Abstract
Objective
To compare the clearance rate of high-risk human papillomavirus (HR-HPV) in patients with a high-grade squamous intraepithelial lesion (HSIL) 12 months after focused ultrasound (FUS) or loop electrosurgical excision procedure (LEEP), and analyze the influencing factors.
Methods
A retrospective cohort was established in HSIL patients with HR-HPV infection treated with FUS or LEEP from 2015 to 2019. The cohort consisted of 321 patients under 30 years of age, of which 119 patients received FUS and 202 patients received LEEP. The Cox regression model was used to identify the influencing factors for HR-HPV clearance. Kaplan–Meier method was applied to estimate the efficacy of FUS and LEEP in HR-HPV clearance, and the log-rank test was used to compare the efficacy difference between FUS and LEEP.
Results
Multivariate Cox regression analysis showed that both FUS and LEEP were independent influencing factors for HR-HPV clearance. HR-HPV cleared faster in the FUS group than in the LEEP group [the median time to HR-HPV clearance: 6 months in the FUS group (95% CI: 5.492–6.508) and 6 months in the LEEP group (95% CI: 5.734–6.266), p = 0.021]. The HR-HPV clearance rates at 6 and 12 months were 54.6% and 94.1% respectively in the FUS group, and 50.5% and 79. 2%, respectively in the LEEP group (p = 0.001 at 6 months, p = 0.000 at 12 months).
Conclusions
For HPV-positive HSIL patients under 30, FUS had a better HR-HPV clearance effect than LEEP 1 year after treatment. FUS may be a viable modality for the treatment of young HSIL patients.
Introduction
Cervical cancer ranks second among malignant tumors as a lethal disease among women and poses a great threat to women’s health and life, with the morbidity increasing in younger populations [Citation1]. A large number of studies have confirmed that persistent high-risk human papillomavirus (HR-HPV) infection is closely related to high-grade squamous intraepithelial lesion (HSIL) or cervical intraepithelial neoplasia grades 2–3 (CIN 2–3) and that HR-HPV is an important factor for the progression of CIN 2–3 to invasive cervical cancer [Citation2]. Human papillomavirus (HPV) is a common sexually transmitted infection in life with the peak incidence observed among sexually active women [Citation3]. The major peak of HPV infection occurs in women aged 26–30 years [Citation4], who are more likely to be infected with the high carcinogenic types [Citation5].
In most cases, infected cells are eliminated by the immune system [Citation6]. However, over time, the transient infection may persist if the viral genome and/or the infected host cell changes. If persistent HR- HPV infection is not detected and cleared by the immune system, it may develop into CIN [Citation7]. CIN usually takes 10–20 years to develop into cervical cancer [Citation8,Citation9]. If there is no intervention before the age of 30, CIN may eventually develop into cervical cancer.
In addition, HPV-positive women with normal or abnormal cytology have a significantly increased and higher risk of developing anxiety disorders. Continuous anxiety increases the psychological burden on patients and reduces their quality of life, which in turn affects disease progression [Citation10]. A positive HPV result may have a negative impact on patients’ mental health, and this anxiety may trigger worries about sexual relations [Citation11]. Therefore, identifying persistent HR-HPV infection risk factors as soon as possible and adopting effective treatments to prevent the disease development are crucial for treating HSIL due to persistent HPV infection. Ultimately this will reduce the morbidity of cervical cancer and improve the mental health of patients [Citation1].
For histological diagnoses of CIN 2–3, WHO guidelines recommend ablative or excisional treatment; observation is unacceptable [Citation12]. In this context, it is vital to find the best treatment for effective HPV clearance and minimal surgical harm. A large number of studies have shown that LEEP is the most common and effective treatment method for HPV-infected cervical lesions [Citation13–15]. However, LEEP can change the cervical structure, and patients who have undergone LEEP treatment have a higher rate of spontaneous abortion [Citation16]. In the recent decade, FUS has emerged as a promising treatment technique. After years of development, FUS technology has achieved clinical efficacy in the treatment of recurrent cervicitis with HPV infection [Citation17] and low-grade squamous intraepithelial lesion (LSIL) with HPV infection [Citation18,Citation19]. However, it is unclear whether FUS can achieve a similar clinical effect for HSIL patients.
With the availability of real-world data about patients treated with FUS or LEEP for HSIL, we aim to demonstrate the efficacy of LEEP and FUS in HPV clearance for cervical lesions and to identify the factors affecting the effect of LEEP and FUS in HPV clearance.
