Abstract
Objective
To evaluate the clinical efficacy and safety of low-intensity ultrasound (LIUS) in promoting uterine involution and relieving postpartum pain.
Methods
The randomized controlled clinical trial in this study was conducted at five centers in three regions across China from June 2014 to December 2014. A total of 498 subjects were randomly divided into two groups. The LIUS group received ultrasound treatment, and the control group received sham ultrasound treatment. The fundal height and visual analogue scale (VAS) scores of the subjects following cesarean section were recorded separately before and after five treatments. The incidence of adverse events was recorded, while the records on lochia duration were obtained by telephone follow-up. The Full Analysis Set (FAS) comprised all subjects randomized who received at least one treatment. The Per-Protocol Set (PPS) comprised all patients who did not seriously violate the study protocol and had good compliance with complete report forms. Efficacy analyses were performed based on the FAS and PPS. All safety analyses were performed based on the safety set (SS), which included all patients who received at least one treatment.
Results
In the analysis of PPS and FAS, the LIUS group performed better than the control group in reducing the fundal height, shortening the duration of lochia, and relieving postpartum pain, with a significant difference between the two groups (p < 0.0001). In the SS analysis, there were no treatment-related adverse events observed in either group.
Conclusions
The LIUS therapy is safe and effective, which contributes to uterine involution and the alleviation of postpartum pain.
Introduction
The uterine changes greatly during pregnancy and returns to its pre-pregnant function and size after delivery. This process of self-repairing called uterine involution takes 6–8 weeks. If the uterus fails to return to the non-pregnant state within the 6-week period following delivery, poor uterine involution may occur. The main clinical symptoms of poor uterine involution include uterine atony, prolonged duration of bloody lochia, and abdominal pain. Uterine atony is responsible for 50–80% of all cases of postpartum hemorrhage (PPH) [Citation1,Citation2]. Hemorrhage due to uterine atony is a much more common cause of bleeding than placental abruption, placenta accreta, placenta praevia, and peripartum hysterectomy [Citation3]. The main symptoms of abnormal bleeding/lochia after cesarean section include bleeding volume greater than 500 mL or more [Citation4]/bloody lochia more than 4 days, the total duration of lochia more than 42 days with odorous discharge, and bloody lochia greater than menstrual flow. In recent years, poor uterine involution becomes more common due to the increase of the average female childbearing age [Citation5] and the high rate of cesarean section [Citation6], with the incidence rate of about 22–39% [Citation7]. If poor uterine involution cannot be treated in a timely manner, unclean lochia, repeated intermittent blood lochia and uterine pain may occur, resulting in endometritis, and ultimately increasing menstrual volume and prolonged menstrual time, and even secondary infertility, which seriously affects maternal health both physically and mentally. Therefore, reducing the incidence of poor uterine involution is an urgent clinical need, and postpartum care is essential to accelerate uterine involution and avoid complications.
The current treatment methods for uterine involution include physical therapy and drug therapy. Oxytocin is recognized as the preferred drug [Citation8], but it has a receptor saturation effect. Excessive doses of oxytocin will no longer increase uterine contraction, but lead to hypertension and accelerated pulse rate [Citation9]. Uterine massage [Citation10] and electroacupuncture [Citation11] in physical therapy have been used in clinical practice, but the efficacy varies due to different operators, and the onset time is longer. Microwave irradiation has a certain effect on uterine involution, but long-term or high-power irradiation often inhibits the body’s immune function [Citation12]. The above treatments have both advantages and disadvantages, but the overall effectiveness remains poor.
Ultrasound therapy is widely used in clinical practice as a new noninvasive treatment technology, and its effectiveness and safety have been proven [Citation13–15]. As shown in mouse models, Low-intensity ultrasound (LIUS) promotes the contraction of uterine smooth muscle and can increase the frequency and amplitude of spontaneous uterine contractions [Citation16]. Nizard et al. showed that high-intensity focused ultrasound exposure of the postpartum uterine arteries in ewes reduced the diameter of the target vessel, resulting in a slowing of blood flow downstream of the vessel. The above research suggested that ultrasound can cause vascular smooth muscle to contract, which may play a role in postpartum hemorrhage [Citation17]. Nevertheless, the clinical benefits of LIUS on human uterine involution have received less attention. In this study, we evaluated the clinical efficacy and safety of LIUS in promoting uterine involution and alleviating postpartum pain by directly measuring the fundal height following LIUS treatment and recording the duration of lochia and the score changes in the visual analogue scale (VAS). Overall, this study aimed at providing an insight into the clinical treatment of postpartum uterine involution.
