Abstract
Purpose
To investigate the long-term efficacy of radiofrequency ablation (RFA) with a multiple-electrode switching system (MESS) in the treatment of early hepatocellular carcinoma (HCC) and evaluate the patterns and risk factors of intrahepatic recurrence of HCC after RFA.
Methods
In total, 139 patients with early HCC who underwent RFA with MESS as primary treatment at multiple centers were prospectively enrolled according to the inclusion criteria. We evaluated the local tumor progression (LTP), intrahepatic distant recurrence (IDR), the incidence of cumulative disease-free survival (DFS), LTP-free survival, IDR-free survival, and overall survival. We also analyzed the associated risk factors.
Results
A total of 139 patients were included in the study and the median follow-up time was 64 months, ranging from 11 to 72 months. The complete ablation rate was 98.56%. Sixty-nine (49.64%) were found to have intrahepatic recurrence (LTP, n = 15; IDR, n = 55) during follow-up. The 1-year, 3-year and 5-year cumulative DFS, LTP-free survival, and IDR-free survival rates were 74.82, 94.46 and 78.75%; 54.68, 88.03 and 61.79%; and 51.80, 85.67 and 60.17%, respectively. In the multivariable analysis, tumor size > 4 cm was the only important risk factor for LTP. The alkaline phosphatase (ALP) level and the number of tumors were independent risk factors for IDR; α-fetoprotein (AFP) level > 400 µg/L and recurrence interval were risk factors for the overall survival period.
Conclusions
The MESS-RFA is an effective method for local control of tumors in early HCC. Early HCC with multiple high-ALP tumors has a higher rate of recurrence, which mainly occurs in an IDR pattern. Early HCC with high AFP levels and a shorter initial recurrence interval resulted in a poorer prognosis. Thus, treatments such as liver transplantation or surgical resection may be a good strategy in those cases.
ClinicalTrials.gov ID
NCT02046356.
Introduction
Although the prognosis of hepatocellular carcinoma (HCC) has improved in recent years, the median survival of the patients just ranged from 2.1 to 12.1 months, which inflicts a heavy burden on the patients [Citation1]. Partial hepatectomy and liver transplantation were considered the primary treatment regimens for HCC with marked improvement on the prognosis of the HCC patients. However, due to the anatomical position of the tumor, the number of tumors, the advanced clinical characteristics of the tumor and the shortage of liver donor sources, many HCC patients miss the opportunity for surgical treatment [Citation2,Citation3]. Thus, many clinicians have tried to seek other alternative nonsurgical therapies. In recent years, radiofrequency ablation (RFA) has become the most widely used local thermal ablation method due to its technical convenience, safety, reproducibility and minimal invasiveness [Citation4–7].
Generally, percutaneous RFA is recommended for early HCC patients with a tumor diameter less than 3 cm, and the complete ablation rate was proven to exceed 90%. Other studies tried to expand the indication for this treatment to HCC patients with a tumor diameter of 3–5 cm, but the complete ablation rate was reported to range from 73.1 to 82.5% [Citation8,Citation9]. Whether the results were conclusive or not remains controversial as the efficiency of RFA is expected to improve through the enhancement of its technical parameters. For example, the use of clustered electrodes and an improved motor can provide higher power. Furthermore, the multiple-electrode switching system-RFA (MESS-RFA) uses various methods, including using multiple electrodes to increase the ablation area, clustered internally cooled electrodes to increase the accessible area and multipolar controllers to provide the synergy of multiple applicators, to create a sufficient ablation zone [Citation10]. To obtain better clinical outcomes for early HCC with a tumor diameter larger than 3–5 cm, we need to update further the management strategy for the RFA treatment of early HCC.
Having a better understanding of the prognosis and the associated factors for patients who underwent RFA is essential for clinicians to tailor appropriate individual treatment regimens. However, the reported results are not consistent, limiting the application of RFA in clinical practice to some extent [Citation11,Citation12].
