Abstract
Irreversible electroporation (IRE) ablation is gaining popularity over the last decade as a nonthermal alternative to thermal ablation technologies such as radiofrequency ablation (RFA) and Microwave ablation (MWA). This review serves as a practical guide for applying IRE to colorectal cancer liver metastases (CRLM) for interventional radiologists, oncologists, surgeons, and anesthesiologists. It covers patient selection, procedural technique, anesthesia, imaging, and outcomes.
Keywords:
Introduction
Irreversible electroporation (IRE) ablation is gaining popularity over the last decade as a nonthermal alternative to thermal ablation technologies such as radiofrequency ablation (RFA) and Microwave ablation (MWA). This review serves as a practical guide for applying IRE to colorectal cancer liver metastases (CRLM) for interventional radiologists, oncologists, surgeons, and anesthesiologists. It covers patient selection, procedural technique, anesthesia, imaging, and outcomes.
Background
Colorectal cancer primarily metastasizes to the liver; 20–35% of patients present with CRLM at the time of diagnosis, and 50–70% develop metastasis during the disease [Citation1–3]. The median survival of patients with untreated CRLM is only 6–12 months [Citation1]. Resection of oligo-CRLM in conjunction with the latest chemotherapy and biologics improves the 5-year median survival rate to more than 50% [Citation1].
Unfortunately, most patients (70–80%) are ineligible for surgical resection due to comorbidities, extrahepatic disease, or location, size, or number of metastases [Citation4]. Ablation is an effective alternative for oligo-CRLM, for which R0 resection is achievable. Most notably, the CLOCC trial, a randomized phase II clinical trial in 119 patients, demonstrated that aggressive local treatment with ablation could prolong progression-free survival (PFS) and overall survival (OS) in patients with unresectable oligo-CRLM (<10 lesions, no extrahepatic disease) [Citation2].
IRE is a newer ablation technology compared to RFA or and MWA. Unlike RFA and MWA, it is nonthermal. By delivering low energy, high voltage direct electrical pulses to tumor cells, IRE creates permanent holes in the cellular membrane, disrupts homeostasis, triggers apoptosis, and ultimately leads to controlled cell death. Its ability to specifically treat CRLM was demonstrated in COLDFIRE-1, an ablate-and-resect study [Citation5], and COLDFIRE-2 [Citation6], a phase II open-label clinical trial. Unlike RFA and MWA, IRE can be safely performed adjacent to critical vessels and central biliary structures [Citation7–9]. With greater availability of IRE in the United States and abroad, this review aims to inform physicians from all facets of medicine and surgery of the unique applicability of IRE in CRLM ().
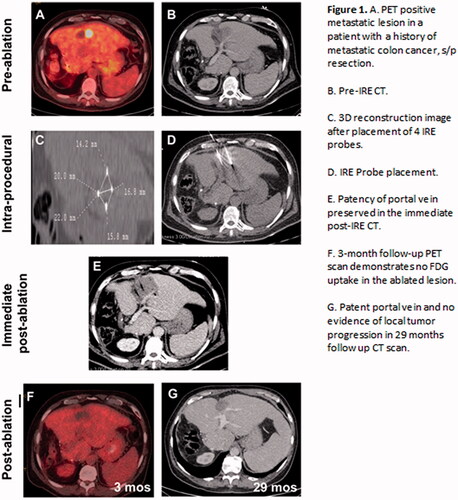
Figure 1. (A). PET positive metastatic lesion in a patient with a history of metastatic colon cancer, s/p resection. (B) Pre-IRE CT. (C) 3D reconstruction image after placement of 4 IRE probes. (D) IRE Probe placement. (E) Patency of portal vein preserved in the immediate post-IRE CT. (F) 3-month follow-up PET scan demonstrates no FDG uptake in the ablated lesion. (G) Patent portal vein and no evidence of local tumor progression in 29 months follow up CT scan.
Patient selection
Consideration of anatomic factors – proximity to vessels
In general, IRE can be performed in nearly all cases where RFA and MWA can be used and in most cases where ablative stereotactic body radiotherapy (SBRT) can be used. However, its main advantage lies in the treatment of tumors in anatomic locations that are unsafe for RFA, MWA, and SBRT (). The latest consensus multidisciplinary consensus guideline recommends IRE use for perihilar and/or perivascular CRLM [Citation10].
