Abstract
Objective
This study was aimed at comparing the outcomes of high-intensity focused ultrasound (HIFU) with those of uterine artery embolization (UAE) and traditional surgeries for treating symptomatic uterine fibroids.
Materials and methods
We searched the following databases from their beginning to 5 November 2021: PubMed, Medline, Embase and Cochrane Library.
Results
Overall, 21 studies were included in this meta-analysis. The results revealed that HIFU had a higher re-intervention rate than UAE (relative risk [RR] = 4.06, 95% confidence interval [CI]: 2.47–6.69) and offered no significant advantages in reducing the symptom severity score (SSS) (mean difference [MD] = 17.01, 95% CI: 10.25–23.77) and improving the health-related quality of life (HRQoL) score (MD= −18.32, 95% CI: −24.87 to −11.78) in the treatment of symptomatic uterine fibroids. However, compared with UAE, HIFU may be associated with a higher pregnancy rate (RR = 17.44, 95% CI: 2.40–126.50) and may have a significant advantage in shortening pregnancy interval and preserving ovarian function. Moreover, upon comparing HIFU with traditional surgical treatments, the HIFU group showed significantly improved HRQoL score (MD = 2.25, 95% CI: 1.15–3.35), but the re-intervention rate (RR = 1.65, 95% CI: 0.59–4.57), pregnancy rate (RR = 1.01, 95% CI: 0.90–1.13), SSS and ovarian function did not significantly differ between the two groups.
Conclusions
Although HIFU has relatively high re-intervention rate, it may offer a higher pregnancy rate and shorter pregnancy interval with little influence on ovarian function, thus making it an attractive option for treating symptomatic fibroids in young women who wish to plan a pregnancy in the future.
1. Introduction
Uterine fibroids are the most prevalent genital benign tumors in women. They usually occur in women aged 30–50 years and cause many adverse effects. They particularly occur during reproductive age, with an incidence of 20–35% [Citation1], and may cause several complications, such as infertility, miscarriage, premature delivery and menorrhagia. At present, according to the fertility requirement, age and the size and location of fibroids, uterine fibroids are treated with various approaches, such as traditional surgical treatment, conservative drug treatment (mifepristone) and uterine artery embolization (UAE). Owing to technological advancements, minimally invasive surgeries with greatly reduced trauma are a superior alternative to traditional surgery; furthermore, they fulfill the requirement of young women who want to preserve the uterus. In recent years, UAE has emerged as a minimally invasive surgery to treat uterine fibroids. It functions by blocking the blood supply to fibroids, thus intercepting the nutrient supply and eventually leading to apoptosis of the fibroid. Notably, although UAE offers the advantage of causing minimal trauma, it may affect ovarian function and fertility and increase the risk of thrombogenesis.
In China, the first focused ultrasound surgery treatment was administered to a man with tibia osteosarcoma in 1997, and then, its application was gradually expanded to other solid tumors, such as liver cancer, breast cancer, pelvic malignant tumors and uterine fibroids [Citation2]. In 2004, magnetic resonance-guided high-intensity focused ultrasound (MR-guided HIFU) was approved by the Food and Drug Administration (FDA) for treating symptomatic uterine fibroids in humans [Citation3,Citation4]. This technology increases the temperature of fibroids, thus resulting in the degeneration, necrosis and atrophy of target tissues and alleviation of the clinical symptoms; this is complimented by MRI guidance, which allows for accurately focusing the ultrasound energy to a small area while monitoring the temperature simultaneously. Over the last two decades, ultrasound-guided HIFU (USgHIFU) has also been widely used to treat uterine fibroids; it has proven to be effective, safe and cost-effective [Citation5,Citation6]. Thus far, several clinical studies have corroborated the efficacy of HIFU in the treatment of uterine fibroids while accentuating its positive aspects of causing fewer adverse effects and complications than traditional surgeries [Citation7–9]. Particularly for women planning a pregnancy, it is even more advantageous as it does not increase the risk of uterine rupture and pregnancy-related complications [Citation10,Citation11]; however, findings in this regard are controversial. For example, a meta-analysis conducted by Sandberg et al. suggested that after 5 years of treatment, the re-intervention rates of the myomectomy (MY), UAE and HIFU were 12.2%, 14.4% and 54%, respectively, suggesting an association between HIFU and higher risk of reintervention [Citation12]. Therefore, this study was aimed at evaluating the association of HIFU with the alleviation of the symptom severity score (SSS), the improvement of the health-related quality of life (HRQoL) score, the re-intervention rate of symptomatic uterine fibroids, preservation of ovarian function and impact of the procedure on pregnancy and delivery by comparing HIFU with UAE and traditional surgeries via a meta-analysis.