In this study, we aim to conduct a multicenter prospective case series study to evaluate the long-term efficacy of MESS-RFA as a primary method for treating early HCC to provide evidence for the management strategy of RFA in the treatment of HCC. Moreover, we divided the intrahepatic recurrence after RFA into local tumor progression (LTP) and intrahepatic distant recurrence (IDR) to further investigate the prognosis and associated factors for recurrence and survival.
Materials and methods
Patients
It is a multicenter prospective case series study, and it has been approved by the institutional review board of the Army Medical University (Approval number: 2013-047). Early HCC patients who were newly diagnosed in the First Affiliated Hospital, the Second Affiliated Hospital and the Third Affiliated Hospital of the Army Medical University were prospectively recruited based on the following inclusion criteria: 1) HCC diagnosis was based on either histological findings (2 nodules) or the noninvasive criteria proposed by AASLD and EASL [Citation13,Citation14]; 2) there were ≤ 3 malignant hepatic nodules without extra-hepatic metastasis or large vascular invasion; 3) the largest tumor was ≤ 5 cm in diameter; 4) MESS-RFA was used as the initial treatment and had been used again after the recurrence. The patient should not have received other treatments, such as transarterial chemoembolization (TACE), sorafenib, surgery, etc.; 5) the liver function was determined to be Child-Pugh class A or B.5, the pro-thrombin activity was above 40%, and the platelet count was more than 5 × 109/L; 6) the patient was only infected with HBV; and 7) HCC that was close to the main blood vessels (distance ≤ 10 mm), or with a large amount of ascites were considered relative contraindications for MESS-RFA treatment [Citation15]. Perivascular tumors were defined as index tumors contacting the first- or second-degree branches of the portal or hepatic veins 3 mm or greater in axial diameter [Citation16]. Subcapsular HCC was defined when the distance between tumor margin and the liver surface was < 10 mm [Citation17].
This analysis included all study participants who met the inclusion criteria from December 2013 to January 2015, and subsequent follow-up was conducted until 31 January 2020.
MESS-RFA parameter
All interventional procedures were performed by the three operators (Ma, Li and Chen) from the Army Medical University, who have more than 15 years of experience in liver ablation procedures. Preoperative informed consent of the patient was obtained, and all operations were completed with anesthesia and monitoring. The dose of injected sufentanil citrate (Humanwell Pharmaceutical Co., Ltd., Yichang, China), which was used for analgesia, was 0.1–0.2 mg, while the dose of injected dexmedetomidine hydrochloride (Heng Rui Medicine Co., Ltd., Jiangsu, China), which was used for sedation, was 50–100 μg. Furthermore, lidocaine hydrochloride injection (Zhao Hui Pharmaceutical Co., Ltd., Shanghai, China) was used for local anesthesia. During the operation, the patient's blood pressure, pulse, electrocardiogram and oxygen saturation were continuously monitored.
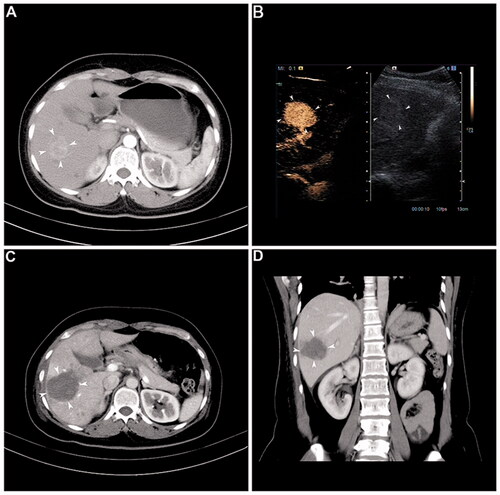
MESS-RFA was conducted with the radiofrequency electrode (Covidien LLC, Mansfield, MA) being placed at the tumor site under ultrasound guidance (S2000, Siemens Ltd, Munich, Germany). Furthermore, all ablation used MESS (Cool-tip™ RF Ablation System and Switching Controller; Valley Lab, Boulder, CO). During RFA, the switching machine was set to auto-mode, and all electrodes worked alternately and switched off automatically after any impendence surge. Continuous ablation lasted from 12 to16 min until the impedance shut-off cycle was at approximately 15 s and after an adequate hyper-echoic change of the ablated area was achieved [Citation18]. In general, to obtain sufficient ablative volume to cover the entire tumor fully, an ablative margin of >0.5 cm beyond the index tumor was required. Overlapping ablation guided by fusion imaging technology was used. The hepatic tumor’s size, shape and location determined the number of electrodes used for RFA. One RFA electrode was suitable for tumors sized ≤ 1 cm, two electrodes were suitable for tumors ≤ 3 cm, while two or more RFA electrodes were needed for tumors > 3 cm. Two or three RFA electrodes were placed at a spacing of 1–2 cm away from each other. The first electrode was inserted along the medial margin of the targeted tumor, the second electrode was inserted along the plane of the first electrode, and the third electrode was inserted parallel to the first two electrodes but in different planes; the distance between the two electrodes was 2.0–2.5 cm. Overlapping ablation is necessary to achieve sufficient ablation in different planes of the index tumor. Before the withdrawal of the needle, the system was set to manual mode at 35 W for ablation of the needle tract [Citation19] ().