Table 1. Indications and contraindications for IRE of CRLM.
IRE is preferred over RFA and MWA when treating tumors encasing or abutting important vessels for two reasons: averting heat-sink effect and maintaining vessel patency. Both MWA and RFA cause heat-induced coagulative necrosis. The efficacy of MWA and RFA near large vascular structures is reduced by the heat sink effect, the local cooling effect from adjacent circulating blood. While MWA protocols may be adjusted to overcome the heat-sink effect with a bigger ablation zone or longer duration of ablation [Citation11], IRE is not limited by the heat sink effect because it is nonthermal. It is efficacious even for lesions directly abutting or encasing large high-flow vascular structures such as portal veins, hepatic arteries, or hemangiomas.
More importantly, whereas all tissues within and near the ablation zone are affected by heat indiscriminately during thermal ablation, IRE limits direct heat to only tissues with direct contact to its electrodes. Cellular death within treatment zone occurs as a result of cellular wall disruption and apoptosis. [Citation12,Citation13]. It kills the vascular mesenchymal cells but entirely maintains the wall’s collagen and elastin structures. This allows the vessel to remain patent until mesenchymal cells repopulate the vessel wall [Citation14]. Multiple clinical studies have validated the safe use of IRE near vessels. Narayanan et al. observed vascular changes in only 4.4% of 158 vessels after IRE ablation of hepatic tumors [Citation7]. Notably, the treated tumors abutted 50 and encased 10 studied vessels [Citation7]. Distelmaier et al. reported no occlusion or narrowing after treating 43 tumors adjacent to major hepatic or portal veins [Citation8]. Tamura et al. demonstrated that when hepatic or portal veins did occlude after IRE, the occlusions were subclinical without ramifications. Most occluded veins were <4 mm. No hepatic vein >4 mm occluded, including 14 veins within the treatment zone [Citation9]. Temporary vessel narrowing caused by reactive spasm have been described, lasting weeks after IRE [Citation15].
Consideration of anatomic Factors - Proximity to biliary structures and bowel
Another common application of IRE is ablation near bile ducts [Citation16], gallbladder [Citation17] and bowel [Citation18]. The safety of IRE within 1.0 cm of these critical structures is well demonstrated. Dollinger et al. successfully ablated 53 hepatic lesions adjacent to 55 major bile ducts. While biliary ductal changes, including mild stenosis or dilatation, were observed on imaging in 15 out of 55 ducts, only three patients developed transient cholestasis that resolved without intervention. Notably, 33 tumors encased the assessed ducts, and 14 were directly abutting the ducts [Citation16].
Biliary manipulation or a history of bilioenteric anastomosis poses a higher risk of postoperative liver abscess for both thermal and nonthermal ablations. This can be mitigated by aggressive preoperative antibiotics [Citation19] starting 2 days before the procedure and continuing for 14 days after the ablation [Citation20].
Consideration of tumor size
IRE demands significant technical expertise in image-guided ablation. Electrode misplacement by a few millimeters may lead to incomplete tumor ablation. Some studies suggest that clinical outcomes are best for lesions ≤3 cm [Citation21,Citation22]. Most recently, a phase II, two-center, single-arm clinical trial (COLDFIRE-2) successfully treated 21 patients with tumors 3-5 cm, and did not detect a higher LTP compared to tumors ≤3 cm (hazard ratio, 1.7; p = .22) [Citation6]. The authors of COLDFIRE-2 attributed the lack of difference in LTP to placement of electrodes bracketing the tumor rather than in the center of the tumor as it is done with thermal ablation [Citation6]. The multidisciplinary consensus group recommended treatment of tumors 3–5 cm if further downsizing systemic therapy is not safe or feasible [Citation10].