2. Materials and methods
2.1. Literature search and inclusion criteria
We identified eligible studies from PubMed, Medline, Embase and Cochrane Library databases from the beginning of database up to 5 November 2021. The following keywords were used in the literature search in abstract or full text: (leiomyoma OR fibroid OR uterine myoma OR leiomyomata) and (HIFU OR high-intensity focused ultrasound OR uterine artery embolization OR UAE OR transcervical resection of myoma [TCRM] OR laparoscopic myomectomy [LM] OR hysterectomy [HY] OR MY OR hysteroscopic myomectomy). Furthermore, through references of published articles, a manual search of the literature was also conducted. The inclusion criteria were as follows: (1) studies published in English language, (2) studies that included women with symptomatic uterine fibroids, (3) studies that were randomized controlled trials (RCTs), non-RCTs or observational studies (cohort studies and case-control studies), (4) studies that included quantitative data of outcomes of interest, and (5) studies that were designed for comparing HIFU with UAE or surgical treatment of uterine fibroids. The following were the exclusion criteria: (1) studies for which the original datasets were not accessible, (2) studies designed to compare HIFU with percutaneous microwave ablation or drug treatment (e.g., mifepristone), and (3) studies published as letters, comments, case reports and literature reviews.
2.2. Data extraction and outcomes
The following data were extracted from the studies: year of publication, age, sample size, methodology, study groups, first author and follow-up length and outcomes data. Herein, the SSS, re-intervention rate and HRQoL scores were used to determine the effectiveness of each intervention. Secondary outcome measures were the pregnancy rate, pregnancy-related complications, outcomes of pregnancy and delivery and the impact on ovarian function.
2.3. Quality assessment
The quality of RCTs was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias. The following sources of bias were associated with study participation: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. The quality of non-RCTs was assessed using methodological index for non-randomized studies (MINOS). The Newcastle–Ottawa Scale (NOS) was used for assessing the quality of cohort and case–control studies. Studies with ≥5 points were considered as high-quality studies.
2.4. Statistical analysis
The Cochrane Q statistic, which includes quantitative I2 and qualitative p value, was used to assess heterogeneity among the results of studies. When p was <.1 and I2 was >50%, the heterogeneity between studies was considered significant. For dichotomous data, we used relative risk (RR) with 95% confidence interval (CI). Furthermore, when data were provided as mean and standard deviation, we used mean difference (MD) with 95% CI for continuous data. Sensitivity analyses were performed by sequentially excluding one study at a time to assess the stability of this meta-analysis. Publication bias was checked for by using the funnel plot. Because the included studies that showed other outcomes were too few for an analysis, we only evaluated the publication bias of the re-intervention rate. Review Manager version 5.4 (RevMan, Review Manager 5.4) was used to perform all statistical analyses.
3. Results
3.1. Literature search
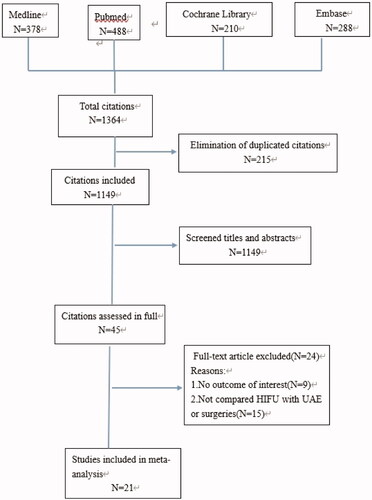
Our initial search yielded a total of 1364 potentially relevant citations. All citations were reviewed and data extraction was performed independently by two authors (Y. L and L. JW). Any disagreement was resolved by seeking the opinion of a third author (H. HM). After applying the inclusion and exclusion criteria, 21 studies encompassing 29,661 patients were included in our meta-analysis (). Among these 21 studies, 6 studies were RCTs, 2 studies were non-RCTs and others were cohort studies. Furthermore, 8 studies compared HIFU with UAE, and 14 studies compared HIFU with traditional surgical treatments (e.g., TCRM, LM, HY and MY). The general information of each study is shown in . After excluding non-RCTs, all 19 studies scored ≥5 points when rated as per the Cochrane Collaboration’s tool and the NOS, which is indicative of their high quality. The average score according to MINOS for the two non-RCTs was 15, suggesting moderate quality.