We present the following article in accordance with the CONSORT reporting checklist.
Methods
Standard protocol approvals, test centers and patient consents
The prospective randomized controlled clinical trial was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital on 19 June 2014 (approval No. 2014-20). This study was conducted from June 2014 to December 2014 in the First Affiliated Hospital of Chongqing Medical University, the Second Affiliated Hospital of Chongqing Medical University, Xinqiao Hospital Army Medical University, Shanghai First Maternity and Infant Hospital, Peking Union Medical College Hospital in accordance with the Declaration of Helsinki (2013 edition). All subjects can understand the study and have signed informed consent forms.
Inclusion criteria: 37 weeks of pregnancy and above; no serious complications in prenatal checkup; breastfeeding and exercise as prescribed by doctors after delivery; transverse uterine incision;.
Exclusion criteria: vertical uterine incision; concurrent malignant tumors, skin diseases, cutaneous sensory disorder, collagenous connective tissue diseases during pregnancy; scar constitution; intrahepatic cholestasis of pregnancy (ICP); coagulation disorders; packing with gauze in the uterine cavity; severe abdominal distension or obesity leading to the inaccessibility to the uterine fundus, etc.
Discontinuation criteria: Patients who require to withdraw from the trial due to the aggravation of clinical symptoms: in this case, the efficacy should be recorded. Patients who withdraw from the clinical observation due to serious adverse effects: in this case, the efficacy is not evaluated, but the safety should be recorded.
Study design
All subjects who complied with the study design criteria were assessed. Subsequently, patients’ medical history, age, height, weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, heart rate, intraoperative blood loss, number of deliveries, and infant birth weight were recorded. Patients were divided into LIUS group and control group at a 1:1 ratio in a random and double-blinded manner. Randomization was performed using a computer- generated list of random numbers with equal numbers in each group. A statistician who was unaware of the enrollment status assigned subjects consecutively to treatment codes that corresponded to labels on other identical concealed containers. Patients, investigators, and analysis staff were blinded to the treatment for the study.
Therapeutic method
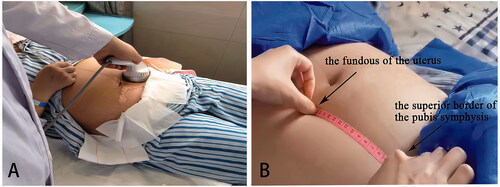
Both the LIUS group and the control group received routine obstetric treatment. The subjects in the LIUS group received Model-CKC100 Ultrasound Therapeutic Device for Involution of Uterus (Chongqing Haifu Medical Technology Co., Ltd, Chongqing, China) for ultrasound therapy. Parameters: Operating frequency 0.7 MHz–1.0 MHz; input power ≤100VA. Focus area: 0.09–0.12 cm2, spatial peak time average sound intensity: <3 W/cm2. There are four gears in terms of pulse duration: 2 ms, 4 ms; 6 ms; 8 ms. Patients were instructed to empty their bladders before every treatment and lie on the treatment bed in the supine position with a layer of ultrasound coupling agent applied to the treatment area (i.e., the surface projection area of the uterine). The ultrasound treatment probe was covered with a disposable acoustically transparent protective membrane, and then pressed on the treatment area to the fundus of the uterus (). The default gear at startup is the second gear. The treatment probe worked in a mobile way and we asked about the patient’s feelings after one minute. If the patient has no obvious pain or heat, the gear can be adjusted up. If the patient suffered from unbearable pains or heat, we then increased the dose of the ultrasound coupling agent, adjusted the moving speed of the treatment probe and adjusted down the gear. The treatment process was based on the principle that the patient has no obvious discomfort. The operation method and time of the control group were consistent with the LIUS group, but the control group did not output ultrasound energy. A total of five treatments were performed with two treatments a day and each one lasting 30 min. The first treatment was 6 h after the cesarean section and the others were on the second and third day respectively. All patients received 20 U of intravenous oxytocin once a day for the first 2 days after cesarean section. Certain personnel was designated to regularly measure the fundal height before and after treatments. The patient was asked to lie flat on the treatment bed after emptying the bladder. Then, the external contour of the uterus was massaged and made contracted and harden, and then the fundal height was measured by a soft ruler (the distance from the midpoint of the superior border of the pubis symphysis to the fundus of the uterus measured along the linea mediana ventralis) (). Finally, lochia assessment, VAS score changes and adverse events recordings were conducted.
