Abstract
Objective
To investigate the efficacy and safety of a novel method of hyperthermic intraperitoneal chemotherapy (HIPEC) as adjuvant therapy for stage-III gastric cancer.
Methods
Patients with stage-III gastric cancer who underwent D2 radical gastrectomy were randomly assigned to the HIPEC or control group four weeks after surgery. The HIPEC group was treated with cisplatin (60 mg/m2) administered with a HIPEC device on days 1 and 3 (30 mg/m2 each time), along with oral S-1, 40–60 mg, twice daily, for 14 days. The control group was treated with cisplatin (60 mg/m2) administered intravenously plus oral S-1 (40–60 mg, 2/d for 14 days). The primary outcome of the study was disease-free survival (DFS).
Results
Total 114 patients were included in the study, with 57 patients in each group. The median DFS was 29.0 months in the HIPEC group, which was significantly longer than that in the control group (15.0 months, p = 0.006). The two-year DFS rate in the HIPEC group was higher than that in the control group (50.4% vs. 25.5%). Median OS was 42.0 month in the HIPEC group and 31.0 month in the control (p = 0.042). Peritoneal metastasis occurred in six patients in the HIPEC group (10.5%) and 12 patients in the control (21.1%, p = 0.198). No significant difference in the incidence of adverse event except for thrombocytopenia.
Conclusion
HIPEC with cisplatin plus oral S-1 is a safe and effective adjuvant therapy for patients with advanced gastric cancer following D2 radical gastrectomy. Trial registration: This study was registered at ClinicalTrials.gov with the identifier (NCT number): NCT02396498
Background
Gastric cancer was the third leading cause of cancer-related death worldwide according to the GLOBOCAN data 2018 [Citation1]. In China, gastric cancer is the second most common cancer and the third cause of cancer-related death [Citation2,Citation3]. Though prognosis of advanced gastric cancer has been improved by D2 radical gastrectomy, the five-year recurrence rate of advanced gastric cancer is still up to 25–40% [Citation4–6]. Perioperative systemic chemotherapy is accepted as the standard therapy for treating patients with advanced gastric cancer. Longer overall survival was reported for perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel (FLOT) in advanced, resectable gastric or gastroesophageal junction adenocarcinoma compared to that with fluorouracil or capecitabine plus cisplatin and epirubicin [Citation7]. Adjuvant chemotherapy has also been reported to improve the outcomes of patients with gastric cancer after D2 radical surgery [Citation8,Citation9].
Peritoneum is the most frequent site of gastric cancer metastasis after radical surgery, especially for Borrmann types III and IV [Citation10]. Patients with postoperative peritoneal metastasis have significantly shorter survival time [Citation11–15]. Prevention and treatment of postoperative peritoneal metastasis is crucial to improve patient’s survival and quality of life for gastric cancer patients. However, conventional intravenous chemotherapy has limited effects on peritoneal metastasis. Hyperthermic intraperitoneal chemotherapy (HIPEC) is a topical chemotherapeutic method for treating peritoneal malignancies, which can be applied during or after surgery [Citation16,Citation17]. The combination of hyperthermia and intraperitoneal chemotherapy has significantly increased the efficacy of chemotherapeutic drugs [Citation18]. Cytoreductive surgery (CRS) plus HIPEC has now become a recognized therapeutic option for pseudomyxoma peritonei (PMP), mesothelioma and peritoneal metastasis in colon cancer [Citation17,Citation19].
A recent study suggested that prophylactic HIPEC using open circulation (coliseum) technique could improve survival of patients with advanced gastric cancer treated by D2 radical gastrectomy and showed a lower rate of peritoneal metastasis [Citation20]. We have previously reported a novel method of bedside HIPEC with closed circulation and confirmed that the method was safe in 5,759 times of HIPEC treatment for 985 patients with malignant pleural effusion or ascites [Citation21]. The current study was to investigate the efficacy of this bedside HIPEC combined with oral S-1 treatment as adjuvant therapy for stage-III gastric cancer patients after D2 radical surgery.
