Abstract
Purpose
Transarterial chemoembolization (TACE) was obtained acceptable benefit for advanced hepatocellular carcinoma (HCC). Here in this study, we compared the benefit of TACE combined palliative thermal ablation with TACE alone for HCC with portal vein tumor thrombus (PVTT).
Methods
Patients with HCC and PVTT were retrospectively analyzed from January 2012 to December 2017, who accepted treatment of TACE alone (TACE group) or TACE plus palliative thermal ablation (TACE + P-ablation group). Propensity score matching (PSM) was applied to balance differences between the two groups. Overall survival (OS) and progression-free survival (PFS) rates were compared between groups.
Results
Median follow-up time was 7.4 (3.0–60.0) months. In the cohort, 142 patients were enrolled in TACE group and 86 patients were enrolled in TACE + P-ablation group. The pre-PSM estimated 6-, 12-, and 18-month OS rates for the TACE + P-ablation group were 70.9, 46.5, and 31%, respectively, whereas rates for the TACE group were 57, 23.1, and 10%, respectively. After PSM, OS and PFS rates remained coincident with the pre-PSM. Risk factors for poor OS included PVTT type III and type II relative to type I (HR = 1.76; 95% CI, 1.13–2.74; p = .01) and (HR = 1.86; 95% CI, 1.2–2.88; p = .006), TACE alone (HR = 1.40; 95% CI, 1.01–1.96; p = .04), a single TACE treatment (HR = 2.69; 95% CI, 1.79–4.03; p < .001), 2 or 3 TACE treatments (HR = 2.02; 95% CI, 1.32–3.09; p = .001).
Conclusions
The combination of TACE and palliative thermal ablation for HCC with PVTT could obtain delayed progression and longer survival.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world [Citation1]. The incidence of portal vein tumor thrombus (PVTT) has been reported to range from 44 to 62% [Citation2]. The presence of PVTT is an indicator of particularly poor prognosis [Citation3]. Based on the Barcelona Clinic Liver Cancer (BCLC) staging system, HCC patients with PVTT were considered to advanced stage, and the first-line treatment option for these patients is system therapy [Citation4,Citation5]. Relative to patients without PVTT, those with PVTT tended to have worse liver function, higher risk of complications, and poorer tolerance to treatment [Citation6]. Because of this, most patients are offered either palliative treatment with system therapy, or best supportive care [Citation6]. A phase III, randomized, controlled trial showed that the median survival time for patients with advanced HCC treated with sorafenib was only 6.5 months [Citation7].
Patients with PVTT actually consist of a variety of heterogeneous subgroups. In acknowledgment of this, classification systems have been proposed to differentiate prognose based on the invasion of PVTT into portal vein [Citation8]. Patients could be offered different treatment that are more individualized. For some patients, more aggressive treatments, including surgical resection and locoregional treatment, can be considered [Citation9]. Locoregional options may include thermal ablation, transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) [Citation10].
For advanced HCC, system therapy is recommended [Citation9,Citation11]. The IMbrave150 trial has demonstrated that Atezolizumab–bevacizumab are offered as first-line treatment for patients with advanced HCC now [Citation12]. The combination showed encouraging antitumor effect. However, patients with locally advanced disease may be candidates for liver-directed therapies, including TARE and TACE [Citation13]. However, thermal ablation including radiofrequency ablation (RFA) and microwave ablation (MWA) could be considered if possible. Studies have demonstrated that insufficient ablation could promote HCC progression through the up-regulation of HIF-1alpha and stress-induced phosphoprotein 1 (STIP1) [Citation14,Citation15]. However, thermal ablation could be used in advanced HCC which provided local disease control, reduced tumor burden [Citation11,Citation16,Citation17]. Palliative thermal ablation has been shown to be safe and effective in obtaining local control of advanced HCC [Citation18,Citation19]. A randomized trial involving patients with large HCC and PVTT who received additional RFA has demonstrated significantly prolonged survival and progression-free survival (PFS) [Citation20]. Many studies have proved that thermal ablation in advanced HCC have resulted in improved local control of tumor, and ablation could also be applied for distant metastatic sites [Citation21,Citation22].
TACE and TARE are usually recommended for unresectable HCC [Citation23]. Even in the setting of PVTT, TACE and TARE still play important roles [Citation24,Citation25]. However, TARE is relatively difficult to available and TACE is the main local treatment for HCC patients with PVTT in China [Citation26]. TACE combined other therapies could improve the outcome [Citation27]. Because HCC patients with PVTT often present with large tumor burden, and system therapy such as sorafenib or immunotherapy was recommended [Citation28]. Therefore, it is clinically necessary to combine different therapeutic strategies to improve the efficacy. Evidence has proved that addition of ablation on HCC accompanied PVTT significantly prolonged the long-term survival [Citation19]. It is accepted that HCC patients with PVTT could obtain survival benefit from the alleviated tumor burden after TACE plus ablation [Citation29]. We hypothesized that addition of thermal ablation therapy after TACE may result in better local control and longer survival than TACE alone for HCC patients with PVTT. To test this hypothesis, we calculated and compared the PFS and overall survival (OS) in HCC patients with PVTT.