Consideration of patient factors
Since IRE ablates cancer by delivering electrical pulses, ablations close to the heart can induce ventricular arrhythmias [Citation23]. Therefore, a thorough cardiac history and an electrocardiogram should be obtained preoperatively. History of ventricular arrhythmias, pacemaker, and implantable cardiac devices (ICD) are contraindications. However, atrial fibrillation does not preclude the patient from IRE [Citation15]. The cardiac gating device with IRE synchronizes with the heart rhythm and delivers each pulse during the absolute refractory period after each R wave [Citation24]. Pacer pads are recommended on all patients.
Epilepsy and a history of seizures are listed contraindications for IRE since it is speculated that its electrical pulses may provoke a seizure. However, a study has yet to report seizure triggered by IRE [Citation24]. Interestingly, IRE have been performed in canine intracranial meningioma models [Citation25]. Nevertheless, more trials are necessary before treating patients with epilepsy.
Cardiac and epileptic considerations aside, the patient selection for IRE is the same as that of RFA and MWA. IRE is recommended for patients with oligo-CRLM who are not resection or transplant candidates. However, it should be limited to patients with Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and American Society of Anesthesiologists score of 0–3 [Citation15]. As per the Society of Interventional Radiology consensus guideline, an INR of ≤1.5–1.8 and platelet level >50 × 10^9/L is recommended. If coagulopathy is not correctable, IRE should not be performed [Citation26].
Procedure
Equipment and techniques
The current manufacturer for IRE (NanoKnife, AngioDynamics, Latham, NY) provides a generator, a footswitch, and a cardiac synchronization device with five leads. Two to six 19-gauge unipolar electrodes connect to the generator. Each electrode is covered by a sheath, which can be retracted to expose up to 40 mm of active electrode tip in 5-mm increments.
IRE requires careful planning and placements of electrodes in often very challenging locations. To prevent central narrowing of the ablation zone created by long inter-electrode distance, the electrodes should be no more than 2.5 cm apart. Moreover, the electrodes should be parallel, not deviating more than 10 degrees, with electrode tips all at same plane [Citation12].
The ablation zone extends 5-mm outward from the probes [Citation27]. Treatment margin is equally important with IRE as with thermal ablation, and should be at least 5–10 mm beyond the edge of the tumor[Citation11,Citation12]. A 10 mm margin was associated with no LTP [Citation11]. Therefore, to achieve >5-mm margin, the electrodes should be placed at the edge or just outside of the tumor, bracketing the targeted tumor. The exposed active tip should be 2.0 cm in liver ablations to prevent under- or overcurrent. If the tumor is larger than 2.0 cm along the access trajectory, the electrodes can be pulled back in 0.5–1 cm increments for repeat ablation of the more superficial portions [Citation12,Citation13].
Before delivering any electrical pulses, one must verify with the anesthesia team that full neuromuscular relaxation is achieved (see Anesthesia Care). Ten test pulses are then delivered between each electrode pair with a target initial current level of 20–50 Amperes. The remainder 70 pulses are then administered, with a goal increase of 12–15 Amperes from baseline. While there is no consensus, most cases have been performed with a 70 or 90 µs pulse length [Citation5].
Intraoperative CT commonly demonstrates a hypodense ablation zone with gas at the electrode tips due to electrolysis of water into oxygen and hydrogen [Citation28]. The shape and size of the ablation zone on CT are accurate predictors of the treatment zone on pathologic specimens [Citation12].
Anesthesia care
IRE has unique anesthesia needs, including preparation for possible arrhythmias, hemodynamic swings, and the need for complete muscular relaxation.
Ventricular arrhythmias may occur during IRE even with cardiac synchronization. Systolic and diastolic blood pressure often increases by 20–45 mmHg during ablation [Citation24]. Therefore, essential to safe IRE ablation is briefing the anesthesia team for possible ectopy and hemodynamic changes. External defibrillation pacer pad placement can be considered in high-risk patients [Citation24] and some centers routinely place these pads in all patients. Propofol and remifentanil can mitigate blood pressure and heart rate increases from IRE [Citation5].