Follow up
On the 7, 14, 21, 28, and 35 days after delivery, we followed up by telephone to inquire and record the patients' lochia condition (lochia color, lochia amount) and the duration of lochia. In case of discomforts such as abdominal pain, bloody lochia greater than menstrual flow or odorous discharge, the patients received prompt treatment. On the 42 day after delivery, patients returned to the hospital to evaluate the uterine involution.
Evaluation criteria for efficacy and safety
Efficacy indicators (primary outcome indicators): (1) The degree of fundal height decrease: The fundal heights of subjects of the two groups before and after each treatment were measured. The significant difference in the decrease in the fundal height in the last treatment between subjects in the LIUS group and the control group indicated a treatment efficacy.
(2) The total duration of lochia and the duration of bloody lochia: The significant difference in the decrease in the total duration of lochia and the duration of bloody lochia between subjects in the LIUS group and the control group indicated a treatment efficacy. The total duration of lochia >42 days demonstrated the poor uterine involution [Citation18].
Secondary outcome indicators (postpartum pain score): The VAS scaleplate (10 cm long, millimeter-scale) made under the supervision of the Chinese Association for the Study of Pain was used to determine the pain degree [Citation19]. 0 cm: no pain; below 3 cm: mild and tolerable pain; 4–6 cm: sleep-affected but tolerable pain; 7–9 cm: severe and intolerable pain that affects appetite and sleep; 10 cm: the worst pain. The subjects can adjust the sclae of the VAS scaleplate by the pain degree. The VAS scores were recorded before and after each treatment and the significant difference in the score changes between subjects in the LIUS group and the control group indicated a treatment efficacy.
Safety evaluation criteria: The adverse reactions and adverse events related to clinical trials were observed, including skin damage, abdominal wall discomfort and all other unfavorable events affecting maternal health.
Sample size
According to the results of the preliminary ‘clinical validation’, the fundal height in the LIUS group and the control group decreased by 3.2 ± 1.6 cm and 1.6 ± 1.4 cm respectively after treatments. If the fundal height in the LIUS group decreased at least 1 cm more than that in the control group after treatments, the difference was considered to be a clinical significance. According to our pretrial calculation, with a Type I error rate of 0.05, a Type II error of 0.1 (i.e., a test efficacy of 90%), an estimated sample size of 150 and a 20% drop-out rate, no less than 180 cases would be included in each of the LIUS and control groups and no less than 360 cases would be enrolled in this study. Finally, we expanded the observation sample size according to the actual situation and there were 500 cases in 5 centers with 100 each.
Statistical analysis
Statistical analysis method: All continuous data were presented as a mean value ± standard deviation. For the comparative analysis of the two groups, quantitative data conforming to a normal distribution were tested by t-test (the homogeneity of variance between groups was tested, with 0.05 as the test criteria, data were compared using Satterthwaite t’test when the variance was not homogeneous). χ2 test or Fisher’s exact probability test was used for inter-group comparisons in the dropout rate and the incidence of adverse events. The significance level for all statistical analyses was set at p < 0.05. A repeated measures analysis of variance (ANOVA) with a Greenhouse–Geisser correction was performed to test the difference in the time points (1-, 2-, and 3-day following treatment) between two groups. All statistical analyses were performed using SAS9.13 software.
Statistical analysis data set: According to the intent-to-treat (ITT) principles, the Full Analysis Set (FAS) comprised all subjects randomized who received at least one treatment. The missing data regarding efficacy in the FAS were processed with Last Observation Carried Forward (LOCF). The per-protocol set (PPS) comprised all patients who did not seriously violate the study protocol, had good compliance, and whose case report forms were complete. The FAS and PPS were both used for efficacy evaluation. The Safety Analysis Set (SS) included data of subjects who underwent at least one treatment after randomized grouping with their safety assessments were recorded. This dataset was applied to safety evaluation.