Materials and methods
Study population
This study was approved by the ethics committee of Xiamen Women and Children’s Hospital (Protocol number: KY-2020-002). From January 2015 to December 2019, 321 women were diagnosed with pathologically confirmed HSIL and HR-HPV infection by colposcopic biopsy in the outpatient department of cervical disease of Xiamen Women and Children’s Hospital. Patients were counseled about the treatment options and then chose whether to receive FUS or LEEP.
The inclusion criteria were: (i) By December 2019, patients with complete data who had received at least one follow-up; (ii) Under 30 years of age; (iii) HPV test results were clear; (iv) Non-pregnant and non-lactating women; (v) Endocervical curettage (ECC) was negative. Exclusion criteria were: (i) Unsatisfactory colposcopy or lesion in the cervical canal; (ii) Patients who received adjuvant therapy or other treatment after FUS or LEEP; (iii) Patients who had received physical therapy and surgery for cervical disease in the past year; (iv) Patients with recurrent HSIL; (v) Patients with serious heart, liver, kidney, blood system and autoimmune diseases. Baseline data included patients’ age, HR-HPV types, cytological grade, educational background, marital status, age of first sexual intercourse, and parity.
Therapeutic method
FUS group: FUS treatment was performed using the Model-CZF Ultrasound Therapeutic Device (Chongqing Haifu Medical Technology Co., Ltd, Chongqing, China) with the therapeutic power of 3.5–4.5 W and working frequency of 9.8 MHz and impulse of 1000 Hz. Certified doctors trained in FUS treatment technology performed the procedure. Patients were in the lithotomy position. Their cervixes were disinfected and fully exposed with a speculum. The surface of the cervix was coated with ultrasound coupling gel prior to treatment. The treatment probe was placed in close contact with the cervix and moved around continuously with the cervix as the center and against the skin over the diseased area with 2 mm more of healthy tissue at a speed of 5–10 mm/s, using the uniform linear or circular irradiation mode. The treatment lasted until the lesion presented as a depressed area and the external cervical aperture was moderately introverted.
LEEP group: The lesions around and under the cervical transformation zone were removed under colposcopic guidance. Loop electrodes of different diameters were used according to the lesion range. The resection range included 3 mm of normal tissues around the transformation zone. The resection was performed clockwise with high-frequency electric waves reaching 3.8 Mhz for the treatment. All patients were given a single session of treatment.
Follow-up
Patients were advised to keep the perineum clean, refrain from sexual intercourse, and bathe and use a vaginal douche for 2 months after treatment. 3 months after treatment, patients underwent repeat colposcopy. The suspicious cases underwent cervical biopsy for pathological examination while the patients with normal colposcopy results underwent cytological testing. Patients were followed-up with HPV testing at 6 and 12 months after treatment.
HPV testing
The cervical squamocolumnar junction cells were collected by attending physicians of the Gynecologic Oncology Department and were tested for HPV infection by using polymerase chain reaction (PCR) and flow-through hybridization. The PCR kit was used to detect the 18 types of HR-HPV DNA, including 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82.
Cervical cytology testing, colposcopy, and biopsy
Cervical cytological diagnosis was made in accordance with the Bethesda System (TBS). Attending physicians of the Pathology Department tested the samples and provided reports of the cervical cytology results. Colposcopy was performed using an SLC-2000 electronic colposcope (Goldway, Shenzhen) by colposcopy specialists. For suspected lesions, colposcopy-guided biopsy or cervical curettage was performed. Biopsy specimens of all patients included in this study were reviewed by experienced associate chief physicians of the Pathology Department before the final diagnosis.
Definitions
HR-HPV clearance was defined as all HR-HPV subtypes were negative. The objective was to explore the relationship between baseline and HR-HPV clearance rate. HR-HPV was divided into HPV16/18 infection and non-HPV16/18 infection. Cytology results were classified as negative for intraepithelial lesion or malignancy (NILM)/low-grade squamous intraepithelial lesion (LSIL)/atypical squamous cell of undetermined significance(ASCUS) and atypical squamous cells-cannot exclude HIS (ASC-H)/high-grade squamous intraepithelial lesion (HSIL). Educational background was divided into university degree and above, and below university degree. Marital status was divided into unmarried and married. The age of first sexual intercourse was divided into <18 and ≥18. Parity was divided into 0 and ≥1. Conversion of HR-HPV to negative or not was defined as the outcome variable, and the interval between HR-HPV testing time and conversion time to negative or the interval between HR-HPV testing time and the last follow-up date was defined as outcome time.