Also unique to IRE is the requirement for complete muscular relaxation during ablation. Inadequate paralysis during electrical pulse delivery can lead to generalized muscle contraction [Citation24]. Therefore, immediately before test pulse delivery, the interventionalist should verify that the anesthesiologist completely paralyzed the patient. Even with complete paralysis, IRE triggers local contractions of abdominal wall muscles and sometimes the diaphragm. Lastly, lower-body hot-air blankets are recommended to maintain patient body temperature in the cold CT scanner [Citation29].
Technical limitations
The greatest challenge for widespread adaptation of IRE is that it is often technically demanding, even for experienced physicians. Unlike thermal ablations, it requires careful placement of parallel probes in tumors often adjacent to vessels, bile ducts, and other vital structures, while avoiding ribs and bowel. COLDFIRE-2 demonstrated much higher LTP rate in their first five out of 50 participants [Citation6]. Treatment zone prediction can be challenging within heterogeneous tissues with different tissue conductivities. Therefore, close interval follow-up for possible retreatment may be necessary. Its availability will depend on local resources.
Postprocedural care
Postoperative pain after liver IRE is typically mild and similar to that of RFA [Citation30]. The patient can be discharged home when deemed appropriate as early as the following day.
Transient rapid elevation of AST and ALT within the first 24 h is typical. They resolve spontaneously as early as postoperative day 4. Bilirubin may rise and resolve as quickly as AST and ALT. The etiology of rapid changes in both transaminase and bilirubin is not clear and may be due to the release of cellular contents after electroporation. Rarely, a gradual rise in bilirubin may occur and can indicate cholestasis. In such cases, it can take days or weeks for bilirubin to normalize [Citation31].
Safety and complications
Postoperative complication rates after IRE are comparable to that of RFA [Citation5] and MWA [Citation32], without a difference in the frequency and length of postoperative intensive care unit stay [Citation32]. A 2014 systematic review of 16 studies with 129 IRE treated liver tumors reported an overall complication rate of 16%, all of which were minor (Grade I and II) [Citation5]. Many of the included complications were directly related to probe punctures, including hemothorax, pneumothorax, and pleural effusions. Only 3 cases of biliary obstructions were identified and included in the complication analysis [Citation5]. Notably, two of these cases were due to local tumor progression rather than ablation-induced biliary stenosis [Citation33].
More recently, a large single-center retrospective analysis of 85 IRE ablations of 114 liver tumors reported a 7.1% major (Grade III and IV) and 18.8% minor (Grade I and II) complication rate [Citation34]. The most common major complication was liver abscess (4.7%), and incidence correlated with the presence of bilioenteric anastomosis, a known risk factor for liver abscess after ablation or transarterial embolization. This rate is only mildly higher than the reported liver abscess rate after RFA (0.4–3.1%) [Citation34] and may be considered acceptable. In COLDFIRE-2, Meijerink et al. reported higher overall complication rate of 40%, however many of his procedures were concomitantly performed with thermal ablation and surgical resection. Notably, the only reported death in literature was related to an infected biloma [Citation6].
Interestingly, although IRE requires more probes per treatment than RFA and MWA, its bleeding risk is minimal. Out of the 16 studies within the 2014 systematic review, no bleeding complication was reported [Citation5]. In the large retrospective analysis of 114 liver tumors, only 2 cases of bleeding requiring transfusion or embolization were reported [Citation34]. Similarly, another study of 43 hepatic IREs reported only 2 cases of subcapsular hematoma and 1 case of arterioportal fistula [Citation8]. In COLDFIRE-2, one bleeding event requiring transarterial coil embolization was reported [Citation6].
As discussed previously, IRE can be safely used near vessels and bile ducts with reduced vascular or biliary complication rates. The incidence of ventricular arrhythmia is rare, between 0–2.5% [Citation35].
Follow up
Imaging and frequency
After discharge, cross-sectional imaging and new labs should be obtained one month postoperatively, then every 3 months [Citation15]. Contrast-enhanced MRI is the preferred modality, although contrast-enhanced CT is also acceptable [Citation15]. Positron emission tomography (PET) can be performed on postoperative day 0 or 1 if patient has not been given meals or medications in dextrose solutions. Immediate postoperative PET is helpful for assessing the completeness of ablation. However, PET-CT in days, up to months, following ablation can demonstrate a rim of radioactivity, making it challenging to distinguish inflammation from local tumor progression.