Results
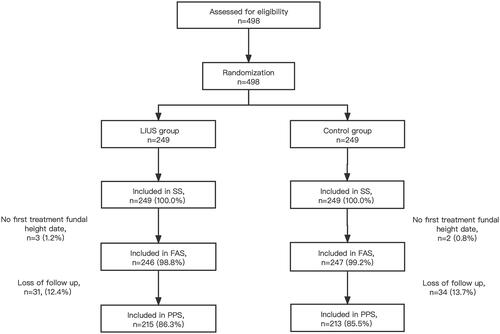
As shown in , a total of 498 subjects from 5 centers in 3 regions were included in this study according to the exclusion and inclusion criteria. Subjects were randomly divided into the LIUS group (n = 249) and the control group (n = 249). 493 FAS cases (246 in the LIUS group and 247 in the control group) accounted for 99.0% of all the included cases. 428 PPS cases (215 in the LIUS group and 213 in the control group) accounted for 85.9%. 498 SS cases (249 in the LIUS group and 249 in the control group) accounted for 100.0%. The drop-out rate of subjects was 13.7% (34/249) in the LIUS group, and 14.5% (36/249) in the control group, and the P value for Fisher's exact was 0.8975.
The baseline demographics and clinical characteristics of the two groups are shown in . In the FAS and the PPS, there were no significant differences in age, height, weight and body mass index between the LIUS group and the control group (p > 0.05), indicating the comparability between the two groups.
Table 1. Baseline demographic and clinical characteristics (FAS).
Primary endpoint of clinical efficacy
As shown in , the baseline fundal height in the LIUS group and the control group was (FAS, 17.58 ± 2.24 cm vs. 17.45 ± 2.15 cm, t = 0.61, p = 0.5439; PPS, 17.73 ± 2.08 cm vs. 17.51 ± 2.19 cm, t = 0.99, p = 0.3204). The differences between the two groups were not statistically significant. After treatment five times, the fundal height in the LIUS group and the control group decreased to (FAS, 14.42 ± 1.80 cm vs. 15.64 ± 2.27 cm, p < 0.0001; PPS, 14.18 ± 1.66 cm vs. 15.66 ± 2.27 cm, p < 0.0001). The fundal height changes in the LIUS group before and after treatment was much significant than that in the control group (p < 0.0001) (). As shown in , the Greenhouse-Geisser correction model was used in the ANOVA of the fundal height after five times of treatments. It was found that there were significant difference between groups (FAS, F = 15.39, p = 0.0001; PPS, F = 15.91, p < 0.0001) and time points (FAS, F = 420.92, p < 0.0001; PPS, F = 495.60, p < 0.0001), and there was a significant interaction between groups and time points (FAS, F = 25.66, p < 0.0001; PPS, F = 37.76, p < 0.0001).
Figure 3. (A) FAS; (B) PPS. Fundal height changes in both groups of subjects before and after five treatments. (C) FAS; (D) PPS. Fundal height difference between the two groups of subjects before and after five treatments. (a) After ultrasound treatment; (b) before ultrasound treatment.
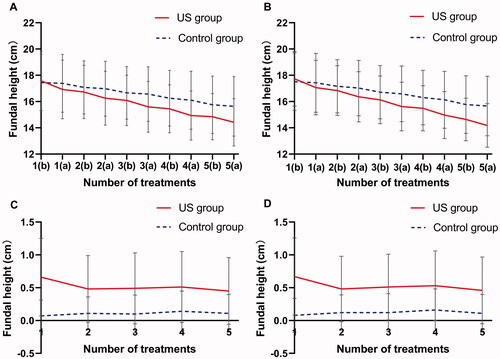
Table 2. Comparison of repeated measurement of uterine height before and after five treatments between the two groups of subjects.
As shown in , the total duration of lochia was significantly shorter in the LIUS group compared to that in the control group (29.88 ± 7.53 days vs. 34.46 ± 9.84 days, p < 0.0001), and the duration of bloody lochia was shorter (5.76 ± 2.98 days vs. 7.06 ± 3.72 days, p = 0.0002), showing that the difference between the two groups was statistically significant. Compared with the control group, the LIUS group achieved better outcomes in uterine involution (Fisher p = 0.0001) ().
Figure 4. Comparison of the effect of uterine involution between the two groups of subjects. Good uterine involution, the total duration of lochia? 42 days; Poor uterine involution, the total duration of lochia >42 days.
