Abstract
Introduction
MRI guided focused ultrasound (MRgFUS) is a noninvasive technique for treating uterine fibroids. The presence of abdominal scars can limit the number of women eligible for the procedure, due to absorbance of beam energy. The goals of this study were to assess the number of women that fit the procedure and to compare outcomes among women with or without abdominal scars.
Material and methods
A prospective cohort study of all women that were interested in MRgFUS in a single University-Affiliated Hospital between November 2012 and December 2019. Rates of women that were referred to further screening, fulfilled selection criteria and underwent the procedure were compared between patients with or without abdominal scars. We evaluated the treatment parameters of the two groups and used linear regression model predict non-perfused volume (NPV) at the end of the process.
Results
Out of 701 patients, 21.8% were suitable for MRgFUS. Women with scars had significant lower NPV compared with women without scars (60% versus 82.4%, p = 0.021). No serious adverse events were reported in both groups. Linear regression models showed that fibroids' volume, stopping the treatment due to severe pain and the presence of abdominal scars had a statistically significantly negative effect on NPV (betas: −11.51, −6.96, and −6.29, p-values: <0.001, 0.003, and 0.007 respectively), while number of sonication had a statistically significantly positive effect on NPV (beta = 5.98, p = 0.011).
Conclusion
Regardless of strict inclusion criteria, MRgFUS treatment is less efficient among women with abdominal scars, although still feasible for those who are interested in noninvasive option.
Introduction
The number of women seeking minimally invasive alternatives for the treatment of uterine fibroids has increased over the last decade [Citation1–4]. Magnetic resonance-guided focused ultrasound (MRgFUS) is a noninvasive thermo-ablative, technique, performed in outpatient clinics and designed to improve fibroid-associated symptoms with a very low risk profile compared to surgical procedures [Citation5,Citation6].
MRgFUS is not pertinent in all patients with symptomatic leiomyomas as technical limitations limit the number of available candidates [Citation7]. The presence of a scar in the desired ultrasound beam pass could potentially restrict its applicability and increase the risk of adverse effects. Fibrotic tissue could absorb the US energy and cause intolerable pain and even skin burns [Citation8–11].
Given the growing number of women with previous laparotomies seeking noninvasive treatments for fibroid associated symptoms, the goal of the current study was to compare the suitability of women with or without abdominal scars for MRgFUS treatment and to assess the effect of uterine scars on treatment outcome among the two groups.
Materials and methods
The study was approved by the Institutional Review Board of our hospital (SMC #1987-15). As patients' identifiers were removed and data was collected routinely as part of the policy follow-up of our patients, our IRB waived the requirement for signed informed consent. All women with symptomatic uterine fibroids that presented for MRgFUS treatment in a single university-affiliated medical center between November 2012 and December 2019 were prospectively followed.
Inclusion criteria for MRgFUS therapy in our center were previously published [Citation12]. In brief, pre-menopausal women who had clinically symptomatic uterine fibroids (maximal fibroid diameter 10 cm, as the treatment is less efficient in larger fibroids) without significant medical conditions, claustrophobia or morbid obesity were evaluated [Citation12]. Abdominal scars were not considered exclusion criteria. In contrast to previous studies, desire for future fertility was not a contraindication to the treatment in the current study.
Our two steps of screening began with medical and gynecological evaluations including self-reported symptom severity score questionnaire (uterine fibroid symptom-quality of life, UFS-QOL-SSS) [Citation13]. Those patients who were found to be clinically eligible were scheduled for pelvic screening MRI with gadolinium (Dotarem, Guerbet, France) [Citation14,Citation15].
MRI study with the administration of intravenous (IV) gadolinium contrast was performed, including multi-planar T2W images and T1W images pre- and post-IV gadolinium contrast administration. The parameters considered on MRI included the number and volume of the fibroids, their location and their signal intensity. Signal intensity was compared to the myometrium as was heterogeneity on T2- and T1-weighted MRI. Fibroid volume was calculated using the ellipsoid formula: (length × width × height) × 0.523 [Citation16]. Women with fibroid diameter of >8 cm were offered 3 months of long acting GnRH analogues before the treatment. MRI was performed 3 months after the first injection. Only cases in which the fibroid(s) shrank and their diameter was smaller than 8 cm were eligible for treatment. Upon MRI performance we excluded women with massive abdominal scarring.