Criteria for progression
The modified Response Evaluation Criteria in Solid Tumors (m-RECIST) is the most commonly used method to track local response. However, enhancement and size on imaging do not fully represent pathologic response after IRE. Padia et al. have shown that large areas of peripheral enhancement may represent areas of reversible electroporation, which can involute over time [Citation36], albeit this study evaluated only IRE of hepatocellular carcinoma.
The Metabolic Imaging and Marker Integration (MIAMI) criteria is an alternative which includes PET-activity and carcinoembryonic antigen levels. A small retrospective analysis in 29 patients demonstrated that MIAMI was a better predictor for PFS and OS than RECIST and m-RECIST [Citation18]. This has not yet been confirmed on a larger scale.
Efficacy and outcomes compared to other modalities
The randomized phase II CLOCC trial placed percutaneous ablation on the map for patients with oligo-CRLM. Combining ablation (with or without resection) with systemic treatment improved OS. The median PFS in the combined treatment arm was 16.8 months compared to 9.9 months in the systemic-only treatment arm.
IRE extends ablation as a therapeutic option for many patients who are not RFA or MWA candidates. The improved survival outcomes from the CLOCC trial have been extrapolated to IRE in clinical practice. Unfortunately, because IRE is typically reserved for patients who aren’t RFA or MWA candidates, a direct, matched and unbiased comparison between IRE and thermal ablation is not feasible. In addition, most IRE studies are small, observational, and include a heterogeneous population of liver tumors including HCC, CRLM, and other metastasis, making analysis limited to CRLM patients difficult.
Hosein et al. reported 36% complete response, 21% partial response, 25% stable disease, and 18% progressive disease with a median follow up of 11 months in 58 CRLM following IRE [Citation18]. Repeat ablations were often performed, with a subsequent local control rate of 74–96% [Citation6]. Other studies with more heterogeneous pathologies reported total local tumor progression (LTP) rate of 13.4–37% at one year [Citation35]. Despite inherently biased patient selection, IRE has an acceptable LTP rate compared to those of RFA (3–30%) and MWA (3–13%) [Citation35].
Some studies showed that IRE is more effective in smaller tumors [Citation22, Citation35, Citation37]. This is not a surprise given how technically demanding this procedure is, requiring multiple precisely placed electrode to achieve a complete ablation. Cannon et al. treated 44 patients with 48 CRLM, HCC, and other liver metastasis with IRE and found a local recurrence-free survival (LRFS) of 98% at 1 year for lesions <3 cm, but an overall LRFS of 60% for all lesions [Citation22]. Freeman et al showed a 1-year LRFS of 100% for tumors <2 cm, and 83.6% for all lesions (mean 2.0 cm, range 1.0–5.0 cm) [Citation21]. Some did not find a difference in LTP by size [Citation18]. Over time, increasing success with larger tumor ablations has been reported and a 5-cm limit has been recommended by a consensus panel [Citation15].
At least two studies have found that body mass index (BMI) is an independent predictor of LTP. A leading theory is that fatty tissue is less electrically conductive and causes IRE to be less effective [Citation35]. In addition, fatty liver may limit delineation of tumor border, and lead to treatment failure [Citation35].
Direct comparison between IRE, surgery and SBRT is difficult. Most participants in IRE clinical trials have been heavily pretreated, and therefore direct comparison of overall survival rates with other technique carries an inherent bias [Citation6]. However, CRLM is often radioresistant, worse in patients who have had chemotherapy [Citation38]. A large Dutch-Belgian registry including 668 liver metastases treated with SBRT showed a local control rate of only 87% at 1 year, 75% at 2 years, and 68% at 3 years. Those with CRLM and lung metastasis responded less to SBRT than other tumor subtypes [Citation38]. The survival benefit in patients with local control is also only seen in patients with a projected OS of more than 12 months [Citation38]. In 2021, the prospective Amsterdam CORE registry including 199 patients demonstrated that thermal ablation has better median OS (27.4 vs 54.0 months), LPFS and local control than SBRT [Citation39]. Often SBRT is performed when thermal ablation is not an option (e.g. perihilar/perivascular location). These lesions can now be treated by IRE primarily or secondarily after SBRT failure. Only patient with very poor health (ECOG 3, ASA 4, or CCI 9–10) remain better SBRT candidates than ablation candidates [Citation10].