Statistical analysis
Descriptive analysis was used to statistically describe the distribution of baseline variables. The number of cases (n) was used for enumeration data and percentage (%) was used for classified variables. χ2 test and t-test were used to compare baseline characteristics between the FUS group and the LEEP group. Rates of HPV clearance at months 6 and 12 were compared between treatment approaches, using χ2 test. The time to clearance was evaluated using the Kaplan-Meier method, with the log-rank test. Cox regression model was used to compare the effect of FUS and LEEP on HPV clearance after adjustment of age, cervical cytology, HR-HPV types, educational background, marital status, age of first sexual intercourse, and parity. Hazard ratio (HR) and 95% confidence intervals (95%CI) were calculated for each factor that potentially impacted the time to HPV clearance. p < 0.05 was considered statistically significant with the two-sided test. The survival curve was performed with GraphPad Prism version 8.0. All data analysis was performed using SPSS 26.0 software.
Results
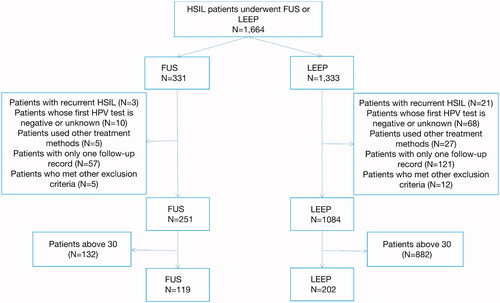
Overall, the medical records of 1664 women who were diagnosed with HSIL and received treatment from 2015 to 2019 were searched. The study population included 331 (19.9%) and 1333 (80.1%) patients who underwent FUS and LEEP, respectively. shows the enrolling process of the patients through the study design.
Baseline demographics and clinical characteristics of the two groups were reported in . A total of 321 patients were included in the HR-HPV infection cohort study, of which 119 patients received FUS and 202 received LEEP. The average age of the patients in the FUS group was lower than that in the LEEP group: 26.11 ± 2.982 years vs 27.14 ± 2.836 years. The unwed rate in the FUS group was higher than that in the LEEP group: 34.5% vs 19.8%. The non-parturition rate in the FUS group was higher than that in the LEEP group: 52.9% vs 40.6%. There were statistically significant differences in age, marital status and parity, but no differences in other baseline data.
Table 1. Baseline demographics and clinical characteristics of the two groups.
As shown in , the HR-HPV clearance rates of both groups increased over time. The cumulative HR-HPV clearance rates at 6 and 12 months were 54.6% and 94.1%, respectively in the FUS group, both higher than those in the LEEP group (50.5% and 79.2% respectively), with significant difference between the two groups (p = 0.001 at the 6th month, p = 0.000 at the 12th month).
Table 2. Accumulated clearance rate of HR-HPV infection in each group.
As shown in , a univariate Cox regression model was used to analyze the relationship between various related factors (e.g., cytology, HPV infection types, etc.) and the HR-HPV clearance rate. The results showed that the effect of the treatment methods [FUS vs LEEP (HR: 1.281 (95%CI: 1.003–1.636), p = 0.047)] and HPV infection types [HPV16/18 infection vs non-HPV16/18 infection (HR: 0.687 (95%CI: 1.144–1.855), p = 0.002)] on the HR-HPV clearance rate was considered statistically significant (p < 0.05). The results of multivariate Cox regression analysis showed that after adjusting for various confounding factors (e.g., cervical cytology, educational background, marital status, age of first sexual intercourse, parity), treatment methods [FUS vs LEEP (HR: 1.296 (95%CI: 1.001–1.677), p = 0.049)] and HR-HPV infection types [HPV16/18 infection vs non-HPV16/18 infection (HR: 0.701 (95%CI: 1.112–1.828), p = 0.005)] were still the influencing factors of HR-HPV clearance. The clearance rate in the FUS group was 1.296 times that in the LEEP group, and the clearance rate of HPV16/18 was 1.426 times that of non-HPV16/18. Cervical cytology, educational background, marital status, age of first sexual intercourse, and parity were not statistically associated with the HR-HPV clearance rate.
Table 3. Univariate and multivariate cox regression analysis of the correlation between the relevant factors of the study object and HR-HPV clearance rate.