All treatments were performed by one experienced radiologist (Y. I.). The MRgFUS system (ExAblate 2100, InSightec, Haifa, Israel) was integrated on a standard 1.5 T MR system (GE Medical Systems, Milwaukee, WI). ExAblate 2100 system incorporates several software and hardware upgrades. This includes a new 3D axis US transducer capable of rotation and tilt as well as movement in the vertical plane, and thus allows the transducer to be placed much closer to the patient's skin surface. This results in a lower energy density in the abdominal wall. This system also allows selective shutdown of transducer elements, thus allowing for sculpting and steering of the US beam. At the end of the treatment, the percentage of non-perfused volume (NPV) of the fibroids was calculated as the non-enhanced volume divided by the total fibroid volume.
Outcome variables
Data collected from patients’ medical records included demographic characteristics, symptoms (abnormal bleeding, abdominal pressure and urinary symptoms), presence of previous abdominal scars, and baseline UFS-QOL-SSS. Analyses of the MRI studies included the number, total volume, and location of the fibroids, as well as their characteristics and texture on the T1- and T2-weighted MRI study before treatment.
For the assessment and comparison of treatment outcomes at the end of the treatment between the groups, we performed a sub-analysis including only patients that had 1–3 fibroids. We excluded women that were pretreated with GnRH analogues, were scheduled for more than one MRgFUS treatment or had multiple fibroids, and were a priory scheduled for the treatment of the dominant fibroid only. Women that were pretreated with GnRH analogues were excluded as the current analysis aimed to assess the effectivity of MRgFUS solely, without the addition of other medications. Cases of multiple fibroids were excluded from the sub-analysis as not all the fibroids were treated, thus leading to under estimation of the NPV (calculated per total fibroids' volume). We compared the rates of technical issues (i.e., need for bowel manipulation), the presence of warming of the rectus muscles or subcutaneous tissue and complaints of intolerable pain that led to premature cessation of the procedure as well as the number of sonication and the NPV at the end of the treatment. The presence of warming of the rectus muscles was based on MRI images at the end of the treatment. Sonication parameters differed according to fibroid parameters (size, location, type, vascularity, etc.), relation of fibroid to other pelvic organs, presence of bowel, nerves, scarring in the beam path, patients ability to endure the pain etc. On an average, sonications were 20–30 s long, with the energy of 2000–6000 Joules.
Statistical analysis
The validity of the data was tested with the use of Q–Q plots and Kolmogorov–Smirnov tests. Data are described as means ± standard deviation for continuous data or median and interquartile range for non-parametric variables. Comparison between continuous variables was conducted with Student t test or Mann–Whitney U test, as appropriate. The Chi-square and Fisher's exact tests were used for comparison between categorical variables. Significance was accepted at a probability value of <0.05. All the tests were two-sided and a p-value ≤ 0.05 was considered statistically significant.
Linear regression was used to predict the percentages of NPV. Data preparation included imputation of missing values using the median of each feature, and normal normalization. Due to co-linearity (Pearson correlation coefficient > 0.85) between abdominal scars and number of laparotomies, and between the number of previous pregnancies and the number of previous deliveries, we did not include the number of laparotomies nor the number of previous deliveries in the linear regression model. Statistical analyses were conducted using MATLAB (version: R2019a).
