Abstract
Objective
To compare the long-term efficacy of microwave ablation (MWA) for subcapsular and non-subcapsular hepatocellular carcinomas (HCCs) using propensity score matching (PSM).
Materials and methods
Using a multicenter database, we enrolled 430 patients (347 men, 83 women; age range, 15–71 years) with HCCs who received percutaneous ultrasound-guided MWA, between January 2012 and December 2018. The patients were grouped as follows, based on whether the tumor was adjacent to the capsule: subcapsular group (n = 142) and non-subcapsular group (n = 142). To evaluate the correlation between subcapsular position and efficacy of MWA, a Cox proportional hazards model was used to calculate disease-free survival (DFS) and overall survival (OS) based on PSM data.
Results
In total, 142 pairs of patients were matched. In the PSM cohort, the 1-year, 3-year, and 5-year DFS rates of the subcapsular and non-subcapsular groups were 84%, 61%, and 47%, respectively, and 85%, 67%, and 58%, respectively, while the 1-year, 3-year, and 5-year OS rates were 98%, 90%, and 84%, respectively, and 98%, 90%, and 88%, respectively. In the PSM cohort, subcapsular position was not an independent risk factor for DFS (hazard ratio [HR] = 1.291, p = 0.196) or OS (HR = 0.926, p = 0.866). Additionally, there were no significant differences in the incidence of local tumor progression, major complications, technical success rate, number of puncture needles, and postoperative hospital stay between the two groups (p > 0.05).
Conclusion
There were no significant differences in DFS, OS, incidence of local tumor progression, and major complications between patients with subcapsular and non-subcapsular HCCs treated with MWA.
Introduction
According to GLOBOCAN 2018 [Citation1], hepatic carcinoma (HC) has become the sixth most common type of cancer worldwide and the fourth leading cause of cancer-related deaths. The main pathological type of HC is hepatocellular carcinoma (HCC). With the rapid advances in interventional radiology, thermal ablation based on microwave or radio wave frequency has become the preferred method for treating small HCs because of its advantages of preserving liver function and achieving local treatment effects similar to surgical resection [Citation2,Citation3]. Thermal ablation has been recommended in international guidelines for treating primary HC [Citation4–6]. Previous studies have discussed the treatment effects of percutaneous thermal ablation for HC adjacent to the gallbladder [Citation7], gastrointestinal tract [Citation8], and diaphragm [Citation9]. However, due to direct puncture, which easily leads to bleeding and metastasis, interference of lung gas, and unsatisfactory location, the therapeutic efficiency of thermal ablation has always been controversial.
Recent studies have shown no significant difference in the treatment effects of thermal ablation for subcapsular and non-subcapsular HCCs. However, the biases of retrospective study design, small sample size, and short-term follow-up in most of these studies have made the results flawed [Citation10,Citation11]. Therefore, we adopted propensity score matching (PSM) based on a multicenter database to compare the long-term efficacy of percutaneous microwave ablation (MWA) for subcapsular or non-subcapsular HCCs of ≤3 cm in diameter.
Materials and methods
Patient selection
This study was approved by the institutional review board of the Chinese PLA General Hospital (S2020-464-01). The requirement for informed consent was waived owing to the study’s retrospective design.