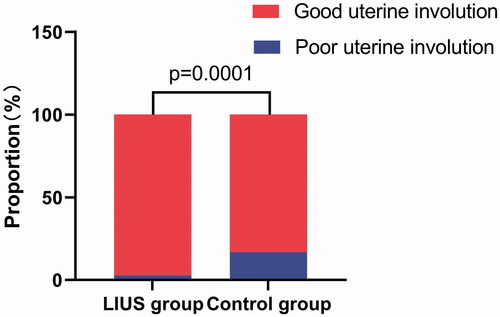
Table 3. Comparison of the total duration of lochia and the duration of bloody lochia between the two groups of subjects (PPS).
Secondary endpoints of clinical efficacy
As shown in , the baseline pain VAS scores in the LIUS group was higher than that in the control group (FAS, 5.95 ± 2.11 vs. 5.30 ± 1.46, t = 3.83, p = 0.0001; PPS, 6.17 ± 1.94 vs. 5.44 ± 1.39, t = 4.33, p < 0.0001), indicating that the difference between the two groups was statistically significant. After five times of treatments, the pain VAS scores of the LIUS group and the control group decrease to (FAS, 1.86 ± 1.27 vs. 4.10 ± 1.13, p < 0.0001; PPS, 2.17 ± 1.08 vs. 4.07 ± 1.12, p < 0.0001), which indicated a statistically significant difference between the two groups. The VAS scores changes of the LIUS group before and after treatments are much significant than that of the control group (p < 0.0001) ()). As shown in , the Greenhouse-Geisser correction model was used in the ANOVA of the VAS scores after five times of treatments. It was found that there were significant difference between groups (FAS, F = 55.16, p = 0.0001; PPS, F = 96.55, p < 0.0001) and time points (FAS, F = 277.82, p < 0.0001; PPS, F = 307.91, p < 0.0001), and there was a significant interaction between groups and time points (FAS, F = 194.45, p < 0.0001; PPS, F = 231.45, p < 0.0001).
Figure 5. (A) FAS; (B) PPS. Pain VAS scores changes in both groups of subjects before and after five treatments. (C) FAS; (D) PPS. Pain VAS scores difference between the two groups of subjects before and after five treatments. (a) After ultrasound treatment; (b) before ultrasound treatment.
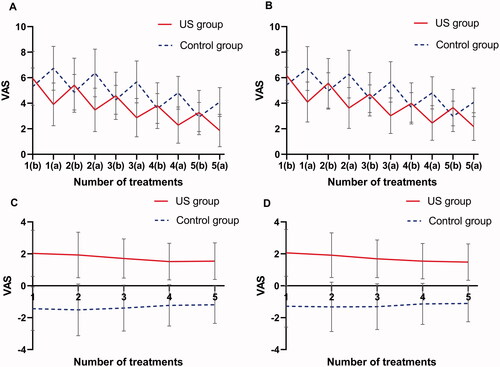
Table 4. Comparison of repeated measurement of VAS scores before and after five treatments between the two groups of subjects.
Safety
All subjects were well tolerated during the treatments, without mild adverse reactions such as skin redness and abdominal wall discomfort in the treated area and other severe adverse events such as nerve injury and skin infection. As of the end of the research, no treatment-related adverse events occurred in patients.
Discussion
PPH is divided into primary and secondary types. Primary PPH (early PPH) occurs within 24 h after delivery, and secondary PPH (late or delayed PPH) occurs 24 h to 12 weeks after delivery, with a blood loss of 500 mL or more [Citation4]. It has been estimated that 32% (997/3164) of maternal deaths in China are related to PPH [Citation20]. Uterine atony is the most common cause of PPH and the main symptom of poor uterine involution. Increasing the frequency of uterine contractions can minimize the risk of postpartum complications. This study was based on real data and demonstrated a sharper decline of the fundal height, a shorter total duration of lochia and a shorter duration of bloody lochia in the LIUS group compared with the sham-LIUS group. Thus, promoting uterine contraction effectively is proved as an effective way to prevent and treat PPH. Although patients experienced a temporary increase in VAS due to uterine paroxysmal pain during the treatment, the LIUS group VAS score was significantly lower than the control group (p < 0.0001). Since no treatment-related adverse events occurred throughout the process, the study can give an insight into the enhancement of the well-being of puerpera. Thus, this research provides a potential method to improve uterine involution and relieve postpartum pain after cesarean section.