Figure 1. Representative case showing the usefulness of the multiple-electrode switching system radiofrequency ablation (MESS-RFA) in ablating a large volume at one time. (A) Image before MESS-RFA showing. A 3.5-cm nodule (arrowheads) with enhancement in the arterial phase. (B) Intra-procedural contrast-enhanced ultrasound guiding tumor (arrowheads) targeting and monitoring. (C) Axial CT image immediately after MESS-RFA showing that the ablation zone (arrowheads) covers the index tumor (5.5 cm in size). (D) Coronal CT image reconstructed from the immediate post-procedural CT scan shows that the ablation zone (arrowheads) measures 5.4 cm in its coronal long axis.
Evaluation and follow-up of MESS-RFA
To assess the efficacy and identify any complications, ultrasonography and contrast-enhanced CT of the liver were performed 24 h after MESS-RFA, where the treatment was considered incomplete if the tumor showed any tissue enhancement. When LTP or IDR was confirmed in the enrolled patients during follow-up, MESS-RFA treatment was performed again. Contrast-enhanced ultrasound (CEUS) and serum AFP assays were performed every 2 months during the first postoperative year and every 4 months in the subsequent years. If recurrence was suspected by CEUS, contrast-enhanced CT was performed for further confirmation. The local progression and distant recurrence of the HCC were then determined. LTP and IDR were evaluated using imaging during reexamination [Citation20].
Statistical analysis
The Kaplan–Meier method was used to estimate the cumulative incidence for each type of recurrence (i.e., LTP and IDR) and the survival rate. Univariable and multivariable Cox proportional hazards models were used to estimate the prognosis factors for both LTP and IDR. Additionally, the univariable Cox proportional hazards model was fitted to each variable. The total survival period was defined as the interval between the treatment period and death or last follow-up date, while the recurrence-free survival was defined as the interval between the treatment period and the date of local or distant HCC recurrence. The calculation of rates and incidences was based on each patient. All variables with a p value of less than .05 were included in the multivariable analysis. The stepwise Cox proportional hazards regression model was used to estimate the values used as independent prediction factors. SAS version 9.3 software (SAS Inc., Cary, NC) was used for all statistical analyses.
Results
Technical success
MESS-RFA was used 141 times for early HCC treatment. Of these treatments, 137 patients had complete ablation with one treatment, Furthermore, of the 207 HCCs, 205 achieved technically complete ablation after one treatment (99.03%). Twenty-four hours after the first MESS-RFA, the multiphase contrast-enhanced CT results of the liver were compared with the CEUS results, and we found two cases that did not reach complete ablation with one treatment due to proximity to the diaphragm and needed another session of RFA to achieve complete ablation (). Two months after MESS-RFA, the follow-up imaging showed that all tumors achieved technical success ().
Figure 2. The flow chart shows the first-line treatment results for 139 HCC patients who met the Milan criteria and were treated with a multiple-electrode switching system radiofrequency ablation (MESS-RFA).