Methods
This study was conducted in accordance with the principles of the Declaration of Helsinki [Citation30], and the study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University (No. 2021-hg-ks-21).
Study population
We retrospectively reviewed the medical records of patients who received diagnosis of HCC with PVTT between January 2012 and December 2017 at Second Affiliated Hospital of Guangzhou Medical University. The diagnosis of HCC was based on the American Association for the Study of Liver Disease and the European Association for the Study of the Liver, or based either on positive liver biopsy and/or characteristic findings on imaging (multiphasic computed tomography [CT], or on dynamic contrast-enhanced magnetic resonance imaging [MRI]) combined with elevated serum alpha-fetoprotein (AFP) levels [Citation30]. PVTT was considered to be present on imaging when there was an enhancing intraluminal mass extending into the portal venous system on the arterial phase and a low-attenuation, intraluminal mass noted on the portal phase [Citation31]. PVTT was classified based on the level of extension of the tumor thrombus in the portal venous system: type I, tumor thrombus involving the segmental branches of the portal vein or higher; type II, tumor thrombus involving the right/left portal vein; type III, tumor thrombus involving the main portal vein; and type IV, tumor thrombus involving the superior mesenteric vein [Citation8]. The clinical stage of HCC in each patient was determined according to the BCLC guidelines [Citation32].
Patients were included as follows: (a) were initially diagnosed with HCC with PVTT; (b) received TACE as initial treatment and showed a response to it; (c) had no extrahepatic metastasis; (d) had adequate liver function (i.e., Child–Pugh class A or B liver function); (e) had adequate renal function (i.e., serum creatinine concentration no higher than 1.5 times the upper limit of normal); and (f) had adequate coagulation function (i.e., prothrombin activity > 40%, international normalized ratio (INR) < 1.26 and platelet count > 50 × 109/L); and (g) were age 18–75 years. Patients were excluded from the analysis for any of the following reasons: (Citation1) were under 18 years or over 75 years of age; (Citation2) had no response to TACE; (Citation3) had recurrent HCC; (Citation4) had PVTT extending into the superior mesenteric vein (PVTT type IV) and was therefore not a candidate for TACE therapy; (Citation5) had hepatic vein or vena cava invasion by tumor; (Citation6) were lost to follow-up within 90 d of last treatment; or (Citation7) had information about prognostic variables or follow-up that could not be obtained.
Demographic characteristics of each patient were collected, including sex, age, ECOG performance status, Child–Pugh grade, hepatitis (history of chronic HBV infection and/or positive hepatitis C virus RNA test), portal hypertension (esophageal varices and/or splenomegaly on imaging studies combined with a decreased platelet count [≤ 100 × 109/L]), comorbidity (hypertension, diabetes, coronary disease and/or hemoglobin <100 g/L), and cirrhosis (scarring or diminished size of right or left lobe of liver, compensatory enlargement of caudate lobe of liver and splenomegaly on imaging). We recorded baseline levels of AFP, alanine transaminase (ALT), aspartate aminotransferase (AST), complete blood count, and prothrombin time. Albumin–Bilirubin (ALBI) grade was determined for each patient [Citation33], which is based on a score calculated using the formula: ALBI score = (log10 bilirubin × 0.66) + (albumin × −0.085). The SLBI grade is used to identify different mortality risk subsets of patients, as follows: Grade 1 (lowest mortality risk) for ALBI score ≤ −2.60, Grade 2 (intermediate mortality risk) for ALBI score > −2.60 and ≤ −1.39) and grade 3 (highest mortality risk) for ALBI score > −1.39. Finally, we gathered information for each patient about the number of HCC tumors, HCC tumor sizes, PVTT type, number of TACE treatments, number of P-ablation treatments and complications.
Transarterial chemoembolization (TACE)
For the TACE procedure, a super-selective microcatheter was inserted into the artery supplying each tumor. Then a combination of lipiodol (5–15 mL), lobaplatin (30–50 mg) and pararubicin (30–50 mg) was infused into each tumor. We defined technical success as complete embolization of the tumor-feeding artery resulting in no tumor staining observed by angiogram at the end of procedure. TACE procedures were repeated every 1–2 months thereafter, and they were discontinued when patients do not respond to treatment, poor liver function or patients’ choice. The evaluation of tumor response to TACE was according to modified Response Evaluation Criteria in Solid Tumors standard one month after TACE by contrast-enhanced CT [Citation34]. Tumor response was partial response (PR) and non-response was stable disease (SD) or progressive disease (PD).