Methods
Patient recruitment
From April 2014 through April 2018, total 120 patients with stage-III gastric cancer treated with D2 radical gastrectomy were recruited. Patients were screened at three weeks after the surgery. The inclusion criteria include: (1) patients must meet the diagnostic criteria of stage-III gastric cancer by the Union for International Cancer Control (UICC) as well as the seventh edition of the American Joint Committee on Cancer (AJCC) staging system; (2) No previous history of chemotherapy, radiotherapy, or immunotherapy; (3) Patients’ Eastern Cooperative Oncology Group (ECOG) score was ≤1; (4) The predicted survival time was ≥6 months; (5) No contradiction to chemotherapy as suggested by laboratory tests, electrocardiograph or comorbidities; (6) Hepatitis B virus negative; (7) No deficiency in dihydropyrimidine dehydrogenase; (8) This retrospective study was approved by the Institutional Review Board of Tangdu Hospital, Air Force Medical University of China. The approved protocol number was 2014029. Informed Consents from the participants were waived from the Institutional Review Board of Tangdu Hospital.
Study design
Patients were randomly assigned to either the HIPEC group or the control group at about 4 weeks after surgery using permutated block randomization method. Sequence was generated by computer (block length 4, allocation ratio 1:1). Allocation concealment was done with sealed envelopes. For the HIPEC group, the patients were treated with cisplatin (total dose 60 mg/m2) by HIPEC (30 mg/m2 at day 1 and day 3 respectively, together with oral S-1 (40–60 mg, 2/d for 14 days followed by 1-week interval). For control group, cisplatin (60 mg/m2) was given intravenously, and the same dose of S-1 was given orally. HIPEC or intravenous chemotherapy was done at a three-week interval. Patients received 6–8 cycles of treatment.
Medical history and demographic data were collected at baseline. Physical examination and laboratory tests were done at baseline and each follow-up. Laboratory tests include whole blood count, liver biochemical tests, electrolyte panel, renal function tests, routine urine tests and routine stool tests. Electrocardiography (ECG) was performed before and after each therapeutic course. Chest and abdominal contrast CT as well as ultrasound examination were performed at baseline and every two cycles of the treatment. Each participant was given 6–8 cycles of therapy and patients were followed up every three months until recurrence or death. Laboratory test, ECG, CT and B-mode ultrasound examinations for chest and abdomen were conducted at each follow-up. This study protocol was approved by the Institutional Review Board of the Tangdu Hospital, Air Force Medical University of China (#: 2014029) and has been registered in the ClinicalTrials.gov with the identifier (NCT number: NCT02396498) (https://clinicaltrials.gov/ct2/show/NCT02396498?term=NCT02396498&draw=2 &rank=1).
HIPEC procedure
Before HIPEC, prophylactic antiemetic drugs were used. Intravenous saline was given to prevent renal injury by the chemotherapeutic drugs. The HIPEC device was provided by the Xi'an Good Doctor Medical Science and Technology Co., Ltd. (Xi'an, China, Model: GDPR-2100T). Abdominal access was acquired under B mode ultrasound. Briefly, patients were locally anesthetized, a 16-gauge needle was inserted to the abdominal cavity, 2000–4000ml of saline was then injected into the abdominal cavity before another 16-gauge needle was used to establish the outlet. Hyperthermic perfusion and circulation was performed using the HIPEC machine. The inlet temperature was adjusted to 41 ± 1 °C, and the outlet temperature was 40 ± 1 °C. Cisplatin (30 mg/m2, Qilu Pharmaceutical, Jinan, China) was given at day 1 and day 3. Each course of the HIPEC lasted for 60 min (detailed parameter settings of the perfusion were listed in ). By the end of each treatment, approximately 1500 ml of cisplatin saline solution was retained in the peritoneal cavity. Vital signs were closely monitored during the procedure. Patients changed their body position as needed.
Table 1. Parameters for HIPEC.
S-1 administration
S-1 (tegafur-gimeracil-oteracil potassium capsule, Taiho Pharmaceutical, Tokushima, Japan) was administered orally. The dosage was as following: 40 mg, 2/d, for patient with <1.25 m2 body surface area (BSA); 50 mg, 2/d for patient with ≥1.25 m2 but <1.5 m2 BSA; 60 mg, 2/d, for patient with >1.5 m2 BSA.
Outcome evaluation
Therapeutic outcome evaluation was conducted after every two therapeutic cycles (6 weeks).