Conclusion
In summary, ablation of CRLM in conjunction to systemic therapies improves patient survival. IRE extends this option to more patients because it is non-thermal and is safe to use near major vessels or bile ducts. Attention to cardiac screening, precise electrode placement, and coordinate anesthesia care is unique to IRE and required for safe ablation.
The literature review showed that IRE has acceptable efficacy and complication rate compared to RFA and MWA in the liver in existing studies with IRE mostly reserved for tumors that are poor thermal-ablation candidates. At this time, long-term survival outcomes are limited to small series. Larger matched comparisons between IRE and other treatment options will further substantiate its use in CRLM beyond treatment reserved for non-thermal ablation candidates.
Disclosure statement
YK: Consultant, NeuWave Medical Inc. Consultant, Angiodynamics Inc.; NW: None; GN: Consultant, Angiodynamics
References
- Vera R, González-Flores E, Rubio C, et al. Multidisciplinary management of liver metastases in patients with colorectal cancer: a consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin Transl Oncol. 2020;22(5):647–662.
- Ruers T, Van Coevorden F, Punt CJA, et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. JNCI: Journal of the National Cancer Institute. 2017;109(9):djx015.
- Puijk RS, Ruarus AH, Vroomen LGPH, et al.; COLLISION Trial Group. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) – a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18(1):821.
- Jeyarajah DR, Doyle MBM, Espat NJ, et al. Role of yttrium-90 selective internal radiation therapy in the treatment of liver-dominant metastatic colorectal cancer: an evidence-based expert consensus algorithm. J Gastrointest Oncol. 2020;11(2):443–460.
- Scheffer HJ, Nielsen K, de Jong MC, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25(7):997–1011.
- Meijerink MR, Ruarus AH, Vroomen LGPH, et al. Irreversible electroporation to treat unresectable colorectal liver metastases (COLDFIRE-2): a phase II, Two-Center, Single-Arm clinical trial. Radiology. 2021;299(2):470–480.
- Narayanan G, Bhatia S, Echenique A, et al. Vessel patency post irreversible electroporation. Cardiovasc Intervent Radiol. 2014;37(6):1523–1529.
- Distelmaier M, Barabasch A, Heil P, et al. Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology. 2017;285(3):1023–1031.
- Tamura M, Pedersoli F, Schulze-Hagen M, et al. Predictors of occlusion of hepatic blood vessels after irreversible electroporation of liver tumors. J Vasc Interventional Radiol. 2020;31(12):2033–2042.e1.
- Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION trial group. Cancers. 2020;12(7):1779.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: Ablation with clear margins (A0) provides the best local tumor control. J Vasc Intervent Radiol. 2018;29(2):268–275.e1.
- Scheffer HJ, Melenhorst MCAM, Echenique AM, et al. Irreversible electroporation for colorectal liver metastases. Tech Vasc Interv Radiol. 2015;18(3):159–169.
- Geboers B, Scheffer HJ, Graybill PM, et al. High-Voltage electrical pulses in oncology: Irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology. 2020;295(2):254–272.
- Maor E, Ivorra A, Leor J, et al. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6(4):307–312.
- Ruarus AH, Barabasch A, Catalano O, et al. Irreversible electroporation for hepatic tumors: Protocol standardization using the modified delphi technique. J Vasc Interv Radiol. 2020;31(11):1765–1771.e15.
- Dollinger M, Zeman F, Niessen C, et al. Bile duct injury after irreversible electroporation of hepatic malignancies: Evaluation of MR imaging findings and laboratory values. J Vasc Intervent Radiol. 2016;27(1):96–103.
- Lencioni R, Crocetti L, Narayanan G. Irreversible electroporation in the treatment of hepatocellular carcinoma. Tech Vasc Interv Radiol. 2015;18(3):135–139.
- Hosein PJ, Echenique A, Loaiza-Bonilla A, et al. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J Vasc Interv Radiol. 2014;25(8):1233–1239 e2.