Palliative thermal ablation (P-ablation)
For palliative ablation, for tumor size no more than 7 cm, we could achieve complete ablation after TACE. For tumor larger than 7 cm, complete ablation was difficult to obtain. In this situation, an overriding goal of the thermal ablation treatments used was to maximize the ablation of all HCC tumors, leaving as little residual tumor as possible. In this study, we only ablated the tumor not the PVTT.
All thermal ablation procedures were performed by three physicians who each had at least 5 years of experience with the techniques. For patients in TACE + P-ablation group who elected to receive thermal ablation therapy, they had usually had 1 or 2 TACE procedures, in which lipiodol was noted on CT to be well-deposited before receiving the thermal ablation. The interval between TACE and thermal ablation was 1–2 months according to patients’ liver function and tumor-related situation. Patients were abrosia for 12 h before the procedure. The procedure was conducted under intravenous anesthesia, and vital signs were monitored throughout the procedure and recovery. For the first cycle of ablation, MWA was typically used, because it resulted in a larger ablation volume than RFA. For any additional cycles, MWA or RFA were chosen based on physician or patient preference. For ablation treatments, CT was used to determine the optimal route for MWA or RFA antenna placement, and the antennas were then inserted into each tumor along the predetermined route. We used manufacturer recommendations and operator experience to determine the ablation parameter settings for each procedure. The treatment ablation volume was determined for each tumor and patient, taking into account tumor size and location, as well as liver function. A repeat CT scan was performed immediately after each procedure to evaluate the ablation zone and to determine if there were any complications. After the first thermal ablation procedure and based on subsequent follow-up imaging, TACE was repeated if there was residual tumor volume measuring more than 20% of the original or if new tumors appeared in the liver.
MWA procedure: A microwave delivery system (KY-2000, Kangyou Medical Instrument, Nanjing, China) was used during MWA therapy. This system consisted of microwave generator with a frequency of 2450 MHz, a power output of 10–150 W, a flexible low-loss cable, and a 16-gauge cooled-shaft antenna. Percutaneous MWA was performed under the guidance of CT (SOMATOM 64 Sensation, Siemens). The MWA parameters and number of antennas were usually depended on the tumor volume and location. RFA procedure: RFA was performed with the use of local anesthesia. All procedures were performed percutaneously under CT guidance. A cool-tip RFA system (Radionics) with a 3 cm active tip length was used for ablation. The ablation parameters were determined by the volume of the residual tumor with the aim of achieving an ablative margin of at least 0.5 cm in the normal tissue surrounding the tumor.
Propensity score matching (PSM)
Propensity score matching (PSM) method was used to balance the selection bias. The propensity score was estimated using a multivariate logistic regression model, by inserting the following variables: number of HCC tumors, maximum tumor size, PVTT type, number of TACE treatments, ALBI grade, and both AST and AFP levels. Patients were matched 1:1 using the nearest neighbor method with a caliber of 0.05; this matching process has been described in a previous study [Citation35].
Follow-up
The follow-up was terminated on 30 September 2020. Patients were evaluated one month after treatment. Abdominal imaging (e.g., abdominal CT and/or MRI and/or ultrasound) was performed to evaluate effect of treatment and detect the residual viable tumor. Each follow-up visit also involved checking serum AFP levels. The primary endpoint for the study was overall survival (OS), and the secondary endpoint was PFS. OS was defined as the time from accepting first TACE to death or last follow-up, and PFS was defined as the time from accepting first TACE to disease progression. Progression was defined as more than a 20% increase in the diameter of one or more measurable tumors, or the appearance of new tumors, consistent with the criteria that others have used [Citation27,Citation36]. The extend of PVTT type was considered progression [Citation37].
Statistical methods
The demographic, clinical, tumor-related and treatment-related characteristics were summarized using frequencies and percentages for categorical covariates, means and standard deviations (SD) for continuous covariates. The Fisher exact test was used to compare categorical covariates, while the Wilcoxon rank-sum test was used to compare continuous covariates. OS and PFS rates were calculated using the Kaplan–Meier method. Univariate and multivariate Cox regression analyses were used to determine the impact of risk factors. Variables with p value less than .05 in the univariate analysis were subjected to the multivariate Cox regression model using a forward stepwise variable selection. A 2-tailed p value less than .05 was considered statistically significant for all tests. Statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY).
Results
A total of 228 patients were included, 86 patients were enrolled in the TACE + P-ablation group and 142 patients were enrolled in the TACE group (). Before PSM was applied, when compared to the patients in the TACE group, a significantly larger proportion of patients in the TACE + P-ablation group had lower ALBI grade I (72.1 vs. 42.3%, p < .001). There were no significant differences between the two groups with regards to sex, age, AFP, ALT, hepatitis, portal hypertension, comorbidity, cirrhosis, neutrophil count, platelet count, hemoglobin or prothrombin time ().
Figure 1. Flowchart of the patients selection. TACE: transarterial chemoembolization; P-ablation: palliative ablation; PSM: propensity score matching.