Table 1. Characteristics of individual studies included in the meta-analysis (continued).
3.2. Re-intervention rate
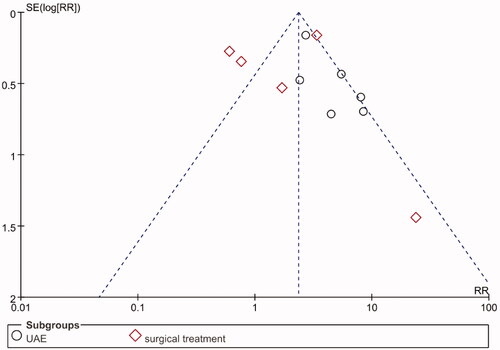
Among the 21 articles, 11 articles were detailed comparative studies on the re-intervention rate of uterine fibroids. Six of these ten articles compared HIFU with UAE [Citation12,Citation14,Citation15,Citation22,Citation28,Citation31] and six articles compared HIFU with traditional surgical treatments [Citation21,Citation24,Citation25,Citation27,Citation29,Citation31]. Funnel plots were used to assess publication bias (). The funnel plot was basically symmetrical, suggesting the absence of a publication bias.
3.2.1. HIFU vs. UAE
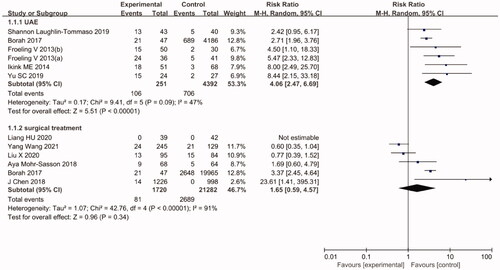
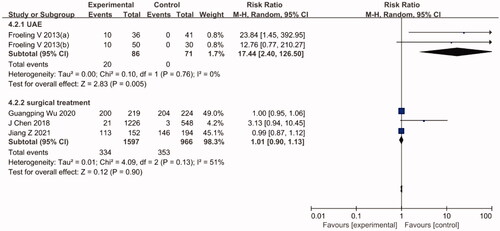
The pooled efficacy results of HIFU vs. UAE are shown in . The re-intervention rate of the HIFU group was significantly higher than that of the UAE group (RR = 4.06, 95% CI: 2.47–6.69). In this regard, the level of heterogeneity observed among studies was relatively low (I2 =47%, p= .09). Furthermore, the notion that the re-intervention rate was higher with HIFU than with UAE remained unchanged after sensitivity analyses.
3.2.2. HIFU vs. surgical treatments
The pooled efficacy results of HIFU vs. surgical treatments are also shown in . According to the results, the re-intervention rate did not significantly differ between HIFU and surgical treatments (RR = 1.65, 95% CI: 0.59–4.57). However, in this regard, the level of heterogeneity observed among studies was relatively high (I2 = 91%, p < .00001). The sensitivity analysis showed that the results remained largely unaffected by the exclusion of any individual study.
3.3. The impact of treatments on SSS and HRQoL scores
3.3.1. HIFU vs. UAE
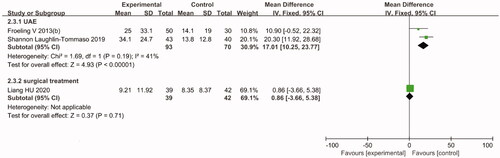
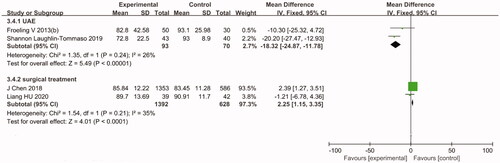
In women with symptomatic uterine fibroids, both treatments could improve the HRQoL score. However, the pooled efficacy results suggested that compared with the HIFU group, the UAE group showed significantly reduced SSS (MD = 17.01, 95% CI: 10.25–23.77) and improved HRQoL score (MD= −18.32, 95% CI: −24.87 to −11.78) after 12 months of follow-up ( and Citation5).
3.3.2. HIFU vs. surgical treatments
The pooled efficacy results suggested that compared with the surgical group, the HIFU group showed significantly improved HRQoL score (MD = 2.25, 95% CI: 1.15–3.35) after 12 months of follow-up (). Moreover, a comparative study by Hu et al. [Citation25] studied how HIFU and TCRM in women with type 2 submucosal fibroids affect the SSS and HRQoL score after 3, 6 and 12 months of follow-up. The results suggested that both treatments are tolerable and effective and significantly resolve SSS and improve HRQoL scores in women with type 2 submucosal fibroids measuring ≤5 cm in diameter. However, there was no statistically significant difference between the two groups (p> .05).