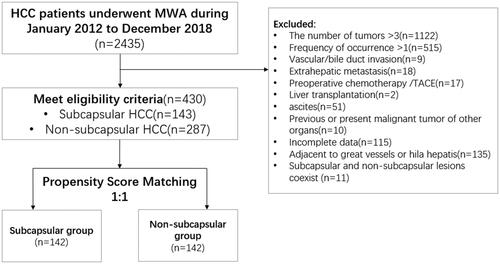
We used data from a multicenter database, which included the Chinese PLA General Hospital, Third Affiliated Hospital of Sun Yat-Sen University, Harbin Medical University Cancer Hospital, the Second Affiliated Hospital of Nanjing University of Chinese Medicine, and Wuhan University of Science and Technology Tianyou Hospital. From January 2012 to December 2018, a total of 430 patients (532 lesions) with HCCs who underwent MWA were screened out. The diagnosis of HCC was based on needle biopsy (n = 293) or typical imaging features (n = 137). The inclusion criteria were as follows: (a) number of nodules of ≤3 and diameter of ≤3 cm, (b) Child-Pugh grade of A or B, (c) initial onset of HCC, and (d) perioperative laboratory test results that met the surgical standards. The exclusion criteria were as follows: (a) vascular and/or bile duct invasion on imaging, (b) preoperative chemotherapy and/or transcatheter arterial chemoembolization (TACE), (c) history of liver transplantation, (d) extrahepatic metastasis, (e) history of other organ malignancies, (f) tumor adjacent to large blood vessels and/or hila, (g) presence of both subcapsular and non-subcapsular tumors, and (h) incomplete data. A subcapsular location was defined as location of a tumor within 5 mm of the liver capsule. According to the tumor location, the patients were divided into subcapsular (n = 143) and non-subcapsular (n = 287) groups ().
MWA equipment and procedure
A 2.4-GHz microwave generator (KY-2000, Kangyou Medical, Nanjing, China) was used with a 15-mm-diameter antenna, and a cold axis for ablation treatment was established under real-time ultrasound guidance to avoid damage to nearby structures such as the intestines, gallbladder, portal vein, and bile duct. When necessary, artificial ascites technology, enhanced-contrast ultrasound, and fusion imaging technology were used in the MWA process to improve the visibility and positioning of the tumor. After subcutaneous infiltration of local anesthesia, the MWA electrode was inserted under ultrasound guidance and confirmed to enter the lesion. Midazolam (0.06 mg/kg) was injected intravenously during ablation to achieve sedation. All treatments were performed by two experienced ultrasound interventional doctors according to the preoperative plans. During the ablation procedure, the ablated hyperechoic area was monitored in real time using conventional grayscale ultrasound. Except for the adjacent subcapsular area, the entire tumor periphery was planned to have an ablation boundary of ≥5 mm. For dangerous locations that could not be completely ablated, temperature measurement technology and alcohol injection treatment were used. When the echogenic area produced by ablation covered the entire tumor and surrounding liver parenchyma, the ablation procedures were halted and the needle tract was routinely ablated to avoid bleeding and implantation during the needle withdrawal process. After ablation, flumazenil (0.5 mg/kg) was injected intravenously to induce recovery from sedation. Antibiotic treatment was given 2 days after the operation, and the treatment was continued for patients with fever. Ablation assistive technologies include artificial ascites [Citation8,Citation12,Citation13], temperature monitoring [Citation14,Citation15], ethanol injection [Citation7], image fusion technology [Citation16], and 3D visualization navigation [Citation17,Citation18]. The operation process is described in detail in previous studies [Citation7,Citation8,Citation14–18].
Post-ablation follow-up
On the third day after ablation, contrast-enhanced ultrasound or enhanced magnetic resonance imaging (MRI) was performed in all patients to observe the ablation area and evaluate the technical success rate and presence of complications. If a residual tumor was found, a second ablation was required. Chest radiography, enhanced MRI, and laboratory examinations were scheduled 1 month after the first discharge, every 3 months for the first 2 years, and every 4–6 months thereafter. Complications were evaluated based on clinical symptoms, imaging results, and laboratory examination records after treatment. All complications were recorded within 1 month after surgery, and tumor metastasis was recorded as a delayed event. Recurrent tumors confirmed during follow-up were treated with the optimal second-line treatment, such as radiofrequency ablation or MWA, TACE, surgical resection, radiotherapy, or targeted therapy.