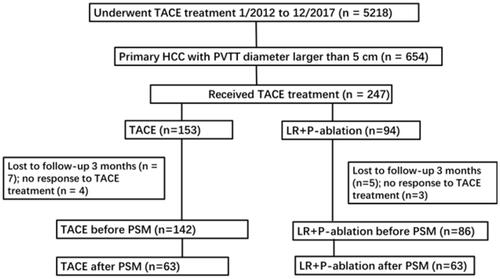
Table 1. Demographic and clinical characteristics of patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before and after propensity score matching (PSM), by type of treatment (TACE + P-ablation vs. TACE), January 2012 to December 2017.
Before PSM was applied, there were total of 240 times of TACE in the TACE group and 216 times in TACE + P-ablation group. The number of HCC tumors, maximum tumor size and number of TACE treatments differed significantly between the two groups (). Compared to the TACE group, higher proportion of patients in the TACE + P-ablation group had a single tumor (43.0 vs. 25.4%, p < .001), tumor diameter less than 10 cm (67.4 vs. 535%, p = .038), and multiple TACE treatments (62.8 vs. 43.7%, p < .001). However, the type of PVTT did not differ significantly between the two groups. After PSM was applied, there were no significant differences in the clinical characteristics () or the treatment-related characteristics ().
Table 2. Tumor- and treatment-related characteristics of patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before and after propensity score matching (PSM), by type of treatment (TACE + P-ablation vs. TACE), January 2012 to December 2017.
Progression-free survival (PFS)
Over the entire study before PSM, patients in the TACE + P-ablation group had significantly higher PFS rates than patients in TACE group (p < .001) (). The median PFS was 3.7 months in the TACE + P-ablation group and 2.4 months in the TACE group. The estimated 2-, 4-, and 6-month PFS rates in TACE + P-ablation group were 76.7, 50.0 and 32.6%, respectively, whereas PFS rates for those in TACE group were 56.2, 22.8 and 15.6%, respectively (p < .001, p < .001 and p = .002) (). Of the 86 patients in TACE + P-ablation group, PFS rates of those receiving more than one time of ablation were significantly higher than the rates of those who had one time (p = .03) ().
Figure 2. Kaplan–Meier curves for progression-free survival (PFS) in 228 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before propensity score matching (PSM), January 2012 to December 2017: (A) The PFS rates of the group of 86 patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of 142 patients who had only TACE (p < .001); (B) Of the group of 86 patients who had both TACE and P-ablation, for patients received more times of ablation had better PFS. Patients received more than 3 times of ablation had the best PFS. The OS rates were in good correlation with the times of P-ablation treatments (p = .03).
![Figure 2. Kaplan–Meier curves for progression-free survival (PFS) in 228 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before propensity score matching (PSM), January 2012 to December 2017: (A) The PFS rates of the group of 86 patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of 142 patients who had only TACE (p < .001); (B) Of the group of 86 patients who had both TACE and P-ablation, for patients received more times of ablation had better PFS. Patients received more than 3 times of ablation had the best PFS. The OS rates were in good correlation with the times of P-ablation treatments (p = .03).](/cms/asset/35c70fdb-a18e-46a6-b80b-778e9be8d541/ihyt_a_2021303_f0002_c.jpg)
Table 3. Overall survival (OS) and progression-free survival (PFS) rates in patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before and after propensity score matching (PSM), by type of treatment (TACE + P-ablation vs. TACE), January 2012 to December 2017.
Based on the multivariate analysis, risk factors for poorer PFS were ALBI grades II and III (HR = 1.38; 95% CI, 1.03–1.85; p = .03), PVTT type II (HR = 1.74; 95% CI, 1.12–2.70; p = .01) and PVTT type III (HR = 2.00; 95% CI, 1.24–3.23; p = .005), and TACE treatment (HR = 1.56; 95% CI, 1.13–2.16; p = .007) (). Also, a single TACE treatment (HR = 1.81; 95% CI, 1.26–2.6; p = .001), 2 or 3 TACE treatments (HR = 1.71; 95% CI, 1.16–2.52; p = .007) were also significant independent risk factors.
Table 4. Multivariate analyses of impact of clinical, tumor-related and treatment-related characteristicsa on progression-free survival (PFS) in 228 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before and after propensity score matching (PSM), by type of treatment (TACE + P-ablation vs. TACE), January 2012 to December 2017.
Overall survival (OS)
The median follow-up period was 17.1 (range, 3.0–60.0) months. Over the entire period, 86.0% (74 of 86) in the TACE + P-ablation group and 96.5% (137 of 142) in the TACE group died. Before PSM, the OS rates in the TACE + P-ablation group were significantly higher than the TACE group (p < .001) (). The median OS was 10.6 months in the TACE + P-ablation group and 6.5 months in the TACE group. The estimated 6-, 12-, and 18-month OS rates in TACE + P-ablation group were 70.9, 46.5 and 31.0%, whereas the OS rates in TACE group were 57.0, 23.1 and 10.0% (p = .02, p < .001 and p < .001) ().