Ultrasound is a kind of mechanical wave with a frequency of above 20 kHz [Citation21,Citation22], which has good tissue penetration, positioning performance and energy deposition. In addition, it can penetrate the surface tissue and focus on the target tissue at a specific depth to produce biological effects. Depending on the sound energy applied, ultrasonic waves can produce thermal or non-thermal effects, both of which have their own application ranges. Through instant hyperthermia, coagulation necrosis, protein denaturation, and cell apoptosis appear in the tissues in the target area [Citation23]. High-intensity ultrasound does not damage the normal tissues around the target area, which is mainly used in the treatment of solid tumors, such as gynecological tumors [Citation24,Citation25]. LIUS is a kind of ultrasound with low frequency and intensity. That mainly provides non-thermal effects, including cavitation, mechanical stimulation and shock waves [Citation26]. It is not destructive and is often used to promote bone healing [Citation27], cartilage healing and regeneration [Citation28], inflammation inhibition [Citation29]. Moreover, LIUS has been clinically proven effective in promoting uterine contractions. However, its underlying mechanisms have not been fully depicted.
Ca2+ influx by store-operated calcium entry was required to maintain Ca2+ release events [Citation30], the increase of intracellular Ca2+ is one of the signals that initiate the smooth muscle excitation-contraction coupling mechanism [Citation31]. Previous experiments have shown that ultrasound therapy can enhance the absorption of Ca2+ at an intensity of 0.5–1.0 w/cm2 [Citation32]. LIUS changes the permeability of cell membranes to Ca2+ by opening the L-type calcium channels and activating the Ca2+ signaling pathway, thus promoting the release of Ca2+ and the influx of Ca2+ to generate action potentials to accelerate smooth muscle contraction [Citation33].
Oxytocin (OT) is the first-line drug to treat uterine atony [Citation7]. OT works through the binding of receptors whose amount affects the response of the uterus to OT [Citation34]. However, excessive doses of OT did not increase the expression of oxytocin receptor (OTR) expression in uterine smooth muscle. Large-dose injections will result in significant side effects and prolonged exposure to OT will cause desensitization [Citation35,Citation36]. However, Zhang et al. showed that after ultrasound exposure of SD rats, the uterine tissue was removed to detect the level of OTR by immunohistochemistry, and it was found that LIUS promoted the increase of OTR in myometrium cells and endometrial cells of SD rats [Citation37]. Cavitation causes shear force, microjets, microstreams, and reactive radicals that increase the permeability of the cell membrane, thereby favoring drug influx [Citation38–40], and enhancing the effect of OT on uterine contraction. The result of this study showed a sharper decline of the fundal height in the LIUS group receiving OT accompanied by ultrasound than that of the control group.
It is clinically suggested that uterine massage can be used to stimulate uterine contractions [Citation9], indicating that mechanical stimulation can effectively promote uterine smooth muscle contraction. We can use the mechanical effect of low-intensity ultrasound to stimulate uterine contractions, thus avoiding the inconsistencies and irregularities in the timing, speed, force and technique of manual uterine massage. The contraction of the uterus is not as strong as in vaginal deliveries due to injury, so it is even more important to take active and effective measures to restore the uterus in women who have had a cesarean section.
Women suffer different types of pain and discomfort after delivery, including incision pain after cesarean section and uterine involution pain [Citation41]. Acute and chronic pain caused by severe tissue trauma after delivery may increase the risk of postpartum depression and affect women to return to normal activities and care for their babies [Citation42]. The incidence of chronic pain ranges from 1% to 18% [Citation43], many scholars are paying more attention to the postpartum pain of parturients [Citation44,Citation45]. Ultrasound therapy can downregulate inflammatory mediators to promote extracellular matrix production [Citation46], and LIUS shows significant effects on cell proliferation and collagen deposition [Citation47]. Xin et al. reported that soft tissue regeneration can be accelerated by promoting cell proliferation in the disease [Citation48]. Jia et al. also confirmed that ultrasound in the treatment of knee osteoarthritis (KOA) has been proved to be a safe and effective treatment, which can relieve the pain of patients with KOA [Citation49]. The effect of reducing pain is remarkable when treating soft tissue injuries by ultrasound therapy. In this study, the VAS score of the LIUS group shows a sharper decline than that of the control group, with a significant difference (p < 0.0001). We believe that ultrasound has the same ability to provide postpartum analgesia for the smooth muscle of the uterus, as a type of soft tissue.