3.4. The impact of treatments on pregnancy and delivery
The pooled efficacy results of HIFU vs. UAE are shown in . According to the analysis, the postoperative pregnancy rate of the HIFU group was significantly higher than that of the UAE group (RR = 17.44, 95% CI: 2.40–126.50). In this regard, the level of heterogeneity observed among the studies was low (I2 =0%, p= .76). The pooled efficacy results of HIFU vs. surgical treatment are also shown in . According to the analysis, the postoperative pregnancy rates of both groups were not significantly different (RR = 1.01, 95% CI: 0.90–1.13). In addition, a study by Guangping Wu et al. [Citation26] drew comparisons between the outcomes of pregnancy and delivery after HIFU and LM. The results suggested that after 1–8 years of follow-up, although the pregnancy rate did not significantly differ between the two groups, the interval between fibroid treatment and the following pregnancy was significantly shorter in the HIFU group than in the LM group (13.6 ± 9.5 months vs. 18.9 ± 7.3 months, p< .05). Furthermore, the rate of cesarean section was also significantly lower in the HIFU group than in the LM group (41.6% vs. 54.9%, p< .05). Regarding the complications associated with pregnancy and delivery, the incidence of placenta implantation and placenta previa was significantly lower in the HIFU group than in the LM group (p< .05). However, the two groups showed no statistically significant differences in terms of fetal distress, preterm birth, the incidence of hypertensive disorders during pregnancy, amniotic fluid embolism, puerperal infection, gestational diabetes and intrahepatic cholestasis during pregnancy.
3.5. The impact of treatments on ovarian function
A study by Laughlin-Tommaso et al. [Citation22] reported that after 24 months of follow-up, the median absolute change in anti-Müllerian hormone (AMH) levels was significantly larger in the UAE group than in the HIFU group (−0.6 [−1.2–0.4] vs. −0.2 [−0.4–0.4]; p= .03). Cui et al. [Citation23] compared the changes in ovarian sex hormone levels (i.e., estradiol [E2], follicle stimulating hormone [FSH] and luteinizing hormone [LH]) as well as the resistance index (RI) and the pulsatility index (PI) of the uterine arterial blood flow after HIFU and myomectomy (including MY, LM and TCRM). The results showed no significant changes in PI, RI, LH, FSH and E2 levels before and after 6 months of treatment between the two groups and within the same group (p> .05). This suggested that HIFU treatment of uterine fibroids had no significant effect on endometrial receptivity and ovarian function.
4. Discussion
There are various ways to treat uterine fibroids. Although traditional surgical treatments can resolve the clinical symptoms, they have many disadvantages, such as relatively large trauma, high chances of postoperative complications and long operation time. In recent years, owing to its noninvasive properties, HIFU has attracted a lot of attention for uterine fibroid treatment. Many studies have reported that it offers high treatment efficacy while being safe with few adverse effects. Łozinski et al. [Citation10] conducted a single-center retrospective cohort study with 276 patients and reported that HIFU resolved clinical symptoms of patients with uterine fibroids without increasing spontaneous abortion and other pregnancy-related complications. Moreover, a prospective observational open-label multicenter study conducted by Qu et al. [Citation33] showed that the AMH levels did not significantly change after HIFU treatment with 6-month follow-up, suggesting that HIFU does not affect ovarian function. In 2020, a review conducted by Ciebiera et al. prompted that HIFU is a relatively safe, noninvasive method and is not detrimental to fertility [Citation34]. These properties make HIFU an attractive option for young women with fibroids who wish to plan a pregnancy in the future. However, to know if HIFU is superior to other treatment methods needs further study.