Figure 3. Kaplan–Meier curves for overall survival (OS) in 228 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before propensity score matching (PSM), January 2012 to December 2017: (A) The OS rates of the group of 86 patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of 142 patients who had only TACE (p < .001); (B) Of the group of 86 patients who had both TACE and P-ablation, for patients received more times of ablation had better OS. Patients received more than 3 times of ablation had the best OS. The OS rates were in good correlation with the times of P-ablation treatments (p = .025).
![Figure 3. Kaplan–Meier curves for overall survival (OS) in 228 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before propensity score matching (PSM), January 2012 to December 2017: (A) The OS rates of the group of 86 patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of 142 patients who had only TACE (p < .001); (B) Of the group of 86 patients who had both TACE and P-ablation, for patients received more times of ablation had better OS. Patients received more than 3 times of ablation had the best OS. The OS rates were in good correlation with the times of P-ablation treatments (p = .025).](/cms/asset/0eaf64be-e15d-4bab-9b00-185a23eb1ae1/ihyt_a_2021303_f0003_c.jpg)
Median OS with different types of PVTT was further analyzed. As shown in , before PSM, the median OS of type I of PVTT was 16.47 months in TACE + P-ablation group and 10.6 months in the TACE group. Medium OS of type of II was 10.57 months in TACE + P-ablation group and 6.4 months in the TACE group for, OS of type III was 7.57 months in TACE + P-ablation group and 5.27 months in the TACE group. Of 52 in TACE + P-ablation received one-time ablation, 17 received two times and 17 received 3 or more times. Over the entire study period, the OS rates of those receiving more than one time of ablation were significantly higher than the one time (p = .03) ().
Table 5. Median overall survival (OS) in patients with different type of portal vein tumor thrombus (PVTT)a, before and after propensity score matching (PSM), by type of treatment (TACE + P-ablation vs. TACE), January 2012 to December 2017.
Based on the multivariate analysis, risk factors for poorer OS included PVTT type III and type II (HR = 1.76; 95% CI, 1.13–2.74; p = .01) and (HR = 1.86; 95% CI, 1.2–2.88; p = .006), TACE treatment (HR = 1.40; 95% CI, 1.01–1.96; p = .04) (). Also, relative to more than 3 times of TACE, one time of TACE (HR = 2.69; 95% CI, 1.79–4.03; p < .001), 2 or 3 times TACE (HR = 2.02; 95% CI, 1.32–3.09; p = .001) were risk factors.
Table 6. Multivariate analyses of impact of clinical, tumor-related and treatment-related characteristicsa on overall survival (OS) in 228 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), before and after propensity score matching (PSM), by type of treatment (TACE + P-ablation vs. TACE), January 2012 to December 2017.
Subgroup analyses
We further analyzed the subgroup of tumor size no more than 7 cm. A total of 33 patients in the TACE group and 26 patients in the TACE + P-ablation group were included. As shown in , the PFS was different between the two groups (p = .048). The median PFS was 4.9 months in the TACE + P-ablation group and 3.2 months in the TACE group. Patients obtained obvious longer survival after palliative ablation, and the median OS was 13.6 months in the TACE + P-ablation group and 6.8 months in the TACE group. There was obvious significance in the OS between the two groups (p = .01) ().
Figure 4. Kaplan–Meier curves for (A) progression-free survival (PFS) and (B) overall survival (OS) in 59 patients with tumor size no more than 7 cm and portal vein tumor thrombus (PVTT), January 2012 to December 2017. PFS and OS rates for the group of 26 patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of 33 patients who had only TACE (p = .048 and p = .001, respectively).
![Figure 4. Kaplan–Meier curves for (A) progression-free survival (PFS) and (B) overall survival (OS) in 59 patients with tumor size no more than 7 cm and portal vein tumor thrombus (PVTT), January 2012 to December 2017. PFS and OS rates for the group of 26 patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of 33 patients who had only TACE (p = .048 and p = .001, respectively).](/cms/asset/4b9d1988-1bb5-4773-8fca-c0d0f2b27d54/ihyt_a_2021303_f0004_c.jpg)
Results after propensity score matching (PSM)
The PSM analysis resulted in 63 pairs ( and ). After PSM, OS rates remained significantly higher in the TACE + P-ablation group than in the TACE group at 6 months (74.6 vs. 63.5%, p = .04), 12 months (41.3 vs. 26.1%, p = .03), and 18 months (28.1 vs. 12.2%, p = .01) (). The PFS was significantly higher in the TACE + P-ablation group than in the TACE group (p < .001) (). OS rates remained significantly higher in the TACE + P-ablation group than in the TACE group (p = .001) (). Similarly, after PSM, PFS rates were significantly higher in the TACE + P-ablation than the TACE group at 4 months (47.6 vs. 25.9%, p = .005) and 6 months (30.2 vs. 17.8%, p = .04); however, there was no significant difference at 2 months (74.6 vs. 61.6%, p = .09) ().