There are some limitations in this study: (i) The description of lochia might be affected by patients’ individual cognitions and subjective feelings in the telephone follow-up, which may cause bias in the results. Therefore, further study should adopt more objective quantitative criteria. (ii) we speculated from previous studies that LIUS could promote uterine involution and relieve pain through different mechanisms. Our next plan is to further study the mechanism of ultrasound through more animal experiments.
In conclusions, the present study has shown that LIUS is a safe and effective treatment for uterine involution. We believe that LIUS contributes to uterine contraction after cesarean section, shortens the duration of lochia and relieves postpartum pain, thus shortening the progress period of uterine involution, decreasing the incidence of poor uterine involution and reducing vaginal bleeding. We should pay more attention to non-drug therapy such as LIUS treatment, which has no side effects, and provide strong clinical trial support for its wide application in promoting uterine involution. Overall, the present study makes a contribution that may have important implications for future ultrasound research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- World Health Organization. WHO recommendations: uterotonics for the prevention of postpartum haemorrhage. Geneva: World Health Organization 2018.
- Bateman BT, Berman MF, Riley LE, et al. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;2(2):CD007412.
- Diaz V, Abalos E, Carroli G. Methods for blood loss estimation after vaginal birth. Cochrane Database Syst Rev. 2018;9(9):CD010980.
- Herstad L, Klungsøyr K, Skjaerven R, et al. Elective cesarean section or not? Maternal age and risk of adverse outcomes at term: a population-based registry study of low-risk primiparous women. BMC Pregnancy Childbirth. 2016;16:230.
- Mi J, Liu F. Rate of caesarean section is alarming in China. Lancet. 2014;383(9927):1463–1464.
- Guan-Yue SU, Jia-Jie YU, You-Ping LI. Effect of motherwort injection in promoting postpartum involution of uterus: a Meta-analysis. Chin J Evid-Based Med. 2016;016(011):1313–1321.
- Stadler B, Whittaker MR, Exintaris B, et al. Oxytocin in the male reproductive tract; the therapeutic potential of oxytocin-agonists and-antagonists. Front Endocrinol (Lausanne). 2020;11:565731.
- Phaneuf S, Asbóth G, MacKenzie IZ, et al. Effect of oxytocin antagonists on the activation of human myometrium in vitro: atosiban prevents oxytocin-induced desensitization. Am J Obstet Gynecol. 1994;171(6):1627–1634.
- Muñoz M, Stensballe J, Ducloy-Bouthors AS, et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus. 2019;17(2):112–136.
- Ma W, Bai W, Lin C, et al. Effects of Sanyinjiao (SP6) with electroacupuncture on labour pain in women during labour. Complement Ther Med. 2011;19 (Suppl 1):S13–S8.
- Szmigielski S. Reaction of the immune system to low-level RF/MW exposures. Sci Total Environ. 2013;454–455:393–400.
- Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189(1):48–54.
- Feng Y, Hu L, Chen W, et al. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem. 2017;36:139–145.
- Li C, Bian D, Chen W, et al. Focused ultrasound therapy of vulvar dystrophies: a feasibility study. Obstet Gynecol. 2004;104(5 Pt 1):915–921.
- Ter Haar G, Dyson M, Talbert D. Ultrasonically induced contractions in mouse uterine smooth muscle in vivo. Ultrasonics. 1978;16(6):275–276.
- Nizard J, Pessel M, De Keersmaecker B, et al. High-intensity focused ultrasound in the treatment of postpartum hemorrhage: an animal model. Ultrasound Obstet Gynecol. 2004;23(3):262–266.
- Xie X, Kong BH, Duan T, et al. Obstetrics and gynecology. Beijing: People’s Medical Publishing House; 2018.
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101.
- Feng XL, Zhu J, Zhang L, et al. Socio-economic disparities in maternal mortality in China between 1996 and 2006. BJOG. 2010;117(12):1527–1536.
- Zhu B, Xin C, Li J, et al. Ultrasonic degradation of konjac glucomannan and the effect of freezing combined with alkali treatment on their rheological profiles. Molecules. 2019;24(10):1860.
- Miller DL, Smith NB, Bailey MR, et al. Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4):623–634.
- Toccaceli G, Barbagallo G, Peschillo S. Low-intensity focused ultrasound for the treatment of brain diseases: safety and feasibility. Theranostics. 2019;9(2):537–539.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96(6):707–714.