UAE, which was proposed by Ravina in 1994, is also a minimally invasive method for uterine fibroid treatment [Citation35]. A study by Gabriel-Cox et al. [Citation36] reported that UAE for symptomatic uterine fibroids permitted uterine conservation in more than 80% of women, with no hysterectomies reported after 5 years. UAE resolves clinical symptoms by blocking the blood vessels that supply nutrition to fibroids, thus causing fibroids to shrink. However, its non-targeted embolization may concurrently affect the blood supply to the uterus and ovary, thus affecting the ovarian and reproductive function. In addition, the radiation used in UAE may also adversely affect the reproductive system [Citation37]. Many studies have indicated that AMH levels after UAE treatment are significantly lower than preoperative levels [Citation38,Citation39]. A RCT by Mara et al. [Citation40] compared myomectomy and UAE in terms of fertility and its outcomes. Consequently, the pregnancy rates after myomectomy and UAE were reportedly 78% and 50%, respectively (p< .05). Currently, professional societies like the Society of Interventional Radiology and the American College of Obstetrics and Gynecology consider the desire for future pregnancy as a relative contraindication to UAE [Citation41,Citation42]. However, in a meta-analysis published by Ghanaati et al. [Citation43], it was reported that obstetrical complications, pregnancy loss, preterm labor and low birth weight after UAE are similar to those observed in the general population. Therefore, more studies are needed to illustrate whether UAE has an impact on pregnancy outcomes.
According to the findings of this meta-analysis, in the treatment of symptomatic uterine fibroids, HIFU had a higher rate of re-intervention (RR = 4.06, 95% CI: 2.47–6.69) and offered no significant advantages in reducing the SSS and improving the HRQoL score when compared with UAE. Conversely, HIFU was associated with a higher postoperative pregnancy rate than UAE (RR = 17.44, 95% CI: 2.40–126.50). In addition, another study by Laughlin-Tommaso et al. [Citation22] suggested that the AMH level decreased by a much greater extent in the UAE group than in the HIFU group at 2-year follow-up, suggesting that HIFU offers a significant advantage in preserving ovarian function. However, upon comparing HIFU with traditional surgical treatments, HIFU could significantly improve the HRQoL score, but no significant differences were noted in the reduction of the SSS, the re-intervention rate, the pregnancy rate, ovarian function, changes in endometrial receptivity between the two groups. However, one literature by Guangping Wu et al. [Citation26] suggested that the cesarean section rate, postoperative time before successful pregnancy and the incidence of placenta previa and placenta implantation were significantly lower after HIFU than after LM, indicating that HIFU treatment of uterine fibroids not only offers a shorter time to successful pregnancy since operation but also does not increase pregnancy complications, thus improving the pregnancy outcome.
In 2021, another meta-analysis by Liu L et al. [Citation44] compared HIFU and UAE in treating symptomatic uterine fibroids in terms of symptom scores, QoL, postoperative reintervention rate and pregnancy rate, and their results were similar to ours. However, their meta-analysis had relatively high heterogeneity (I2 >50%). Our meta-analysis simultaneously compared HIFU with UAE and traditional surgeries and revealed the advantages and disadvantages of HIFU in treating symptomatic uterine fibroids. Notably, we also compared the effects of different treatment methods on the outcomes of pregnancy, delivery and ovarian function, which is a novel comparison. Moreover, the heterogeneity of most of our conclusions is relatively low (I2 <50%). The present meta-analysis has several strengths and advantages. We herein evaluated the therapeutic efficacy of HIFU in the treatment of uterine fibroids in terms of the SSS, HRQoL score and re-intervention rate and compared HIFU with UAE and traditional surgeries in this regard. Simultaneously, we also analyzed the impact of different treatments on pregnancy, delivery and ovarian function. These aspects are particularly meaningful for women of childbearing age having symptomatic uterine fibroids. However, this study has several limitations that need to be acknowledged. First, although 19 literatures were included in this study, the outcome indicators adopted in each literature differed, particularly among the few literatures related to the impact on pregnancy, delivery and ovarian function, which made it very difficult to draw better conclusions. Second, although multiple databases were searched in this article, only English literatures were included, which may lead to bias. Third, among the 19 literatures included in this study, only 5 were RCT studies, and differences in research methods, study populations, sizes and types of fibroids and follow-up times may have led to heterogeneity and bias among the cases.
5. Conclusion
Although HIFU may have a relatively high re-intervention rate, it may be associated with a higher postoperative pregnancy rate and shorter pregnancy interval. Furthermore, it has little effect on ovarian function. Taken together, it may be a better option for young women, but we need more high-quality randomized controlled studies in this regard.
Ethical approval and consent from patients
Since this study was only an analysis of publicly available human subject data and not a primary research involving animals or humans, patient consent and ethical approval were not needed.
Acknowledgments
The authors thank The First Affiliated Hospital of Xiamen University. We also appreciate Medjaden Bioscience Limited for editing and proofreading the manuscript.
Disclosure statement
The authors declare to have no potential conflict of interest.
References
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. Ultrasound Med Biol. 2004;30(2):245–260.