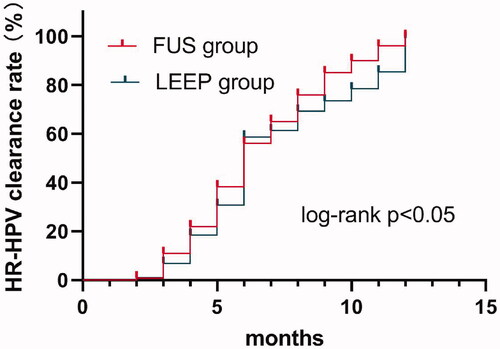
As shown in , the Kaplan-Meier curve representing the HR-HPV clearance rate was calculated according to the treatment method and the efficacy time (months) and showed that the median clearance time of HR-HPV in the FUS group (95%CI: 5.492–6.508) and that in LEEP group were both 6 months (95%CI: 5.734–6.266), p = 0.021 by log-rank test.
Discussion
Using clinical data, we demonstrated that the HPV clearance rate of FUS was higher than that of LEEP for the first time. Considering that FUS causes minimal damage to the cervical structure [Citation17], FUS may serve as a potential treatment option for HSIL patients with fertility needs. We did not find any report on HPV testing for HSIL patients who had received FUS treatment. Hoffman et al. [Citation20] found HPV clearance rates at 6 and 12 months after LEEP to be 79% and 79%, respectively, while we found clearance rates of 50.5% and 79.2%, respectively. This difference may be explained by sample size, patient compliance, and incomplete data due to the limitations of retrospective studies. Compared with other treatments, the FUS treatment in this study has certain advantages in terms of HPV clearance rate. Choi et al. [Citation21] reported that HPV DNA was no longer detected in 87.0% (40/46) of patients with CIN 2–3 12 months after photodynamic therapy (PDT). A report from China found the HPV clearance rate to be 80.4% in female patients who underwent thermal ablation [Citation22]. However, we found in this study the HPV clearance rate to be 94.1% 12 months after FUS treatment. Fu et al. [Citation18] reported that the HPV (including high-risk and low-risk) clearance rate was 85.71% at 3 months after FUS treatment, whereas our study only focused on HR-HPV-positive patients. The reason for this difference may be differences in the sample size (our sample size was 119, and that of the previous study was 28). The follow-up strategy of our study is at 6 months and 12 months after treatment, so we did not know the HPV clearance rate at 3 months. Wang et al. [Citation19] showed that after FUS treatment for LSIL patients with HR-HPV infection, the accumulated clearance rate of HR-HPV at 6 months was 47.3%. In our report, the cumulated clearance rate of HR-HPV infection in HSIL patients at 6 months was 54.6%. The difference from our findings may be due to different regions, different levels of physician practice, and different age range of patients. We also found that the clearance rate of HPV16/18 was higher than that of non-HPV16/18, which was also demonstrated in the following study. A report from Australia showed that HPV16/18 cleared faster than other HPV types regardless of single infection or multiple infections [Citation23].
It has been demonstrated that the risk of progression to vaginal or cervical cancer could be reduced to 0.7% after conventional treatment of HSIL in the cervix [Citation9]. However, some HSIL patients are still infected with HPV after treatment [Citation24]. Previous studies have shown that persistent HPV infection after treatment of HSIL may lead to residual or recurrent disease with an increased risk of further deterioration [Citation25,Citation26]. Kjaer et al. reported that the absolute risk of progression to CIN3 or cancer was estimated to be 47.4% when two follow-up visits were both HR-HPV positive [Citation27]. It was suggested that the reason for CIN recurrence may be treatment failure of precancerous cervical lesions or persistent HPV infection after treatment [Citation20].
For postoperative clinical testing of CIN patients, the follow-up strategies recommended by the American Society for Colposcopy and Cervical Pathology (ASCCP) include HPV testing, cytology testing, and colposcopy, either alone or in combination. However, compared to cytology negative results, HR-HPV negative results can ensure a lower risk of failure to detect HSIL among women. It can be used alone as a method of cervical cancer screening in place of cytology testing [Citation28,Citation29]. Gosvig et al. [Citation30] found that HR-HPV testing was more sensitive than cytology in predicting HSIL recurrence after treatment. HR-HPV test is an objective test of molecular biology, with uniform and stable standards, while cytology is a subjective interpretation of morphology, which depends in part on the tester. The changes in the anatomical structure of the cervix after treatment may restrict the cytology evaluation and affect the test results. The sensitivity and negative predictive values of the HPV test at 6 months to detect recurrence are close to 98% [Citation31]. The purpose of HPV testing is to detect recurrent diseases early [Citation32], and therefore, HPV monitoring of HSIL patients is vital after HSIL treatment.