Outcome assessment
The primary outcomes of the study were local tumor progression (LTP) and disease-free survival (DFS); the secondary outcomes were overall survival (OS) and major complication rates. OS was calculated as the interval from the initial MWA treatment to the outcome of the event or the last follow-up before 25 March 2021. DFS was calculated as the interval from MWA therapy to tumor progression or death. Intrahepatic recurrence was defined as the appearance of new tumor foci in the liver parenchyma, far from the ablation area, whereas LTP was defined as the appearance of new tumor foci in or adjacent to the ablation area. Major complications were clinical events that led to extra therapeutic interventions, prolonged hospital stay, or unexpected increases in the level of care, and were classified as immediate or delayed complications [Citation19].
PSM analysis
We applied PSM to reduce the influence of selection bias on survival analysis. The propensity score model included the following variables: age, sex, age-adjusted Charlson comorbidity index (aCCI), etiology, cirrhosis, tumor size, tumor number, serum alpha-fetoprotein (AFP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), serum albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), blood glucose (GLU), hemoglobin (Hb), platelet (PLT) count, red blood cell (RBC) count, and prothrombin time (PT). These variables were applied to the logistic regression model to calculate the propensity scores. A one-to-one nearest-neighbor matching algorithm with an optimal caliper of 0.1 without replacement was used to generate 142 pairs of patients ().
Statistical analysis
SPSS software (version 26; IBM Corp., Armonk, NY) was used for statistical analysis. Continuous variables were expressed as means ± standard deviation or medians and compared using the independent sample t test or Mann–Whitney U test. Categorical variables were expressed as numbers (percentages) and compared using Pearson’s Chi-square test or Fisher’s exact test. The OS and DFS curves were drawn using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards regression analysis was used to evaluate the independent predictors of OS and DFS, and variables with p-values of <0.05 in the univariate analysis were entered into a Cox proportional hazards model for multivariate analysis. The differences were statistically significant when the two-tailed p-value was <0.05.
Results
Patient characteristics before PSM
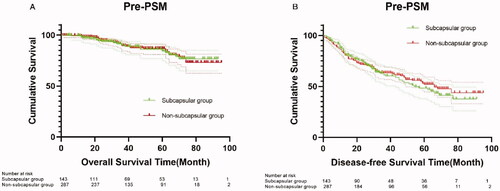
A total of 430 patients were included before PSM, including 143 in the subcapsular group and 287 in the non-subcapsular group (). The characteristics of these patients are shown in . The two groups showed significant differences (p < 0.05) in etiology, blood glucose level, and hemoglobin level. The median follow-up time for all patients was 38 months. There were no significant differences in OS between the subcapsular and non-subcapsular groups at 1 year (98% versus 99%), 3 years (90% versus 90%), and 5 years (84% versus 87%) (p = 0.972) (). There were also no significant differences in DFS between the subcapsular and non-subcapsular groups at 1 year (84% versus 83%), 3 years (61% versus 63%), and 5 years (48% versus 52%) (p = 0.668) ().
Table 1. Baseline characteristics of patients before and after propensity score matching.
Patient characteristics after PSM
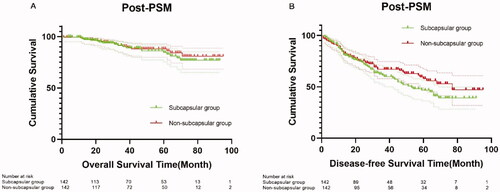
A total of 142 patients were included after PSM. The baseline characteristics of patients in the two groups were similar (), including age, aCCI, cause of liver disease, tumor size, liver function, and other laboratory test results (p > 0.05). The median follow-up time of patients was 39.5 months in the two groups. There were no significant differences in OS between the subcapsular and non-subcapsular groups at 1 year (98% versus 98%), 3 years (90% versus 90%), and 5 years (84% versus 88%) (p = 0.601) (). There were also no significant differences in DFS between the subcapsular and non-subcapsular groups at 1 year (84% versus 85%), 3 years (61% versus 67%), and 5 years (47% versus 58%) (p = 0.199) ().