- Zhang R, Chen JY, Zhang L, et al. The safety and ablation efficacy of ultrasound-guided high-intensity focused ultrasound ablation for desmoid tumors. Int J Hyperthermia. 2021;38(2):89–95.
- Delalande A, Bastié C, Pigeon L, et al. Cationic gas-filled microbubbles for ultrasound-based nucleic acids. Biosci Rep. 2017;37(6):BSR20160619.
- Leighton R, Watson JT, Giannoudis P, et al. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): a systematic review and Meta-analysis. Injury. 2017;48(7):1339–1347.
- Song BW, Park JH, Kim B, et al. A combinational therapy of articular cartilage defects: rapid and effective regeneration by using Low-Intensity focused ultrasound after adipose tissue-derived stem cell transplantation. Tissue Eng Regen Med. 2020;17(3):313–322.
- Nakao J, Fujii Y, Kusuyama J, et al. Low-intensity pulsed ultrasound (LIPUS) inhibits LPS-induced inflammatory responses of osteoblasts through TLR4-MyD88 dissociation. Bone. 2014;58:17–25.
- Drumm BT, Rembetski BE, Cobine CA, et al. Ca2 + signalling in mouse urethral smooth muscle in situ: role of Ca2 + stores and Ca2 + influx mechanisms. J Physiol. 2018;596(8):1433–1466.
- Matthew A, Shmygol A, Wray S. Ca2+ entry, efflux and release in smooth muscle. Biol Res. 2004;37(4):617–624.
- Martin EM, Duck FA, Ellis RE, et al. Ultrasound-induced contraction of the carotid artery in vitro. Ultrasound Med Biol. 2010;36(1):166–172.
- Ren Y, Zhu Y, Liu L, et al. Ultrasound induces contraction of the bladder smooth muscle. Int Urol Nephrol. 2016;48(8):1229–1236.
- Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol. 2014;26(6):356–369.
- Balki M, Erik-Soussi M, Kingdom J, et al. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552–561.
- Magalhaes JK, Carvalho JC, Parkes RK, et al. Oxytocin pretreatment decreases oxytocin-induced myometrial contractions in pregnant rats in a concentration-dependent but not time-dependent manner. Reprod Sci. 2009;16(5):501–508.
- Zhang Y, Guo J, Lin C, et al. Effect of low-intensity focused ultrasound on endothelin-1, nitrogen monoxide and oxytocin receptor in the uterine tissues of Sprague-Dawley rats following abortion. Biomed Rep. 2016;4(3):340–344.
- Burgess A, Hynynen K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11(5):711–721.
- Huang D, Wang L, Dong Y, et al. A novel technology using transscleral ultrasound to deliver protein loaded nanoparticles. Eur J Pharm Biopharm. 2014;88(1):104–115.
- May JP, Li SD. Hyperthermia-induced drug targeting. Expert Opin Drug Deliv. 2013;10(4):511–527.
- Deussen AR, Ashwood P, Martis R, et al. Relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev. 2020;10(10):CD004908.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140(1):87–94.
- Landau R, Bollag L, Ortner C. Chronic pain after childbirth. Int J Obstet Anesth. 2013;22(2):133–145.
- Gaudet C, Wen SW, Walker MC. Chronic perinatal pain as a risk factor for postpartum depression symptoms in Canadian women. Can J Public Health. 2013;104(5):e375-87.
- Lim G, LaSorda KR, Farrell LM, et al. Obstetric pain correlates with postpartum depression symptoms: a pilot prospective observational study. BMC Pregnancy Childbirth. 2020;20(1):240.
- Jia L, Chen J, Wang Y, et al. Focused low-intensity pulsed ultrasound affects extracellular matrix degradation via decreasing chondrocyte apoptosis and inflammatory mediators in a surgically induced osteoarthritic rabbit model. Ultrasound Med Biol. 2016;42(1):208–219.
- Bohari SP, Grover LM, Hukins DW. Pulsed low-intensity ultrasound increases proliferation and extracelluar matrix production by human dermal fibroblasts in three-dimensional culture. J Tissue Eng. 2015;6:204173141561577. 2041731415615777.
- Xin Z, Lin G, Lei H, et al. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol. 2016;5(2):255–266.
- Jia L, Wang Y, Chen J, et al. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci Rep. 2016;6:35453.