Figure 5. Kaplan–Meier curves for (A) progression-free survival (PFS) and overall survival (OS) (B) in 126 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), after propensity score matching (PSM), January 2012 to December 2017. PSM was used to create clinically comparable cohorts and to correct for potential biases, with patients matched 1:1 using the nearest neighbor method with a caliber of 0.05. OS and PFS rates for the group of patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of patients who had only TACE (p = .001 and p < .001, respectively).
![Figure 5. Kaplan–Meier curves for (A) progression-free survival (PFS) and overall survival (OS) (B) in 126 patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT), after propensity score matching (PSM), January 2012 to December 2017. PSM was used to create clinically comparable cohorts and to correct for potential biases, with patients matched 1:1 using the nearest neighbor method with a caliber of 0.05. OS and PFS rates for the group of patients who had transarterial chemoembolization (TACE) + palliative (P)-ablation (microwave ablation [MWA] and/or radiofrequency ablation [RFA]) were significantly higher than for the group of patients who had only TACE (p = .001 and p < .001, respectively).](/cms/asset/1b2df373-727e-4026-8be2-e073d76480c1/ihyt_a_2021303_f0005_c.jpg)
After PSM, multivariate analysis of OS, PVTT type III (HR = 2.31; 95% CI, 1.40–3.80; p = .001), TACE as treatment (HR= 1.56; 95% CI, 1.04–2.23; p = .03), one time of TACE (HR = 2.31; 95% CI, 1.40–3.80; p = .001) were risk factors for poorer OS (). Significant independent risk factors for poor PFS were more than three tumors (HR = 1.81; 95% CI, 1.20–2.73; p = .005), PVTT type II (HR = 2.69; 95% CI, 1.44–5.03; p = .002), PVTT type III (HR = 2.27; 95% CI, 1.20–2.73; p = .01), TACE treatment (HR = 1.88; 95% CI, 1.27–2.80; p = .002), and one time of TACE (HR =1.75; 95% CI, 1.08–2.85; p = .02) ().
Complications
In the TACE + P-ablation group, six patients (7.0%) had complications, including moderate pleural effusion (n = 2), biliary tract injury (n = 1), colonic obstruction (n = 1), esophageal variceal bleeding (n = 1) and abdominal infection (n = 1). In the TACE group, nine patients (6.3%) had complications, including moderate pleural effusion (n = 3), abdominal infection (n = 2), esophageal variceal bleeding (n = 2), hepatic abscess (n = 1) and hepatic failure (n = 1).
Discussion
In this retrospective study, we compared the OS and PFS rates for patients with HCC and PVTT who received TACE + P-ablation or TACE. We found that the addition of palliative ablation after TACE resulted in delayed progression and longer OS. A variety of treatments have been administered in an attempt to improve the prognosis of patients with HCC with PVTT, including sorafenib, lenvatinib, hepatic arterial infusion chemotherapy (HAIC) and combinations [Citation6]. The recommended first-line option recently for HCC and PVTT are atezolizumab plus bevacizumab and the combination resulted in better overall and PFS outcomes than sorafenib [Citation38–42]. Alternatively, TACE has been the preferred treatment for patients with HCC and PVTT in China and other countries in Asia [Citation26,Citation43]. The median OS for patients with HCC and PVTT who have had no intervention has been reported to be as low as 2–4 months [Citation9]. In contrast, the median OS has been reported to range from 6 to 7 months for sorafenib [Citation40,Citation41]. The OS of TACE alone was ranging from 6 to 7.5 months [Citation26,Citation40,Citation43]. Yttrium-90 (90 Y) radioembolization has recently been a wide use for patients with HCC and PVTT [Citation44]. The median OS were 6.1 and 13.4 months of main and branch PVTT patients, respectively [Citation13,Citation45]. The median OS in the TACE group in our study was 6.5 months, which was consistent with the findings of others. The median OS (10.6 months) for TACE + P-ablation was longer than TACE alone. Some studies have proven that the classification of PVTT significantly stratified the prognosis of patients treated with tyrosine kinase inhibitor (including lenvatinib, sorafenib, etc.) (median OS: type I, 14.4 months; type II and III, 5.5 months) [Citation46]. In our study, the median OS for type I, type II and type III of PVTT was 16.47, 0.57 and 7.57 months in TACE + P-ablation. The median OS for type I or II/III of PVTT in TACE + P-ablation group was a little longer than OS reported above.