Treatment efficacy of the PSM cohort
There were no significant differences in the local curative effect and incidence of major complications between the subcapsular and non-subcapsular groups (). Compared with the non-capsulated subgroup, the application rate of assistive technologies in the subcapsular group was significantly higher (p < 0.05). There were no significant differences between the subcapsular and non-subcapsular groups in terms of technical success rate, number of puncture needles, postoperative hospital stay, complications/major complications, technical efficiency, and LTP (p > 0.05).
Table 2. Perioperative outcomes of patients in the subcapsular and non-subcapsular groups.
Subgroup analysis
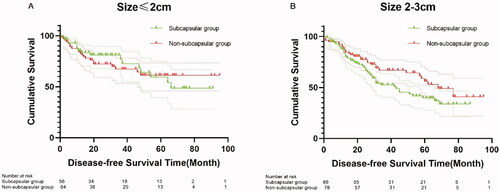
In the subgroup with tumor diameters of ≤2 cm (120 cases), we found no significant difference in DFS between the subcapsular and non-subcapsular groups at 1 year (85% versus 83%), 3 years (77% versus 67%), and 5 years (59% versus 61%) (p = 0.855) (). In the subgroup with tumor diameters of 2–3 cm (164 cases), there were also no significant differences in DFS between the subcapsular and non-subcapsular subgroups at 1 year (84% versus 87%), 3 years (52% versus 67%), and 5 years (40% versus 56%) (p = 0.094) ().
Univariate and multivariable analyses of OS and DFS after PSM
In the univariate and multivariate analyses, age, tumor size, DBIL, Hb level, intrahepatic metastasis, extrahepatic metastasis, and temperature monitoring were significant risk factors for OS (p < 0.05) (). In the univariate analysis, aCCI, tumor size, and RBC count were significant risk factors for DFS (p < 0.05). In the multivariate analysis, aCCI and tumor number were significant risk factors for DFS (p < 0.05) ().
Table 3. Univariate and multivariate analyses of overall survival after PSM.
Table 4. Univariate and multivariate analyses of disease-free survival after PSM.
Discussion
With the development of thermal ablation technology, the role and curative effect of percutaneous thermal ablation in the treatment of HCCs are constantly improving. The effect of ablation therapy for HCC depends on the adequate ablation of the tumor and the surrounding 5–10 mm of liver parenchyma, as adequate ablation margins are associated with a low risk of LTP [Citation20,Citation21]. Subcapsular HCCs are usually difficult to puncture smoothly and are mostly adjacent to vulnerable key organ structures, which can lead to insufficient tumor ablation. Therefore, the application of thermal ablation in the treatment of subcapsular HCCs remains controversial. However, with the development of ablation equipment, guidance methods, and assistive technologies (such as hydrodissection, image fusion, and 3D visualization [Citation22]) and advances in knowledge and skills of ablation doctors, recent studies have shown that the application of thermal ablation technology in the treatment of subcapsular HCCs has achieved exciting results. Our research showed that there were no significant differences in the long-term efficacy (in terms of OS, DFS, technical efficiency, and major complication rate) of MWA in the treatment of HCCs of ≤3 cm in diameter between the subcapsular and non-subcapsular groups.