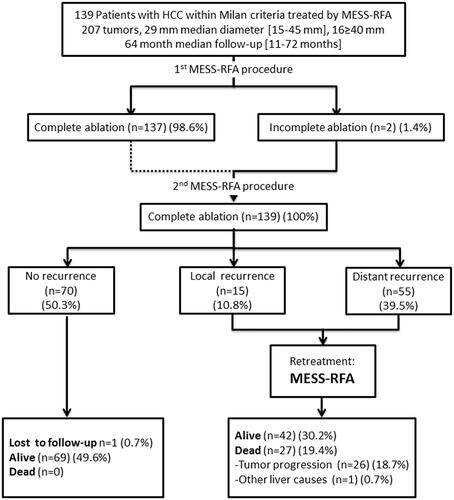
Table 1. Baseline characteristics of the 139 patients with HCC treated by percutaneous MESS-RFA.
Recurrence and survival
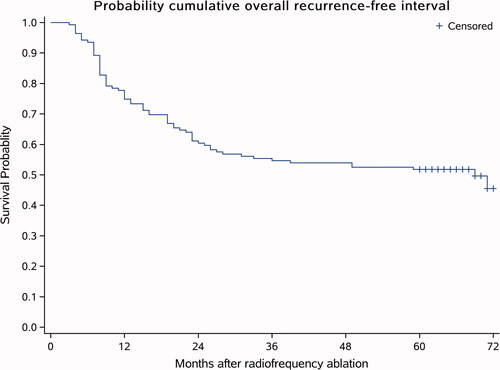
The median follow-up time of the 139 patients (207 tumors) was 64 months and ranged from 11 to 72 months (average, 59.33 months). Sixty-nine (49.64%) out of the 139 patients were found to have an intrahepatic recurrence (LTP and/or IDR) during follow-up. These recurrences were found at a median of 60 (range, 3–72) months after ablation. The 1-year, 3-year and 5-year recurrence-free survival rates were 74.82, 54.68 and 51.80%, respectively (). Patients with a recurrence underwent MESS-RFA again.
LTP
Among the 139 successfully treated HCC patients, the average follow-up time was 64 months (ranging from 11 to 72 months). During follow-up, LTP was found in the follow-up imaging of 15 patients (10.79%). The 1-year, 3-year and 5-year cumulative incidence of LTP were 3.72, 8.04 and 9.62%, respectively. In univariable and multivariable analysis, tumor size > 4 cm (p = .015) was the only significant risk factor for the occurrence of LTP ().
Table 2. Risk factors for LTP in univariate and multivariate analysis.
IDR
Fifty-five (39.56%) of the 139 patients had IDR, and the estimated 1-year, 3-year and 5-year IDR-free survival rates were 78.75, 61.79 and 60.17%, respectively (). In univariable analysis, the alkaline phosphatase (ALP) level (p < .001) and the number of tumors (p < .001) were important predictors for IDR. In the multivariable analysis of the Cox proportional hazards model, both were still considered risk factors for IDR ().
Figure 4. IDR-free survival curve. Fifty-five (39.56%) patients out of the 139 patients had IDR, and the estimated 1-year, 3-year and 5-year IDR-free survival rates were 78.75, 61.79 and 60.17%, respectively. The average follow-up time was 64 months (ranging from 11 to 72 months).
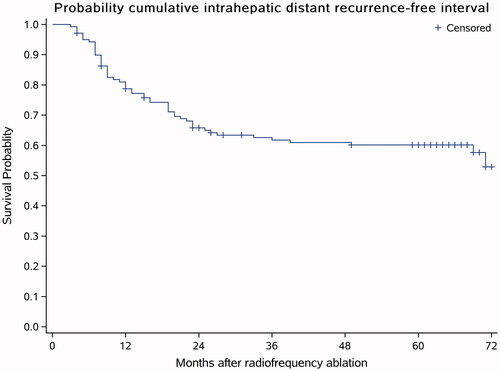
Table 3. Risk factors for IDR in univariate and multivariate analysis.