A report suggests that HPV16/18 infection is highly carcinogenic [Citation27]. These highly carcinogenic types were detected in a greater proportion of CIN2 and CIN3 lesions in young women (<30 years of age), among whom the proportion of HPV 16 and/or 18 positive lesions was 44% and 75%, respectively [Citation5]. WHO requires such patients to undergo surgery. LEEP, a widely-used and effective treatment method, has some limitations. A report showed that LEEP directly or indirectly exerts physical effects, such as anatomical changes of the cervical structure with subsequent inflammation and healing processes that could lead to a microenvironment not conducive to pregnancy [Citation33]. Ciavattini et al. [Citation34] pointed out that if the time interval between LEEP and post-LEEP pregnancy was less than 12 months, the risk of spontaneous abortion for women could increase. Frega et al. [Citation35] showed that the length and volume of the removed cervical tissue were shown to be directly related to the risk of preterm delivery. Compared with women who had not received LEEP treatment, women who had received LEEP treatment had an increased risk of preterm delivery. Moreover, the residual rate, recurrence rate, and positive rate of resection margins were still controversial [Citation36]. FUS causes thermal, mechanical, and cavitation effects, promoting necrosis of the lesion tissues. This leads to targeted destruction of the lesion, which is later replaced by the surrounding normal healthy tissue [Citation37]. As a non-invasive treatment, FUS does not damage normal tissues and preserves the integrity of the cervical tissue structure. It enables the cervix to return to its normal appearance and elasticity after treatment. FUS has better efficacy, lower recurrence rate, and fewer side effects than traditional surgical treatment [Citation38]. It can maintain the normal physiological functions of the cervix. FUS is repeatable and does not produce smoke or ionizing radiation pollution. According to the baseline data, the average age and parity of the patients in the FUS group were both lower than those in the LEEP group, and the proportion of unmarried women in the FUS group was larger. Therefore, since FUS is more effective, it may be a better choice for women who want to preserve fertility.
This study has the following limitations: (i) This study only used data from one hospital, which might impact the representativeness of our samples. One needs to be cautious when applying the results of this study to the general population. (ii) This study could not evaluate surgery-related complications and postoperative pregnancy outcomes. (iii) We could not distinguish whether patients had persistent HR-HPV infection or non-persistent HR-HPV infection. Multi-center, prospective randomized clinical trials of HPV-DNA positive combined with HSIL are necessary to evaluate the prognosis of patients and the impact of fertility.
In conclusion, this study evaluated a group of women who underwent treatment for HSIL of the cervix. Our analysis showed that the HPV clearance rate at 12 months after treatment in the FUS group was higher than that in the LEEP group and that the median time to clearance was the same in both groups. Compared to the patients in the LEEP group, the patients in the FUS group had a slightly decreased risk of HPV persistence. Considering FUS treatment causes minimal damage to the cervical structure, FUS may be a better treatment option for HSIL patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sahlgren H, Elfström KM, Lamin H, et al. Colposcopic and histopathologic evaluation of women with HPV persistence exiting an organized screening program. Am J Obstet Gynecol. 2020;222(3):253.e1–253.e8.
- Sand FL, Kjaer SK, Frederiksen K, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int J Cancer. 2020;147(3):641–647.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325(7364):572.
- Chan PK, Ho WC, Wong MC, et al. Epidemiologic risk profile of infection with different groups of human papillomaviruses. J Med Virol. 2009;81(9):1635–1644.
- Baandrup L, Munk C, Andersen KK, et al. HPV16 is associated with younger age in women with cervical intraepithelial neoplasia grade 2 and 3. Gynecol Oncol. 2012;124(2):281–285.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723.
- Hildesheim A, Gonzalez P, Kreimer AR, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016;215(2):212.e1–212.e15.
- Castle PE, Schiffman M, Wheeler CM, et al. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113(1):18–25.
- McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434.
- McBride E, Marlow LAV, Forster AS, et al. Anxiety and distress following receipt of results from routine HPV primary testing in cervical screening: the psychological impact of primary screening (PIPS) study. Int J Cancer. 2020;146(8):2113–2121.
- McCaffery K, Waller J, Forrest S, et al. Testing positive for human papillomavirus in routine cervical screening: examination of psychosocial impact. BJOG. 2004;111(12):1437–1443.
- WHO. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd ed. Geneva (Switzerland): World Health Organization; 2021.
- Wu J, Jia Y, Luo M, et al. Analysis of residual/recurrent disease and its risk factors after loop electrosurgical excision procedure for high-grade cervical intraepithelial neoplasia. Gynecol Obstet Invest. 2016;81(4):296–301.