Furthermore, the estimated 6-, 12-, and 18-month OS rates for patients in TACE + P-ablation were 70.9, 46.5 and 31.0%, respectively, and these were significantly higher than TACE alone which was 57.0, 23.1 and 10.0%. Difference remained significant even after PSM. The 12-month OS rate for sorafenib was 20–30% [Citation27,Citation28], and TACE alone was 20–46% [Citation26,Citation27,Citation40,Citation43]. A study involving 60 patients treated with TACE + MWA for HCC with PVTT demonstrated 1-year OS rate was 48%, which was similar to our findings [Citation2]. Their results suggested a benefit from the addition of thermal ablation therapy after TACE. Adding palliative ablation after TACE for HCC with PVTT could reduce the tumor burden, and as a result might improve survival. The reason was that patients with HCC and PVTT usually had a large tumor burden, which may lead to early progression and poor survival [Citation47–49]. Our results suggested that in a selected patient with adequate hepatic function, the addition of ablation after TACE for HCC with PVTT could obtain longer PFS and OS.
In offering treatment with the combination of TACE and thermal ablation, we also kept in mind that incomplete ablation can potentially promote tumor growth and metastasis, and that sublethal levels of heat could potentially induce malignant transition [Citation50]. So, an overriding goal of the ablation treatments used was to maximize the ablation of all HCC tumors, leaving as little residual tumor as possible. That said, before ablation was offered to patients, they must have had a substantial response to TACE; if not, we did not offer ablation to them. In addition, in patients who had HCC with PVTT but presented with extrahepatic growth, especially in the peritoneal cavity, ablation was not recommended to avoid the diffuse growth of residual tumor without the restriction of surrounding normal liver tissue.
In this study, we also sought to identify independent risk factors for poorer OS. We found that PVTT level III (tumor thrombus involving the main portal vein), TACE treatment, and one time of TACE treatment were independent predictors of poorer OS. Similarly, Huang et al. also reported that PVTT type of III was a predictor of poorer survival [Citation26]. Although we found that patients who received multi times of TACE had better OS, this finding need to be tempered by the fact that multiple TACE treatments may increase the incidence of hepatic failure in patients with HCC and PVTT [Citation43]. So multiple TACE treatments were often avoided in our patients with PVTT type of III.
Several limitations of this study should be noted. First, although we attempted to match the characteristics of the two groups through the PSM, selection bias was inevitable. Second, we included only patients with Child-Pugh classes A and B liver function. The exclusion of those with severe liver disease and PVTT level IV may have biased our results. However, those with severe liver disease and PVTT level IV tended not to be candidates for TACE treatments or the addition of thermal ablation therapy. Third, the ablation used for patients in this study involved two different technologies (MWA and RFA) and the techniques were not standardized. In addition, it needs to point out that there were 112 patients received only one time of TACE (79 in TACE group and 33 in TACE + P-ablation group). All patients in TACE and TACE + P-ablation group were response to TACE treatment. Even patients were response to TACE, patients could continue the TACE treatment after first cycle especially in TACE + P-ablation group. However, there was a retrospective study, there was many reasons influence the treatment included the doctors and patients. Ultimately, prospective studies combining system (atezolizumab–bevacizumab, lenvatinib), TACE, and thermal therapy could provide much needed evidence to guide treatment of patients with HCC and PVTT.
Conclusions
In a selected group of patients with HCC and PVTT, the TACE combination palliative therapy could obtain longer survival. TACE plus palliative ablation therapy could have better oncological outcomes than TACE alone in patients with HCC and PVTT.
Author contributions
Conceptualization: J.L., K.Z.; Data curation: Q.Z., T.L., Z.L., F.Z.; Formal analysis: Q.Z, R.L.; Data analysis: R.L., Q.Z.; Funding acquisition: K.Z., Q.Z.; Investigation: Q.Z., J.L., K.Z.; Methodology: Q.Z., T.L., C.W.; Project administration: Q.Z., K.Z.; Resources: Q.Z., J.L., K.Z.; Original draft: Q.Z., J.L.; Writing – review and editing: J.L., and K.Z.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Long J, Zheng JS, Sun B, et al. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int. 2016;10(1):175–184.
- Cao F, Shen L, Qi H, et al. Tree-based classification system incorporating the HVTT-PVTT score for personalized management of hepatocellular carcinoma patients with macroscopic vascular invasion. Aging. 2019;11(21):9544–9555.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853.
- Chan SL, Chong CC, Chan AW, et al. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016;22(32):7289–7300.
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
- Chen ZH, Zhang XP, Lu YG, et al. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int. 2020;14(5):754–764.
- Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis. 2018;38(3):242–251.
- Bouvry C, Palard X, Edeline J, et al. Transarterial radioembolization (TARE) agents beyond 90Y-Microspheres . Biomed Res Int. 2018;2018:1435302.
- Crane CH, O’Reilly EM. Ablative radiotherapy doses for locally advanced: pancreatic cancer (LAPC). Cancer J. 2017;23(6):350–354.
- Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345.
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64.
- Yamada S, Utsunomiya T, Morine Y, et al. Expressions of hypoxia-inducible factor-1 and epithelial cell adhesion molecule are linked with aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy. Ann Surg Oncol. 2014;21(S3):436–442.
- Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–3527.
- Ierardi AM, Biondetti P, Coppola A, et al. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg. 2018;7(2):59–66.