- Odisio BC, Richter M, Aloia TA, et al. Use of prophylactic antibiotics to prevent abscess formation following hepatic ablation in patients with prior enterobiliary manipulation. J Gastrointest Surg. 2016;20(8):1428–1434.
- Chehab MA, Thakor AS, Tulin-Silver S, et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a society of interventional radiology practice parameter update endorsed by the cardiovascular and interventional radiological society of Europe and the canadian association for Interventional Radiology. J Vasc Interv Radiol. 2018;29(11):1483–1501.e2.
- Freeman E, Cheung W, Kavnoudias H, et al. Irreversible electroporation for hepatocellular carcinoma: longer-Term outcomes at a single Centre. Cardiovasc Intervent Radiol. 2021;44(2):247–253.
- Cannon R, Ellis S, Hayes D, et al. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107(5):544–549.
- Sugimoto K, Moriyasu F, Kobayashi Y, et al. Irreversible electroporation for nonthermal tumor ablation in patients with hepatocellular carcinoma: initial clinical experience in Japan. Jpn J Radiol. 2015;33(7):424–432.
- Vieveen JM, Bouwman RA. Anesthetic management during irreversible electroporation procedures, In: Irreversible electroporation in clinical practice. Cham, Switzerland: Springer International Publishing AG; 2018. p. 97–103.
- Latouche EL, Arena CB, Ivey JW, et al. High-Frequency irreversible electroporation for intracranial meningioma: a feasibility study in a spontaneous canine tumor model. Technol Cancer Res Treat. 2018; 17:153303381878528.
- Patel IJ, Rahim S, Davidson JC, et al. Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous Image-Guided Interventions-Part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168–1184.e1.
- Appelbaum L, Ben-David E, Faroja M, et al. Irreversible electroporation ablation: Creation of Large-Volume ablation zones in in vivo porcine liver with Four-Electrode arrays. Radiology. 2014;270(2):416–424.
- Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23(12):1613–1621.
- Ball C, Thomson KR, Kavnoudias H. Irreversible electroporation: a new challenge in "out of operating theater" anesthesia. Anesth Analg. 2010;110(5):1305–1309.
- Narayanan G, Froud T, Lo K, et al. Pain analysis in patients with hepatocellular carcinoma: Irreversible electroporation versus radiofrequency ablation-initial observations. Cardiovasc Intervent Radiol. 2013;36(1):176–182.
- Froud T, Venkat SR, Barbery KJ, et al. Liver function tests following irreversible electroporation of liver tumors: experience in 174 procedures. Tech Vasc Interv Radiol. 2015; 18(3) :140–146.
- Verloh N, Jensch I, Lürken L, et al. Similar complication rates for irreversible electroporation and thermal ablation in patients with hepatocellular tumors. Radiol Oncol. 2019;53(1):116–122.
- Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Intervent Radiol. 2014;25(1):112–118.
- Dollinger M, Beyer LP, Haimerl M, et al. Adverse effects of irreversible electroporation of malignant liver tumors under CT fluoroscopic guidance: a single-center experience. Diagn Interv Radiol. 2015;21(6):471–475.
- Langan RC, Goldman DA, D'Angelica MI, et al. Recurrence patterns following irreversible electroporation for hepatic malignancies. J Surg Oncol. 2017;115(6):704–710.
- Padia SA, Johnson GE, Yeung RS, et al. Irreversible electroporation in patients with hepatocellular carcinoma: immediate versus delayed findings at MR imaging. Radiology. 2016;278(1):285–294.
- Cheung W, Kavnoudias H, Roberts S, et al. Irreversible electroporation for unresectable hepatocellular carcinoma: Initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013;12(3):233–241.
- Klement RJ, Abbasi-Senger N, Adebahr S, et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer. 2019;19(1):173.
- Nieuwenhuizen S, Dijkstra M, Puijk RS, et al. Thermal ablation versus stereotactic ablative body radiotherapy to treat unresectable colorectal liver metastases: a comparative analysis from the prospective amsterdam CORE registry. Cancers. 2021;13(17):4303.