The primary endpoint was disease-free survival (DFS), which was defined as the time between the first treatment and cancer recurrence or death. Recurrence was defined as new lesion of >1 cm in diameter as suggested by CT, or malignant ascites confirmed by ultrasound and ascites cytology.
The secondary endpoint was two-year DFS rate, defined as percentage of patient surviving for two years without cancer recurrence.
Adverse events were documented and evaluated according to Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Data were analyzed using the SPSS software for Windows (version 20.0; SPSS Inc., Chicago, IL, USA). Data are presented as means ± standard deviations. The differences in age and treatment cycles between the perfusion and control groups were assessed using the Student’s t-test or one-way analysis of variance. Pearson’s chi-square test or Fisher’s exact test was used to compare the differences in sex, ECOG score, tumor site, histopathology, tumor stage, and peritoneal metastasis. The Kaplan–Meier survival curve was plotted for survival analysis and the log-rank test was used to identify differences between curves. Statistical significance was set at p < 0.05.
Results
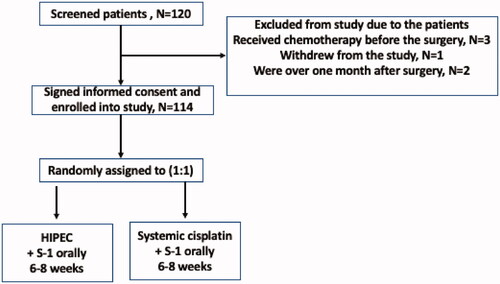
From April 2014 to April 2018, total 120 patients were screened. Six patients were excluded: 3 patients received chemotherapy before surgery, one patient withdrew, and two patients had passed the screening window. One-hundred and fourteen patients were included for final analysis, with 57 patients in each group (). There were no significant differences in age, sex ratio, the ECOG performance status, primary tumor types, and histopathological parameters, but more stage-IIIA patients were included in the control group (p = 0.018) (). The average treatment cycles were 5.9 ± 2.0 cycles in the HIPEC group and 5.9 ± 1.7 cycles in the control group, which was not significantly different (p = 0.959, ).
Table 2. Demographic characteristics of the participants.
Table 3. Treatment cycles.
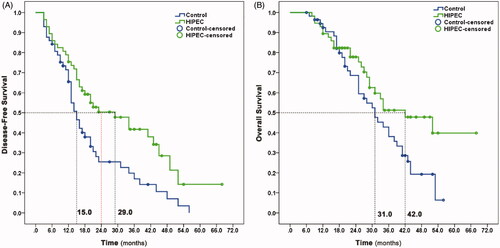
By 31 December 2020, 35 patients in the HIPEC group and 45 patients in the control group had cancer recurrence. The median follow-up duration at the time of data analysis was 44.0 (40.6–47.4) months. Median DFS, the primary endpoint of the study, was 29.0 months in the HIPEC group and 15.0 months in the control group (p = 0.006, ). The risk of recurrence was reduced by 45.5% in the HIPEC group, (hazard ratio = 0.545, 95% confidence interval: 0.349–0.851, p = 0.008). As for second endpoints, the two-year DFS rate was 50.4% in the HIPEC group and 25.5% in the control group; 12 patients in the control group (21.1%) had peritoneal metastasis, compared with six in the HIPEC group (10.5%, p = 0.198). Median OS was 42.0 months in the HIPEC group and 31.0 months in the group (p = 0.042, ). The risk of death was reduced by 40.5% in the HIPEC group, (hazard ratio = 0.595, 95% confidence interval: 0.355–0.995, p = 0.048). Four patients in the control group (7.0%) but none in the HIPEC group showed ovarian metastasis during follow-up. Metastasis to other sites including lung, bone, brain, liver, and abdominal lymph nodes, showed no differences between two groups. Our findings suggested that HIPEC reduced the risk of ovarian and peritoneal metastasis comparing with intravenous cisplatin for advanced gastric cancer patients after D2 gastrectomy.
Figure 2. Kaplan–Meier plot of disease-free survival (Panel A) and overall survival (Panel B) for the patients.