Results
Patients
During the study period, 701 patients attended our MRgFUS outpatient clinic seeking conservative treatment for uterine fibroids. The median age of the patients seeking for the treatment was 44.0 years [interquartile range (IQR) 39.0–48.0], with a median UFS-QOL-SSS of 26.0 (IQR 20.0–31.0). Their main complaints were bleeding; urinary symptoms and/or abdominal pressure. Four hundred and fifty women (65.0%) had experienced at least one pregnancy in the past. One hundred seventy-four (24.8%) of the patients had at least one transverse abdominal scar (range 1–3) (Group 1) and 527 (75.2%) had no previous laparotomies (Group 2). Fibroid associated symptoms (i.e., bleeding, urinary symptoms or pain) as well as infertility rates did not differ between the groups. Patients' demographic are shown in .
Table 1. Patients' demographics.
A total of 455/701 women who attended our outpatient clinic (64.9%) were further referred to diagnostic MRI based on their medical history, fibroid associated symptoms and screening gynecological ultrasound. The percentage of women that fulfilled the inclusion criteria and referred to MRI was significantly lower for women in group 1 compared with group 2 (58.4% versus 67.2%, p = 0.038). Of the 397 women that actually preformed the screening MRI, 153/701 (21.8%) were found to be clinically and anatomically eligible for MRgFUS treatment. Eligibility rates according to the imaging were 16.8% and 23.5% for Groups 1 and 2, respectively (p = 0.06). Ninety-three women (13.3%) scheduled MRgFUS treatment, 54/701(7.7%) decided not to undergo the procedure, mainly due to the costs which are not covered by all Israeli medical insurances. The rates of women who ultimately performed a diagnostic MRI as well as the eligibility for treatment did not differ between the groups. Four treatments were canceled on the day of the procedure due to technical issues with the equipment and another one was stopped at the planning stage due to the patient's request (all in women from Group 2) ().
Table 2. Comparison of rates of eligibility to MRgFUS treatment among patients with or without abdominal scars.
The final treatment analysis included 72 women (15 in Group 1 and 57 in Group 2). Median patients' age and IQR for those with abdominal scars [45.0 years old (43.0–48.0)] or without abdominal scars [43 years old (38.5-47.0)] did not differ between the groups (p = 0.06). The mean fibroid volume of the treated patients was 142 cm3, patients underwent 59 ± 17 sonication during the procedure and the mean ± SD NPV at the end of the process was 70%±23.4. UFS-QOL-SSS, BMI, mean number of fibroids and fibroid volume did not differ between the two groups. Patients' demographics are shown in .
Table 3. Treatment outcomes among women with or without previous laparotomies.
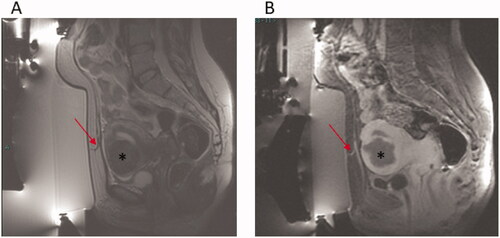
Abnormal areas of enhancement were identified on MRI within the subcutaneous tissue at the end of the treatment without occurrences of skin texture changes or redness were significantly more prevalent in women in group 1 compared with women in group 2 (46.7% versus 15.8%, p = 0.017). and show MRI images of two patients with similar abdominal scars. shows abnormal areas of enhancement within the abdominal scar and surrounding subcutaneous tissue, while shows no significant heating in the abdominal scar and surrounding subcutaneous tissue at the end of the procedure. The mean NPV at the end of the treatment was significantly lower among women from group 1 compared with group 2 (60% versus 82.4%, p = 0.02). No serious adverse events were reported in both groups.
Figure 1. A 44 years old patient with previous C.S. (A) Sagittal T2w image pre-treatment showing scar in abdominal wall (arrow) and uterine fibroid (*). (B) Sagittal CE FS T1w image post-treatment showing marked heating in abdominal scar and surrounding subcutaneous tissue (arrow) and the spotty/small NPV in the uterine fibroid (*).
