apoptosis. Diabetes. 2012;61(4):838–847.
- Kuennen M, Gillum T, Dokladny K, et al. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R524–33.
- Habich C, Sell H, Burkart V. Regulatory role of heat shock proteins in the pathogenesis of type 1 and type 2 diabetes. CIR. 2017;13(1):82–90.
- Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature (Austin)). 2016;3(1):119–145.
- Maley MJ, Hunt AP, Stewart IB, et al. Passive heating and glycaemic control in non-diabetic and diabetic individuals: a systematic review and meta-analysis. PLoS One. 2019;14(3):e0214223.
- Kondo T, Goto R, Ono K, et al. Activation of heat shock response to treat obese subjects with type 2 diabetes: a prospective, frequency-escalating, randomized, open-label, triple-arm trial. Sci Rep. 2016;6:35690.
- Kamioka H, Mori Y, Horiuchi T, et al. Association of daily Home-Based hot water bathing and glycemic control in ambulatory japanese patients with type 2 diabetes mellitus during the COVID-19 pandemic: a multicenter cross-sectional study. DMSO. 2020;Volume 13:5059–5069.
- Nava R, Zuhl MN. Heat acclimation-induced intracellular HSP70 in humans: a meta-analysis. Cell Stress Chaperones. 2020;25(1):35–45.
- Park HS, Lee JS, Huh SH, et al. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. Embo J. 2001;20(3):446–456.
- Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278(37):35272–35278.
- Ely BR, Francisco MA, Halliwill JR, et al. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol. 2019;317(5):R630–R640.
- Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62(2):98–103.
- Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078–802078.
- Booth A, Magnuson A, Fouts J, et al. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig. 2016;26(1):25–42.
- Ghadge AA, Khaire AA. Leptin as a predictive marker for metabolic syndrome. Cytokine. 2019;121:154735.
- Ragino YI, Stakhneva EM, Polonskaya YV, et al. The role of secretory activity molecules of visceral adipocytes in abdominal obesity in the development of cardiovascular disease: a review. Biomolecules. 2020;10(3):374.
- Blüher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64(1):131–145.
- Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22.
- Fioravanti A, Adamczyk P, Pascarelli NA, et al. Clinical and biochemical effects of a 3-week program of diet combined with spa therapy in obese and diabetic patients: a pilot open study. Int J Biometeorol. 2015;59(7):783–789.
- Rość D, Adamczyk P, Boinska J, et al. CRP, but not TNF-α or IL-6, decreases after weight loss in patients with morbid obesity exposed to intensive weight reduction and balneological treatment. J Zhejiang Univ Sci B. 2015;16(5):404–411.
- Oláh M, Koncz A, Fehér J, et al. The effect of balneotherapy on C-reactive protein, serum cholesterol, triglyceride, total antioxidant status and HSP-60 levels. Int J Biometeorol. 2010;54(3):249–254.
- Maeda T, Kudo Y, Horiuchi T, et al. Clinical and anti-aging effect of mud-bathing therapy for patients with fibromyalgia. Mol Cell Biochem. 2018;444(1-2):87–92.
- Hoekstra SP, Bishop NC, Leicht CA. Elevating body termperature to reduce low-grade inflammation: a welcome strategy for those unable to exercise? Exerc Immunol Rev. 2020;26:42–55.
- Byrne HK, Wilmore JH. The effects of a 20-week exercise training program on resting metabolic rate in previously sedentary, moderately obese women. Int J Sport Nutr Exerc Metab. 2001;11(1):15–31.
- James TJ, Corbett J, Cummings M, et al. Timing of acute passive heating on glucose tolerance and blood pressure in people with type 2 diabetes: a randomized, balanced crossover, control trial. J Appl Physiol (1985). 2021;130(4):1093–1105.
- Brunt VE, Howard MJ, Francisco MA, et al. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol. 2016;594(18):5329–5342.
- Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387.
- Harris MB, Mitchell BM, Sood SG, et al. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol. 2008;104(5):795–802.
- Ely BR, Clayton ZS, McCurdy CE, et al. Meta-inflammation and cardiometabolic disease in obesity: Can heat therapy help? Temperature (Austin). 2018;5(1):9–21.