As for safety, adverse events were shown in . Thrombocytopenia was significantly higher in the control group (86.0% vs 36.8%, p = 0.000). No differences in other adverse events were seen between the two groups including nausea or vomiting (p = 0.449), diarrhea (p = 0.524), leukopenia (p = 0.070), fatigue (p = 0.824), renal toxicity (p = 1.000) and liver dysfunction (p = 1.000). In addition, there was no difference (p = 1.000) in the occurrence of severe adverse events (≥3 grade, ). As shown in , HIPEC-associated adverse events included pain at the puncture points (n = 2, 3.5%) and peritonitis (n = 1, 1.8%). No puncture point infection or intestinal perforation was observed in patients treated with HIPEC. No procedure-associated deaths occurred in either group.
Table 4. Comparison of adverse events in the two groups.
Table 5. HIPEC-associated side effects.
Discussion
Gastric cancer is one of the first leading cancer type in China. Although radical surgery improves clinical outcomes of some patients, the overall five-year survival rate of gastric cancer is still < 30% [Citation22], especially for the patients with stage-III gastric cancer [Citation6]. To improve patient survival, adjuvant chemotherapy is applied to stage III or advanced gastric cancer. The current study demonstrated that bedside HIPEC was a more effective adjuvant therapy than systemic chemotherapy for patients with stage-III gastric cancer.
Several retrospective and prospective clinical studies have indicated that adjuvant chemotherapy could benefit patients with stage-III gastric cancer [Citation9,Citation23–25]. However, while D2 radical surgery significantly reduces the occurrence of lymphatic metastasis, peritoneal metastasis becomes a major concern after surgery [Citation10,Citation26]. A retrospective study reported the presence of cancer cells in the peritoneal lavage fluid of 29.7% of T3 and 34.8% of T4 patients after radical surgery, and 59.4% of T3 or T4 patients had peritoneal metastasis within five years after the surgery, while only 21.3% had blood circulatory metastasis [Citation10]. Peritoneal metastasis can lead to intestinal obstruction and malignant ascites, which significantly impaired quality of life and shortened overall survival time. Thus, prevention and treatment of peritoneal metastasis is an important issue for gastric cancer patients, even after radical surgery.
The rationale of HIPEC is that cancer cells are more sensitive to heat than normal cells, and chemotherapeutic drugs and heat can act synergistically to kill cancer cells under hyperthermic conditions (41.5–42.5 °C) more efficiently [Citation18,Citation27]. It has been shown that hyperthermia significantly increases the efficacy of platinum-based anti-cancer drugs [Citation28]. Studies have consistently demonstrated the efficacy of CRS plus HIPEC in patients with gastric cancer during and after surgery. Fujimoto et al. first reported in 1988 that intraperitoneal hyperthermic chemotherapy controlled malignant ascites in nine out of 15 patients with gastric cancer who had peritoneal metastasis after surgery [Citation29]. They further reported in 1999 that surgery plus HIPEC could significantly increase the survival of patients with gastric cancer infiltration into the gastric serosa, and reduced peritoneal metastasis comparing with radical surgery only [Citation30]. Since then, HIPEC has been used not only as a treatment for malignant ascites caused by a variety of abdominal cancers such as colon cancer and ovarian cancer [Citation31–33] but also as a method of preventing peritoneal metastasis in patients with gastric cancer after surgery. In a study by Yu et al [Citation34], 248 patients with stage-II or stage-III gastric cancer were randomly treated with either radical surgery alone or surgery plus HIPEC (HIPEC starting on day 2 after surgery for 5 days with mitomycin plus fluorouracil). They found that the five-year survival rate in patients who had surgery and HIPEC was significantly higher than those treated with radical surgery only (49.1% vs.18.4%). In another randomized study [Citation35], 139 patients with stage II–IV gastric cancer were randomly assigned to be treated with surgery plus HIPEC group (42–43 °C), surgery plus intraperitoneal chemotherapy at normal temperature (37 °C) or radical surgery only. They found that the five-year survival rates were 61% (surgery + HIPEC), 43% (surgery + 37 °C intraperitoneal chemotherapy), and 42% (surgery only) [Citation35]. A more recent study reported the benefit of radical surgery plus HIPEC on DFS comparing with surgery only, as well as reduced risk of peritoneal metastasis [Citation20].