Overall survival
The average follow-up time was 59.33 ± 15.34 months, the median follow-up was 64 months, and one patient was lost to follow-up after 24 months. During follow-up, 27 (19.42%) patients died, and among these 27, 26 (18.70%) were associated with HCC progression and one (0.71%) had hemorrhage from gastric fundal varices. The 1-year, 3-year and 5-year cumulative survival rates were 98.56, 90.64 and 82.60%, respectively (). In the univariable and multivariable analysis, the serum AFP level (p < .001) was the only independent risk factor for overall survival (). The tumor size and the number of tumors had no significant impact on overall survival. For the Cox proportional hazards model, an independent sample t-test indicated that the recurrence interval in the surviving patients was significantly longer than that of the deceased patients ().
Figure 5. Overall survival curve. The average follow-up time was 59.33 ± 15.34 months, the median follow-up time was 64 months, and 1 patient was lost to follow-up after 24 months. During the follow-up, 27 (19.42%) people died. Among the 27 dead patients, the deaths of 26 patients (18.70%) were associated with HCC progression and one patient (0.71%) had gastric fundus hemorrhage.
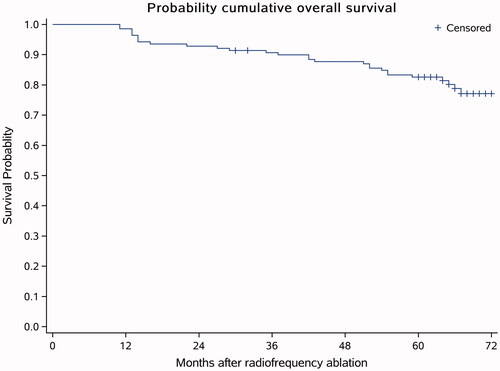
Table 4. Risk factors for overall survival in univariate and multivariate analysis.
Table 5. Comparison of the recurrence time and different survival states.
RFA complications
All ablation-related complications and side effects were defined as described by Ahmed et al. [Citation20]. During the entire study period, two complications were observed, and the incidence of complications was 1.43%. The complications included one case of mild bleeding at the liver puncture site and one case of gallbladder perforation. For the case of mild bleeding at the liver puncture site, clinical observation was performed without special treatment. The complication of gallbladder perforation occurred in the region next to the HCC. The thermal conduction of the RFA electrode led to this gallbladder perforation, which was found and confirmed during the RFA process. As such, cholecystectomy was immediately conducted via laparoscopy. After the operation, the patient healed and was discharged.
Discussion
In our prospective multicenter study, switching from monopolar RFA to using multiple electrodes in patients with HCCs showed a high local tumor control rate (99.03%, 205/207). For complete necrosis with RFA, the treatment outcomes in our study using multiple electrodes were significantly better than those in previous studies in which percutaneous RFA was performed using a conventional overlapping technique with a single electrode [Citation21,Citation22]. In addition, the LTP after multiple-electrode RFA was lower than that of traditional single-electrode RFA. The improved therapeutic efficiency of switching from single-electrode RFA to multiple-electrode can be attributed to the ability of the multiple-electrode set-up to produce a greater ablation volume compared with that of using a single electrode [Citation23,Citation24]. In this study, the recurrence-free survival and the overall survival were similar to those reported previously, which confirmed the efficacy and the relative safety of thermal ablation [Citation25,Citation26].
We divided the intrahepatic recurrence after percutaneous RFA into LTP and IDR. LTP is related to residual tumor cells that have spread microscopically beyond the ablative margin, and LTP may be more strongly associated with the treatment methodology or result rather than the systemic condition of the patient [Citation27]. In contrast to LTP, IDR is most likely associated with systemic factors rather than local factors. The pathogenic mechanism of IDR was considered to be the result of either intrahepatic metastasis of the primary HCC or a multicenter source of HCC [Citation28,Citation29]. Therefore, we conducted an independent multicenter prospective analysis of multiple HCC-associated potential systemic risk factors for IDR recurrence and survival.