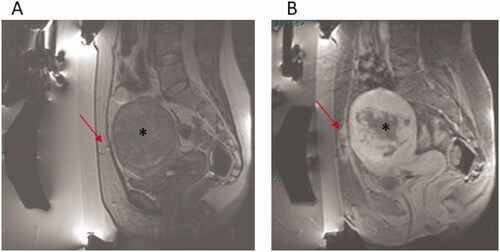
Figure 2. A 50 years old patient with previous C.S. (A) Sagittal T2w image pre-treatment showing scar in abdominal wall (arrow) and uterine fibroid (*). (B) Sagittal CE FS T1w image post-treatment showing no significant heating in the abdominal scar and surrounding subcutaneous tissue (arrow) and the extensive NPV in the uterine fibroid (*).
We further performed both linear and logistic regressions in order to assess the factors that predict successful treatment. Fibroid volume was the feature most contributing to the linear regression, with a negative effect (beta= −11.5, p < 0.001), meaning higher volume leading to lower NPV percentages. Stopping the treatment due to severe pain as well as the presence of abdominal scars had a statistically significantly negative effect on NPV (betas: −6.96, −6.29, p-values: 0.003, 0.007, respectively), while number of sonication had a statistically significantly positive effect on NPV (beta = 5.98, p = 0.011) ().
Table 4. Linear regression to predict NPV at the end of the treatment.
Discussion
In this large cohort of 701 women, evaluated for eligibility for MRgFUS, 22% fulfilled the inclusion criteria after medical intake and imaging and 13.4% underwent the procedure. Significantly fewer women with previous laparotomies were referred to MRI screening, and there was a non-significant trend for lower eligibility for the procedure according to the results among women with scars. In a sub-analysis of women who underwent MRgFUS treatment, there were more cases of excessive warming of the rectus muscles or the abdominal subcutaneous tissue among women with previous laparotomies. Further, the NPV at the end of the treatment in this group was significantly lower, compared to women without abdominal scars. Linear regression results emphasize the significance of and the presence of abdominal scars, stopping the treatment prematurely due to severe pain and the number of sonication on treatment outcome.
One of the main constraints of MRgFUS treatment for uterine fibroids is the relatively low number of patients that can benefit from this therapy due to the strict inclusion criteria [Citation7,Citation17]. The number of referred patients with fibroids appropriate for the procedure in previous studies ranged from 14% to 74% depending on the selection criteria [Citation1,Citation8,Citation18]. Therefore, our aim should be to examine the validity of existing inclusion and try to augment eligibility without affecting the low risk profile of the procedure.
Abdominal scars are well recognized as a challenge for MRgFUS therapy of uterine fibroids [Citation19]. The fibrotic scar tissue has different acoustic properties, it can divert the energy of the ultrasound beam and lead to increased absorption of acoustic energy in the skin and the subcutaneous tissue [Citation9].
The current study was performed using ExAblate 2100 system. This system is an upgrade to the older versions, with many features the can enhance treatment results while increasing both safety and widening the range of fibroids that can be treated. However, we do believe that the results and conclusions of the current study have applicability and generality to other FUS systems and operating teams. In order to minimize the risk of skin burning and excessive warming of the rectus muscles or the subcutaneous tissue, we used different maneuvers for positioning the fibroid away from the scar, such as the use of a step gel pad, and the use of ultrasound gel for filling the rectum and for filling and emptying of the bladder. Indeed, 26.7% of the patients in our cohort needed bladder or bowel-interference mitigation strategies. The need for such maneuvers in our cohort was similar for women with or without previous scars. Although no cases of skin burns occurred among our patients, women with abdominal scars had significantly higher rates of abnormal areas of enhancement and heat in subcutaneous tissue compared with those without operations. Heat absorption in the abdominal wall is probably due to the changes in the tissue post-surgery leading to fibrosis that absorb the ultrasound energy and can lead to heat accumulation and thermal damage. Due to the experience and attention of the operating team and the information supplied by the system, we could avoid significant trauma to the surrounding tissue.
The duration of improvement and shrinkage of fibroids is usually correlated with the NPV achieved at the end of the procedure [Citation15,Citation20,Citation21]. NPV at the end of the procedure was significantly higher among women without previous scars compared with patients with scars and might hint for further treatment efficiency.