In previous studies, some researchers believed that the effect of thermal ablation for treating subcapsular HCCs was unsatisfactory because a safety margin of 5–10 mm could not be obtained [Citation23–25]. However, these studies were retrospective and did not balance the basic clinical features of patients in the subcapsular and non-subcapsular groups. Evidence-based medical results indicated that the subcapsular location was not a risk factor for recurrence after surgical resection of HCC, and the lack of tumor margins adjacent to the capsular side would not increase local recurrence [Citation26]. Kang et al. showed that tumor size, liver function, ablation time, and technical difficulty all affected the prognosis of subcapsular tumors, but the subcapsular location itself had no significant effect on local tumor control after HCC radiofrequency ablation [Citation27]. Although most of these comparative studies did not balance the basic clinical characteristics of patients between the subcapsular and non-subcapsular groups, in this study, we conducted a comparative analysis of the efficacy of both groups after propensity score matching. Here, we found no significant differences in the incidence of LTP (p = 1.000) and DFS (p = 0.199) between the subcapsular and non-subcapsular groups. In the subgroup analysis of tumors with diameters of ≤2 cm and 2–3 cm, the difference in DFS between the subcapsular and non-subcapsular groups was not statistically significant, which is consistent with the results of Worakitsitisatorn and Yu [Citation10,Citation28]. Compared with non-subcapsular tumors, although the subcapsular location of tumors increased the technical difficulty of puncture and led to more frequent application of assistive techniques and prolonged postoperative hospital stay, the subcapsular location itself had no significant effect on LTP and OS after MWA was performed to treat HCCs. In this study, age, tumor size, DBIL, Hb level, intrahepatic metastasis, extrahepatic metastasis, and temperature monitoring were significant risk factors for OS (univariate and multivariate analyses, p < 0.05), while aCCI, tumor size, and RBC count (univariate analysis) and aCCI and tumor number (multivariate analysis) were significant risk factors for DFS (p < 0.05). Kang et al. suggested that the use of hydrodissection for tumors in high-risk locations is an independent risk factor for LTP [Citation27]. This finding may be due to the low number of hydrodissection and LTP cases in their study. Moreover, when the diameter of the tumor is >3 cm, the results need to be further studied.
Another important issue in the use of MWA for subcapsular HCCs is the presence of a major complication. The incidence of major complications has been shown to be significantly higher in subcapsular HCCs than in non-subcapsular HCCs, including pneumothorax, diaphragmatic injury, hemorrhage, gastrointestinal thermal injury, and tumor metastasis [Citation29]. In contrast, our results showed no significant differences in the rate of major complications, such as immediate complications (needle bleeding, pleural effusion, and postoperative infection) and delayed complications (inferior vena cava thrombosis) between the two groups, and there was no severe abdominal hemorrhage or tumor seeding in both groups. This finding illustrates the safety of MWA in treating subcapsular HCCs and is consistent with the findings of Francia [Citation30]. This result may be due to the use of artificial ascites or pleural effusion to separate the liver from neighboring organs in our cohort, and temperature monitoring or alcohol injections in some cases, which may reduce the risk of severe thermal damage to adjacent organs in the subcapsular group [Citation8]. The application of other ablation assistive technologies can improve the display rate of lesions and increase the efficiency of ablation technology. After ablation, to reduce the risk of bleeding and metastasis, the needle track needs to be routinely ablated [Citation31].
Conclusions
This study revealed no significant differences in LTP, DFS, OS, and the incidence of major complications associated with the use of percutaneous MWA as the first-line treatment for subcapsular and non-subcapsular HCCs.
Our study had a few limitations. The study was retrospective; hence, even if PSM was carried out, selection bias was inevitable. Moreover, the diameter of the nodules included in this study was ≤3 cm, and whether the results of the study were suitable for larger tumors requires further research. However, to our knowledge, this is the largest retrospective cohort study on the effects of microwave ablation in hepatocellular carcinoma based on national multicenter data. This study verified the long-term efficacy and safety of MWA in the treatment of subcapsular liver tumors through PSM of randomized controlled trials.
Author contributions
P. L. and X. L. -Y. conceived the study; J. Y., F. Y. -L., Z. Y. -H., Z. G. -C., R. Q. -Z., W. C., Q. W., S. Y. -Y., Y. C. -L., and J. D. -Y. collected the data; B. B. -L., X. H. -W., K. L., P. C., and J. D. -Y. collated and analyzed the data; B. B. -L., X. H. -W., and J. D. -Y. interpreted the data; B. B. -L. and J. D. -Y. searched the literature; and J. D. -Y. wrote the first draft. All authors read and approved the final manuscript.
Disclosure statement
The authors declare no potential conflict of interest.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30(5):2463–2472.
- N CCC, Kit-Fai L, Cheuk-Man C, et al. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: application of ALBI score to patient selection. HPB: Off J Int Hepato Pancreato Biliary Assoc. 2018;20(6):546–554.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463.