To improve the efficacy of RFA in the treatment of HCC, a complete understanding of the risk factors that affect the recurrence and prognosis is both essential and challenging. The study results showed that the ALP level and the number of tumors were the independent risk factors that affect IDR. It is consistent with previous reports showing that the preoperative ALP level was an independent factor that affected the postoperative recurrence of HCC patients [Citation30]. The increase in ALP level was mainly due to cholestasis and bile duct obstruction. The number of tumors affecting IDR may be associated with early intrahepatic micrometastasis and a multicenter source of HCC. Furthermore, patients with multiple tumors are known to have a higher tumor recurrence rate [Citation25]. In our study, LTP was identified in 15 (10.79%) of the 139 patients, and tumor size was the only important predictor of LTP. The 1-year, 3-year and 5-year LTP progression rates were 3.72, 8.04 and 9.62%, respectively. These rates were lower than those in other studies that used a traditional single-electrode RFA system. According to previous reports, the LTP rate was approximately 10% three years after RFA [Citation31,Citation32], suggesting that MESS-RFA provided a higher local tumor control than the traditional single-electrode RFA system. To reduce the incidence of LTP, we suggest that, in addition to using more advanced RFA methods (such as MESS-RFA), the ablation procedure should be carefully performed to ensure a sufficient and safe boundary, and a more detailed RFA procedure may be needed to treat larger tumors. Interestingly, we found that tumor size and the number of tumors were not factors that affected the survival rate. The explanation of this result could be that 1) MESS-RFA can result in a lower local recurrence rate and 2) the majority of these local recurrences were sufficiently limited to be completely ablated by iterative RFA.
The 1-year, 3-year and 5-year overall survival after RFA was estimated to be 98.56, 90.64 and 82.60%, respectively. These results were similar to previous studies that also performed RFA treatment for early HCC [Citation33,Citation34]. In addition, the comparison of the clinical characteristics between the early and late recurrence groups showed that the overall survival after RFA of the early recurrence patients was significantly lower than that of late recurrence patients. Recent studies have shown that the interval from RFA to recurrence was an independent prognostic factor for survival after recurrence [Citation35,Citation36]. This finding demonstrated that early recurrence mainly resulted from intrahepatic metastases, whereas most late recurrences may have a multicentric origin. Our current results may be consistent with the results of the previously mentioned studies.
Combination therapy (TACE plus RFA) is recommended to treat HCC tumors larger than 3 cm [Citation37,Citation38]. Our results are not directly comparable with previous studies dealing with sequential combined therapy since this regimen could lead to a longer hospital stay, more frequent discomfort, and complications than RFA for small HCCs [Citation39]. Still, for a single HCC of 3–5 cm, TACE plus RFA showed comparable therapeutic outcomes, and shorter hospital stays compared with surgical resection [Citation40]. In general, for HCCs > 3 cm, the treatment outcomes of TACE plus RFA are superior to RFA only and comparable to surgical resection [Citation37,Citation40–42]. Microwave ablation (MWA) is also a common approach for treating HCC. The therapeutic principle of MWA and RFA is similar, but MWA induces higher and quicker temperature increases than RFA [Citation43]. A previous meta-analysis suggested that MWA had similar local recurrence rates and overall survival, but a higher major complication rate than RFA when treating HCC [Citation44]. No-touch RFA is also an emerging technology using multiple electrodes [Citation45]. Due to data availability, our study did not explore the efficacy of other treatments, and future studies could compare the different treatments for HCC. When RFA is compared with hepatectomy as an initial treatment for HCC, RFA has some inherent drawbacks. First, the heat-sink effect, the major limitation of RFA, occurs when thermal energy dissipates by blood flow in adjacent vessels, and this could result in an inadequate ablation of tumors located near the main hepatic vessels [Citation46,Citation47]. Second, the LTP risk of RFA is higher than that of hepatectomy [Citation48]. In this study, however, the major complication rate was 1.43% (2 of 139 patients), and this result was consistent with the incidence of complications in other studies [Citation49–51]. All patients with major complications fully recovered without any severe adverse sequelae. For hepatectomy, the reported incidence of complications ranged from 8 to 25% [Citation52–54]. Furthermore, a recent publication exploring the efficacy of MESS-RFA treating HCC nodules (size 3.0–6.0 cm) in Europeans has also reported a low occurrence of procedure-related death or major complications (0%) [Citation55].