While most of the previous studies have compared the outcomes of addition of HIPEC versus radical surgery only [Citation20,Citation30,Citation35,Citation36], a meta-analysis including 18 randomized studies and 2,299 cases of late-stage gastric cancer found that rates of post-operational recurrence and distant metastasis were significantly lower in patients who received HIPEC plus systemic chemotherapy than in those who received systemic chemotherapy only; long-term survival was also increased without significant adverse effects [Citation37]. While most the studies were from Japan or other Asian countries, a randomized control trial is undergoing to investigate the effect of curative surgery plus HIPEC on survival of gastric cancer patients with sera and/lymph node involvement or positive cytology at peritoneal washing in European or Caucasians [Citation38]. This clinical trial recruited patients who had received a radical subtotal or total gastrectomy with D1 or D2 lymph node dissection followed by one cycle of HIPEC, which was carried out by open or closed abdomen technique. Here, we reported a prospective randomized study, which was designed to evaluate the effect of HIPEC and systemic chemotherapy on patients with stage-III gastric who were treated with D2 radical surgery and subsequent oral S-1 adjuvant therapy.
Oral S-1 was approved and used for the treatment of advanced gastric cancer in Japan in 1999. The SC-101 study was conducted in China. Wherein 224 cases of unresectable late-phase or metastatic gastric cancer patients were treated with S-1 plus cisplatin or 5-fluorouracil plus cisplatin [Citation39]. The findings of the SC-101 study suggested that S-1 plus cisplatin was superior to 5-fluorouracil plus cisplatin shown by improved remission rate and median survival. Therefore, S-1 was used in the current study as a pivotal adjuvant therapy, and a comparison between cisplatin administered by HIPEC or intravenously was made. We found significantly better DFS, two-year DFS rate, median overall survival and reduced peritoneal metastasis in HIPEC group, suggesting that cisplatin HIPEC plus oral S-1 might be a superior adjuvant therapy than intravenous cisplatin and plus S-1- not just HIPEC.
HIPEC is an invasive procedure. It has been reported that HIPEC-treated patients had a higher incidence rate of severe adverse events, including leukopenia, peritoneal infection, severe bleeding, and even intestinal perforation [Citation34–36]. However, HIPEC, especially during or immediately after surgery, has been performed under general anesthesia and performed only once [Citation17,Citation40]. Previous studies used catheters or tubes for HIPEC procedures, which has larger lumen diameter inserted by laparoscope during operation. We used a novel HIPEC protocol, in which inlet and outlet of closed HIPEC circulation were established using 16 G puncture needles guided by B mode ultrasound and under local anesthesia. Smaller diameter of the needle and no need for catheter insertion makes the procedure more tolerable. The procedure can be repeated safely. We did not find any differences in the adverse events between the treatment group and control in our study. Only mild procedure-related side effects were observed such as pain at the puncture points and mild peritonitis, which resolved spontaneously. Overall, our study indicated that this bedside HIPEC adjuvant therapy method is effective and safe.
Though our study is a prospective randomized study, there were some limitations. The sample number is relatively small and it is a single-center study. Further evolution of this method in bigger cohort and multiple centers is warranted.
Conclusion
This study demonstrated that bedside HIPEC with cisplatin plus oral S-1 was an effective adjuvant therapy following D2 radical gastrectomy for patients with stage-III gastric cancer. Bedside HIPEC using the puncture technique is safe and can be easily performed for multiple HIPECs.
Ethics approval and waiver of consent from the participate
The protocols for HIPEC and systemic chemotherapy were approved by the Institutional Review Board of Tangdu Hospital, Air Force Medical University, Xi’an, China. The approved protocol number was 2014029. This was a retrospective study and the Informed Consent from each participant was waived by the Institutional Review Board of Tangdu Hospital.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
All data generated in this study were included in this published article.
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Feng R-M, Zong Y-N, Cao S-M, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):22.
- Zheng RS, Sun KX, Zhang SW, et al. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28.
- Songun I, Putter H, Kranenbarg EM-K, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–449.
- Degiuli M, Sasako M, Ponti A, et al. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer. 2004;90(9):1727–1732.
- Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1–27.
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957.
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321.
- Katai H, Ishikawa T, Akazawa K, Registration Committee of the Japanese Gastric Cancer Association, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese gastric cancer association (2001-2007). Gastric Cancer. 2018;21(1):144–154.,
- Sakuramoto S, Sasako M, Yamaguchi T, ACTS-GC Group, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820.,
- Imazawa M, Kojima T, Boku N, et al. Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer. 2009;12(3):153–157.