- Hlavsa J, Procházka V, Andrasina T, et al. Radiofrequency ablation in pancreatic cancer. Rozhl Chir. 2019;98(11):441–449.
- Zheng JS, Long J, Sun B, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation can improve survival of patients with hepatocellular carcinoma with portal vein tumour thrombosis: extending the indication for ablation? Clin Radiol. 2014;69(6):e253–e263.
- Giorgio A, Calisti G, Montesarchio L, et al. Hepatocellular carcinoma invading portal venous system in cirrhosis: long-term results of percutaneous radiofrequency ablation of both the nodule and portal vein tumor thrombus. A case control study. Anticancer Res. 2014;34:6785–6790.
- Ding X, Sun W, Chen J, et al. Percutaneous radiofrequency ablation combined with transarterial chemoembolization plus sorafenib for large hepatocellular carcinoma invading the portal venous system: a prospective randomized study. Front Oncol. 2020;10:578633.
- Lyu N, Kong Y, Pan T, et al. Survival benefits of computed tomography-guided thermal ablation for adrenal metastases from hepatocellular carcinoma. Int J Hyperthermia. 2019;36(1):1003–1011.
- Mu L, Sun L, Pan T, et al. Percutaneous CT-guided radiofrequency ablation for patients with extrahepatic oligometastases of hepatocellular carcinoma: long-term results. Int J Hyperthermia. 2018;34(1):59–67.
- Golfieri R, Bargellini I, Spreafico C, et al. Patients with barcelona clinic liver cancer stages B and C hepatocellular carcinoma: time for a Subclassification. Liver Cancer. 2019;8(2):78–91.
- Schwarz RE, Abou-Alfa GK, Geschwind JF, et al. Nonoperative therapies for combined modality treatment of hepatocellular cancer: expert consensus statement. HPB (Oxford). 2010;12(5):313–320.
- Yu SJ, Kim YJ. Effective treatment strategies other than sorafenib for the patients with advanced hepatocellular carcinoma invading portal vein. World J Hepatol. 2015;7(11):1553–1561.
- Huang M, Lin Q, Wang H, et al. Survival benefit of chemoembolization plus Iodine125 seed implantation in unresectable hepatitis B-related hepatocellular carcinoma with PVTT: a retrospective matched cohort study. Eur Radiol. 2016;26(10):3428–3436.
- Kim JH, Shim JH, Yoon HK, et al. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018;38(9):1646–1654.
- Vitale A, Lai Q, Farinati F, et al. Utility of tumor burden score to stratify prognosis of patients with hepatocellular cancer: results of 4759 cases from ITA.LI.CA study group. J Gastrointest Surg. 2018;22(5):859–871.
- Peng Z, Chen S, Wei M, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;287(2):705–714.
- Oguro S, Yoshimoto J, Imamura H, et al. Clinical significance of macroscopic no-margin hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2018;20(9):872–880.
- Chung SR, Kim JH, Yoon HK, et al. Combined Cisplatin-Based chemoembolization and radiation therapy for hepatocellular carcinoma invading the main portal vein. J Vasc Interv Radiol. 2015;26(8):1130–1138.
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
- Austin PC, Jembere N, Chiu M. Propensity score matching and complex surveys. Stat Methods Med Res. 2018;27(4):1240–1257.
- Hu Y, Qin T, Li S, et al. Efficacy and safety of SBRT combined with camrelizumab and apatinib in HCC patients with PVTT: Study protocol of a randomized controlled trial. Front Oncol. 2020;10:1589.
- Wei X, Jiang Y, Zhang X, et al. Neoadjuvant Three-Dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, Open-Label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905.
- Finn RS, Ikeda M, Zhu AX, et al. Phase IB study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970.
- Lee JM, Jang BK, Lee YJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol. 2016;22(1):160–167.
- Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469–478.
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173.
- Yuan J, Yin X, Tang B, et al. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Biomed Res Int. 2019;2019:1–6.
- Pracht M, Edeline J, Lenoir L, et al. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol. 2013;2013:827649.
- Jia Z, Jiang G, Tian F, et al. A systematic review on the safety and effectiveness of yttrium-90 radioembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Saudi J Gastroenterol. 2016;22(5):353–359.
- Kaneko S, Tsuchiya K, Yasui Y, et al. Strategy for advanced hepatocellular carcinoma based on liver function and portal vein tumor thrombosis. Hepatol Res. 2020;50(12):1375–1385.
- Ibrahim C, Parra N, Macedo FI, et al. Is hepatic resection better than transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis? J Gastrointest Oncol. 2019;10(6):1064–1072.
- Li MX, Zhao H, Bi XY, et al. Total tumor volume predicts survival following liver resection in patients with hepatocellular carcinoma. Tumour Biol. 2016;37(7):9301–9310.
- Hsu CY, Huang YH, Hsia CY, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei integrated scoring system. J Hepatol. 2010;53(1):108–117.
- Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40.