Our study has some limitations. Since the technique is challenging, less experienced operators might find it difficult to insert multiple needles in a pre-determined spatial configuration precisely. Also, patients in this study were only treated with MESS-RFA. Future research should include the control group of patients treated with other therapeutic strategies for comparison.
In summary, MESS-RFA is a safe and effective first-line treatment method for patients with early HCC, mainly due to its significantly reduced procedure-related mortality and morbidity. In this study, the tumor size and the number of tumors did not affect the overall survival of patients, likely due to the advantages of higher local tumor control and the reproducibility of the MESS-RFA. Early HCC with multiple high-ALP tumors had a higher recurrence rate, and the recurrence pattern is mainly LTP. Early HCC with high AFP levels and a shorter initial recurrence interval has a poorer prognosis. Patients with recurrence and eligible to MESS-RFA can undergo repeat MESS-RFA. Otherwise, treatments, such as liver transplantation or surgical resection may be a good strategy in those conditions.
Acknowledgments
The authors thank Chen Shu and all of the medical staff at the Clinical Research Center of Southwest Hospital and the Medical Record Library of Southwest Hospital.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Wallace MC, Preen DB, Short MW, et al. Hepatocellular carcinoma in Australia 1982–2014: increasing incidence and improving survival. Liver Int. 2019;39(3):522–530.
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262(1):43–58.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802.
- Feng K, Ma K, Liu Q, et al. Randomized clinical trial of splenic radiofrequency ablation versus splenectomy for severe hypersplenism. Br J Surg. 2011;98(3):354–361.
- Nishikawa H, Inuzuka T, Takeda H, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: a proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol. 2011;46(12):1418–1426.
- Nishikawa H, Kimura T, Kita R, et al. Radiofrequency ablation for hepatocellular carcinoma. International journal of hyperthermia: the official journal of European society for hyperthermic oncology. Int J Hyperthermia. 2013;29(6):558–568.
- Jiang J, Chen S, Li K, et al. Targeting autophagy enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis for hepatocellular carcinoma. International journal of hyperthermia: the official journal of European society for hyperthermic oncology. Int J Hyperthermia. 2019;36(1):499–510.
- Lupo L, Panzera P, Giannelli G, et al. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford). 2007;9(6):429–434.
- Medhat E, Abdel Aziz A, Nabeel M, et al. Value of microwave ablation in treatment of large lesions of hepatocellular carcinoma. J Dig Dis. 2015;16(8):456–463.
- Woo S, Lee JM, Yoon JH, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a Multiple-Electrode switching System-Mid-term results. Radiology. 2013;268(2):589–600.
- Ryu T, Takami Y, Wada Y, et al. Actual 10-year survival after surgical microwave ablation for hepatocellular carcinoma: a single-center experience in Japan. Ann Surg Oncol. 2019;26(12):4126–4133.
- Zhang W, Luo E, Gan J, et al. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J Surg Oncol. 2017;15(1):122.
- European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the LEASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- N'Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50(5):1475–1483.
- Lee DH, Lee JM, Kang TW, et al. Clinical outcomes of radiofrequency ablation for early hypovascular HCC: a multicenter retrospective study. Radiology. 2018;286(1):338–349.
- Sartori S, Tombesi P, Macario F, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248(2):670–679.
- Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241(1):116–124.
- Wang-Yuan Z, Jiang-Zheng Z, Lu YD, et al. Clinical efficacy of metronomic chemotherapy after cool-tip radiofrequency ablation in the treatment of hepatocellular carcinoma. International journal of hyperthermia: the official journal of European society for hyperthermic oncology. Int J Hyperthermia. 2016;32(2):193–198.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Cabassa P, Donato F, Simeone F, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol. 2006;186(5):S316–S321.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214(3):761–768.
- Weisbrod AJ, Atwell TD, Callstrom MR, et al. Percutaneous radiofrequency ablation with a multiple-electrode switching-generator system. J. Vasc. Interv. Radiol. 2007;18(12):1528–1532.