- Oh SY, Kwon H-C, Lee S, et al. A phase II study of oxaliplatin with low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer patients with malignant ascites. Jpn J Clin Oncol. 2007;37(12):930–935.
- Iwasa S, Goto M, Yasui H, et al. Multicenter feasibility study of combination therapy with fluorouracil, leucovorin and paclitaxel (FLTAX) for peritoneal disseminated gastric cancer with massive ascites or inadequate oral intake. Jpn J Clin Oncol. 2012;42(9):787–793.
- Shirao K, Boku N, Yamada Y, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, et al. Randomized phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J Clin Oncol. 2013;43(10):972–980.,
- Masuishi T, Kadowaki S, Kondo M, et al. FOLFOX as first-line therapy for gastric cancer with severe peritoneal metastasis. Anticancer Res. 2017;37(12):7037–7042.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42.
- Elias D, Goéré D, Dumont F, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer. 2014;50(2):332–340.
- Matsuzaki Y, Shibata K, Yoshioka M, et al. Intrapleural perfusion hyperthermo-chemotherapy for malignant pleural dissemination and effusion. Ann Thorac Surg. 1995;59(1):127–131.
- Gonzalez-Moreno S. Peritoneal surface oncology: a progress report. Eur J Surg Oncol. 2006;32(6):593–596.
- Beeharry MK, Zhu Z-L, Liu W-T, et al. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer. 2019;19(1):932.
- Liu L, Zhang N, Min J, et al. Retrospective analysis on the safety of 5,759 times of bedside hyperthermic intra-peritoneal or intra-pleural chemotherapy (HIPEC). Oncotarget. 2016;7(16):21570–21578.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Chang S-C, Liu K-H, Hung C-Y, et al. Adjuvant chemotherapy improves survival in stage III gastric cancer after D2 surgery. J Cancer. 2018;9(1):81–91.
- Wang G, Zhao J, Song Y, et al. Phase II study of adjuvant chemotherapy with S1 plus oxaliplatin for Chinese patients with gastric cancer. BMC Cancer. 2018;18(1):547.
- Lee C-K, Jung M, Kim HS, et al. S-1 based doublet as an adjuvant chemotherapy for curatively resected stage III gastric cancer: Results from the randomized phase III POST trial. Cancer Res Treat. 2019;51(1):1–11.
- Lee J-H, Son S-Y, Lee CM, et al. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer. 2014;17(3):529–536.
- Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2(2):68–75.
- Goodman MD, McPartland S, Detelich D, et al. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J Gastrointest Oncol. 2016;7(1):45–57.
- Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208(1):36–41.
- Fujimoto S, Takahashi M, Mutou T, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85(3):529–534.
- Nicoletto MO, Padrini R, Galeotti F, et al. Pharmacokinetics of intraperitoneal hyperthermic perfusion with mitoxantrone in ovarian cancer. Cancer Chemother Pharmacol. 2000;45(6):457–462.
- Zhang TING, Pan Q, Xiao S, et al. Docetaxel combined with intraperitoneal hyperthermic perfusion chemotherapy and hyperthermia in the treatment of advanced ovarian cancer. Oncol Lett. 2016;11(5):3287–3292.
- Sugarbaker PH, Mora JT, Carmignani P, et al. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist. 2005;10(2):112–122.
- Yu W, Whang I, Suh I, et al. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg. 1998;228(3):347–354.
- Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48(42):1776–1782.
- Yan TD, Black D, Sugarbaker PH, et al. A systematic review and Meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14(10):2702–2713.
- Li Z, Mi D, Yang K. Evaluation on the safety and efficacy of HIPEC treatment after surgery of progressive stomach cancer. Chinese J Evid Based Med. 2011;11(12):1402–1408.
- Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183.
- Jin M, Lu H, Li J, et al. Ramdomized 3-armed phase III study of S-1 monotherapy versus S-1/CDDP (SP) versus 5-FU/CDDP (FP) in patients (pts) with advanced gastric cancer (AGC): SC-101 study. J Clin Oncol. 2008;26(15_suppl):4533–4533.
- Fan B, Bu Z, Zhang J, et al. Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer. 2021;21(1):216.