- Jiang Y, Chen C, Li L. Comparison of cold-knife conization versus loop electrosurgical excision for cervical adenocarcinoma in situ (ACIS): a systematic review and meta-analysis. PLoS One. 2017;12(1):e0170587.
- Bogani G, DI Donato V, Sopracordevole F, et al. Recurrence rate after loop electrosurgical excision procedure (LEEP) and laser conization: a 5-year follow-up study. Gynecol Oncol. 2020;159(3):636–641.
- Conner SN, Cahill AG, Tuuli MG, et al. Interval from loop electrosurgical excision procedure to pregnancy and pregnancy outcomes. Obstet Gynecol. 2013;122(6):1154–1159.
- Li CZ, Wang ZB, Yang X, et al. Feasibility of focused ultrasound therapy for recurrent cervicitis with high-risk human papillomavirus infection. Ultrasound Obstet Gynecol. 2009;34(5):590–594.
- Fu Z, Fan Y, Wu C, et al. Clinical efficacy and mechanism for focused ultrasound (FUS) in the management of cervical intraepithelial neoplasia 1 (CIN1). Int J Hyperthermia. 2020;37(1):339–345.
- Wang W, Liu Y, Pu Y, et al. Effectiveness of focused ultrasound for high risk human papillomavirus infection-related cervical lesions. Int J Hyperthermia. 2021;38(2):96–102.
- Hoffman SR, Le T, Lockhart A, et al. Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): a systematic review. Int J Cancer. 2017;141(1):8–23.
- Choi MC, Jung SG, Park H, et al. Photodynamic therapy for management of cervical intraepithelial neoplasia II and III in young patients and obstetric outcomes. Lasers Surg Med. 2013;45(9):564–572.
- Zhao XL, Liu ZH, Zhao S, et al. Efficacy of point-of-care thermal ablation among high-risk human papillomavirus positive women in China. Int J Cancer. 2021;148(6):1419–1427.
- Moore EE, Danielewski JA, Garland SM, et al. Clearance of human papillomavirus in women treated for cervical dysplasia. Obstet Gynecol. 2011;117(1):101–108.
- Kreimer AR, Guido RS, Solomon D, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006;15(5):908–914.
- Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013;130(2):264–268.
- Vintermyr OK, Iversen O, Thoresen S, et al. Recurrent high-grade cervical lesion after primary conization is associated with persistent human papillomavirus infection in Norway. Gynecol Oncol. 2014;133(2):159–166.
- Kjaer SK, Frederiksen K, Munk C, et al. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–1488.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182.
- Ronco G, Dillner J, Elfström KM, et al. International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532.
- Gosvig CF, Huusom LD, Deltour I, et al. Role of human papillomavirus testing and cytology in follow-up after conization. Acta Obstet Gynecol Scand. 2015;94(4):405–411.
- Rabasa J, Bradbury M, Sanchez-Iglesias JL, et al. Evaluation of the intraoperative human papillomavirus test as a marker of early cure at 12 months after electrosurgical excision procedure in women with cervical high-grade squamous intraepithelial lesion: a prospective cohort study. BJOG. 2020;127(1):99–105.
- Cuschieri K, Bhatia R, Cruickshank M, et al. HPV testing in the context of post-treatment follow up (test of cure). J Clin Virol. 2016;76(1):S56–S61.
- Kyrgiou M, Koliopoulos G, Martin-Hirsch P, et al. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and Meta-analysis. Lancet. 2006;367(9509):489–498.
- Ciavattini A, Clemente N, Delli Carpini G, et al. Loop electrosurgical excision procedure and risk of miscarriage. Fertil Steril. 2015;103(4):1043–1048.
- Frega A, Santomauro M, Sesti F, et al. Preterm birth after loop electrosurgical excision procedure (LEEP): how cone features and microbiota could influence the pregnancy outcome. Eur Rev Med Pharmacol Sci. 2018;22(20):7039–7044.
- Santesso N, Mustafa RA, Wiercioch W, et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2016;132(3):266–271.
- Wijlemans JW, de Greef M, Schubert G, et al. A clinically feasible treatment protocol for magnetic resonance-guided high-intensity focused ultrasound ablation in the liver. Invest Radiol. 2015;50(1):24–31.
- Li C, Xiong X, Li Y, et al. Therapeutic effects of focused ultrasound in 4014 patients with symptomatic cervical ectopy. Ultrasound Med Biol. 2013;39(4):604–610.