- European Association for the Study of the Liver. Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Huang H, Liang P, Yu XL, et al. Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia. 2015;31(1):40–47.
- Zhang M, Liang P, Cheng ZG, et al. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia. 2014;30(2):134–141.
- Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34(6):863–869.
- Worakitsitisatorn A, Lu DS, Lee MW, et al. Percutaneous thermal ablation of subcapsular hepatocellular carcinomas: influence of tumor-surface contact and protrusion on therapeutic efficacy and safety. Eur Radiol. 2020;30(3):1813–1821.
- Liu F, Yu X, Cheng Z, et al. Comparison of ultrasonography-guided percutaneous microwave ablation for subcapsular and nonsubcapsular hepatocellular carcinoma. Eur J Radiol. 2017;91:93–98.
- Liu C, He J, Li T, et al. Evaluation of the efficacy and postoperative outcomes of hydrodissection-assisted microwave ablation for subcapsular hepatocellular carcinoma and colorectal liver metastases. Abdom Radiol (NY). 2021;46(5):2161–2172.
- Bhagavatula SK, Chick JF, Chauhan NR, et al. Artificial ascites and pneumoperitoneum to facilitate thermal ablation of liver tumors: a pictorial essay. Abdom Radiol (NY). 2017;42(2):620–630.
- Liu SR, Liang P, Yu XL, et al. Percutaneous microwave ablation for liver tumours adjacent to the marginal angle. Int J Hyperthermia. 2014;30(5):306–311.
- Zhi-Yu H, Ping L, Xiao-Ling Y, et al. A clinical study of thermal monitoring techniques of ultrasound-guided microwave ablation for hepatocellular carcinoma in high-risk locations. Sci Rep. 2017;7(1):41246.
- Liu FY, Yu XL, Liang P, et al. Microwave ablation assisted by a real-time virtual navigation system for hepatocellular carcinoma undetectable by conventional ultrasonography. Eur J Radiol. 2012;81(7):1455–1459.
- Liu F, Cheng Z, Han Z, et al. A three-dimensional visualization preoperative treatment planning system for microwave ablation in liver cancer: a simulated experimental study. Abdom Radiol. 2017;42(6):1788–1793.
- Ren H, An C, Liang P, et al. Ultrasound-guided percutaneous microwave ablation assisted by athree-dimensional visualization treatment platform combined with transcatheter arterial chemoembolization for a single large hepatocellular carcinoma 5 cm or larger: a preliminary clinical application]. Int J Hyperthermia. 2019;36(1):44–54.
- Ahmed M, Solbiati L, Brace CL, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- Fukuda K, Mori K, Hasegawa N, et al. Safety margin of radiofrequency ablation for hepatocellular carcinoma: a prospective study using magnetic resonance imaging with superparamagnetic iron oxide. Jpn J Radiol. 2019;37(7):555–563.
- Vogl TJ, Basten LM, Nour-Eldin NA, et al. Evaluation of microwave ablation of liver malignancy with enabled constant spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperthermia. 2018;34(4):492–500.
- Liu F, Liang P, Yu X, et al. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: a preliminary clinical application. Int J Hyperthermia. 2013;29(7):671–677.
- Yang B, Zou J, Xia J, et al. Risk factors for recurrence of small hepatocellular carcinoma after long-term follow-up of percutaneous radiofrequency ablation. Eur J Radiol. 2011;79(2):196–200.
- Komatsu S, Murakami M, Fukumoto T, et al. Risk factors for survival and local recurrence after particle radiotherapy for single small hepatocellular carcinoma. Br J Surg. 2011;98(4):558–564.
- Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38(10):977–981.
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10–24.
- Kang TW, Lim HK, Lee MW, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280(1):300–312.
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66(6):1172–1173.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43(5):1101–1108.
- Francica G, Meloni MF, DE Sio I, et al. Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: safety and efficacy. Eur J Radiol. 2016;85(4):739–743.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940.