- Laeseke PF, Frey TM, Brace CL, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol. 2007;188(6):1485–1494.
- Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107(4):569–577.
- Liang P, Yu J, Yu XL, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012;61(7):1100–1101.
- Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992;15(5):948–963.
- Okuwaki Y, Nakazawa T, Shibuya A, et al. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43(1):71–78.
- Ng KK, Poon RT, Lo CM, et al. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008;12(1):183–191.
- Kim JM, Kwon CH, Joh JW, et al. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40.
- Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967.
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17(3):684–692.
- Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58(1):89–97.
- Takahashi S, Kudo M, Chung H, et al. Outcomes of nontransplant potentially curative therapy for early-stage hepatocellular carcinoma in child-pugh stage a cirrhosis is comparable with liver transplantation. Dig Dis. 2007;25(4):303–309.
- Vouche M, Kulik L, Atassi R, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: imaging analysis from a prospective randomized trial of Y90 +/- Sorafenib. Hepatology. 2013;58(5):1655–1666.
- Rhim H. Recent advance of local ablation for hepatocellular carcinoma. J Korean Med Assoc. 2013;56(11):964–971.
- Takuma Y, Takabatake H, Morimoto Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269(3):927–937.
- Saviano A, Iezzi R, Giuliante F, Hepato CSG, et al. Liver resection versus radiofrequency ablation plus transcatheter arterial chemoembolization in cirrhotic patients with solitary large hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28(11):1512–1519.
- Kim W, Cho SK, Shin SW, et al. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol (NY). 2019;44(6):2283–2292.
- Lee HJ, Kim JW, Hur YH, et al. Conventional chemoembolization plus radiofrequency ablation versus surgical resection for single, Medium-Sized hepatocellular carcinoma: propensity-score matching analysis. J Vasc Interv Radiol. 2019;30(3):284–292. e281.
- Kagawa T, Koizumi J, Kojima S, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010;116(15):3638–3644.
- Bholee AK, Peng K, Zhou Z, et al. Radiofrequency ablation combined with transarterial chemoembolization versus hepatectomy for patients with hepatocellular carcinoma within Milan criteria: a retrospective case-control study. Clin Transl Oncol. 2017;19(7):844–852.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and Meta-analysis. Int J Hyperthermia. 2016;32(3):339–344.
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and Meta-analysis. Int J Hyperthermia. 2019;36(1):264–272.
- Lee DH, Lee MW, Kim PN, et al. Outcome of No-Touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology. 2021;301(1):229–236.
- Quirk MT, Pomykala KL, Suh RD. Current readings: percutaneous ablation for pulmonary metastatic disease. Semin Thorac Cardiovasc Surg. 2014;26(3):239–248.
- Prud’homme C, Deschamps F, Moulin B, et al. Image-guided lung metastasis ablation: a literature review. International journal of hyperthermia: the official journal of European society for hyperthermic oncology. North Am Hyperthermia Group. 2019;36(2):37–45.
- Choi D, Lim HK, Kim MJ, et al. Recurrent hepatocellular carcinoma: percutaneous radiofrequency ablation after hepatectomy. Radiology. 2004;230(1):135–141.
- Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11(6):914–921.
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103(6):1201–1209.
- Cho YK, Kim JK. Sustained complete response and low complication rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis. Hepatology. 2008;47(5):1791–1791.
- Nomi T, Fuks D, Govindasamy M, et al. Risk factors for complications after laparoscopic major hepatectomy. Br J Surg. 2015;102(3):254–260.
- Ratti F, Cipriani F, Ariotti R, et al. Laparoscopic major hepatectomies: current trends and indications. A comparison with the open technique. Updates Surg. 2015;67(2):157–167.
- Komatsu S, Brustia R, Goumard C, et al. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surg Endosc. 2016;30(5):1965–1974.
- Francica G, Altiero M, Laccetti E, et al. Long-term follow-up of unresectable medium-large hepatocellular carcinoma nodules treated with radiofrequency ablation using a multiple-electrode switching system. BJR. 2018;92(1093):20180625.