Our study has several limitations. First, the number of women with a previous abdominal scar treated by MRgFUS for uterine fibroids in this study is relatively small, thus it is possible that it does not estimate correctly the rate of other differences in treatment outcomes between patients with or without scars (i.e., number of women who reported severe pain during the treatment or number of sonication). Further, we excluded from the treatment (based on MRI results) cases with severe abdominal scarring. However, this study has several strengths. To the best of our knowledge, it includes the largest number of women who attended a single medical center interested in MRgFUS treatment. Also, all treatments were performed by a single very experienced physician, thus preventing treatment confounders attributed to the provider.
In conclusion, MRgFUS treatment is a feasible treatment option for women with symptomatic uterine fibroids with previous abdominal scars. However, both patients and physicians should be informed about the possibly lower success rates. Further studies are needed to confirm these results and to assess whether the long-term outcome of these patients is similar to that of patients without laparotomies treated by MRgFUS for uterine fibroids.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Behera MA, Leong M, Johnson L, et al. Eligibility and accessibility of magnetic resonance-guided focused ultrasound (MRgFUS) for the treatment of uterine leiomyomas. Fertil Steril. 2010;94(5):1864–1868.
- Zupi E, Centini G, Sabbioni L, et al. Nonsurgical alternatives for uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:122–131.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22(6):665–686.
- Tan N, McClure TD, Tarnay C, et al. Women seeking second opinion for symptomatic uterine leiomyoma: role of comprehensive fibroid center. J Ther Ultrasound. 2014;2:3.
- Mohr-Sasson A, Machtinger R, Mashiach R, et al. Long-term outcome of MR-guided focused ultrasound treatment and laparoscopic myomectomy for symptomatic uterine fibroid tumors. Am J Obstet Gynecol. 2018;219(4):375 e1–e7.
- Jacoby VL, Kohi MP, Poder L, et al. PROMISe trial: a pilot, randomized, placebo-controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril. 2016;105(3):773–780.
- Duc NM, Keserci B. Review of influential clinical factors in reducing the risk of unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids. Diagn Interv Radiol. 2018;24(5):283–291.
- Zaher S, Gedroyc WM, Regan L. Patient suitability for magnetic resonance guided focused ultrasound surgery of uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):98–102.
- Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(4):1–86.
- Abdullah B, Subramaniam R, Omar S, et al. Magnetic resonance-guided focused ultrasound surgery (MRgFUS) treatment for uterine fibroids. Biomed Imaging Interv J. 2010;6(2):e15.
- Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. Am J Roentgenol. 2004;183(6):1713–1719.
- Machtinger R, Inbar Y, Cohen-Eylon S, et al. MR-guided focus ultrasound (MRgFUS) for symptomatic uterine fibroids: predictors of treatment success. Hum Reprod. 2012;27(12):3425–3431.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30(5):771–777.
- Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85(1):22–29.
- Kroencke TJ, Scheurig C, Kluner C, et al. Uterine fibroids: contrast-enhanced MR angiography to predict ovarian artery supply-initial experience. Radiology. 2006;241(1):181–189.
- Verpalen IM, van 't Veer-Ten Kate M, de Boer E, et al. Development and clinical evaluation of a 3-step modified manipulation protocol for MRI-guided high-intensity focused ultrasound of uterine fibroids. Eur Radiol. 2020;30(7):3869–3878.
- Arleo EK, Khilnani NM, Ng A, et al. Features influencing patient selection for fibroid treatment with magnetic resonance-guided focused ultrasound. J Vasc Interv Radiol. 2007;18(5):681–685.
- Kim YS, Bae DS, Park MJ, et al. Techniques to expand patient selection for MRI-guided high-intensity focused ultrasound ablation of uterine fibroids. Am J Roentgenol. 2014;202(2):443–451.
- Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110(2):279–287.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single Centre. Eur Radiol. 2015;25(5):1317–1328.