- Jenne JW, Preusser T, Gunther M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Z Med Phys. 2012; 22(4):311–322.
- Ringold S. FDA approves ultrasound fibroid therapy. JAMA. 2004;292(23):2826.
- Lyon PC, Rai V, Price N, et al. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: preliminary clinical experience. Ultraschall Med. 2020;41(05):550–556.
- Marinova M, Ghaei S, Recker F, et al. Efficacy of ultrasound-guided high-intensity focused ultrasound (USgHIFU) for uterine fibroids: an observational single-center study. Int J Hyperthermia. 2021;38(2):30–38.
- Kamp JEK, David M, ScheurigMuenkler C, et al. Clinical outcome of magnetic-resonance-guided focused ultrasound surgery (MRgFUS) in the treatment of symptomatic uterine fibroids. Rofo. 2013;185(2):136–143.
- Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol. 2012; 56(4):409–416.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4(3):294–302.
- Łoziński T, Filipowska J, Gurynowicz G, et al. The effect of high-intensity focused ultrasound guided by magnetic resonance therapy on obstetrical outcomes in patients with uterine fibroids - experiences from the main Polish center and a review of current data. Int J Hyperthermia. 2019;36(1):582–590.
- Zou M, Chen L, Wu C, et al. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high intensity focused ultrasound. BJOG. 2017;124(S3):30–35.
- Sandberg EM, Tummers FHMP, Cohen SL, et al. Reintervention risk and quality of life outcomes after uterine-sparing interventions for fibroids: a systematic review and Meta-analysis. Fertil Steril. 2018;109(4):698–707.
- Taran FA, Tempany CM, Regan L, et al. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol. 2009;34(5):572–578.
- Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82(12):2265–2269.
- Froeling V, Meckelburg K, Scheurig-Muenkler C, et al. Midterm results after uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for symptomatic uterine fibroids. Cardiovasc Intervent Radiol. 2013;36(6):1508–1513.
- Wang X, Qin J, Wang L, et al. Effect of high-intensity focused ultrasound on sexual function in the treatment of uterine fibroids: comparison to conventional myomectomy. Arch Gynecol Obstet. 2013;288(4):851–858.
- Wang X, Qin J, Chen J, et al. The effect of high-intensity focused ultrasound treatment on immune function in patients with uterine fibroids. Int J Hyperthermia. 2013;29(3):225–233.
- Wang F, Tang L, Wang L, et al. Ultrasound-guided high-intensity focused ultrasound vs. laparoscopic myomectomy for symptomatic uterine myomas. J Minim Invasive Gynecol. 2014;21(2):279–284.
- Barnard EPD, AbdElmagied Ahmed MM, Vaughan Lisa EMS, et al. Periprocedural outcomes comparing fibroid embolization and focused ultrasound: a randomized controlled trial and comprehensive cohort analysis. Am J Obstet Gynecol. 2017;216(5):500e1–500e11.
- Liu Y, Ran W, Shen Y, et al. High-intensity focused ultrasound and laparoscopic myomectomy in the treatment of uterine fibroids: a comparative study. BJOG. 2017;124(3):36–39.
- Mohr-Sasson Aya, Machtinger Ronit, Mashiach Roy, et al. Long-term outcome of MR-guided focused ultrasound treatment and laparoscopic myomectomy for symptomatic uterine fibroid tumors. Am J Obstet Gynecol. 2018;219(4):375e1–375e7.
- Laughlin-Tommaso Shannon, Barnard Emily PD, AbdElmagied Ahmed M, et al. FIRSTT study: randomized controlled trial of uterine artery embolization vs focused ultrasound surgery. Am J Obstet Gynecol. 2019;220(2):174e1–174e13.
- Cui Y, Dong Y, Guo B, et al. Effect of HIFU on endometrial receptivity and sex hormone level in uterine fibroid patients and analysis of influencing factors for its treatment rate. Exp Ther Med. 2019;17(3):2291–2297.
- Wang Y, Liu X, Wang W, et al. Long-term clinical outcomes of US-Guided High-Intensity focused ultrasound ablation for symptomatic submucosal fibroids: a retrospective comparison with Uterus-Sparing surgery. Acad Radiol. 2021; 28(8):1102–1107.
- Hu L, Zhao JS, Xing C, et al. Comparison of focused ultrasound surgery and hysteroscopic resection for treatment of submucosal uterine fibroids (FIGO type 2). Ultrasound Med Biol. 2020;46(7):1677–1685.
- Wu G, Li R, He M, et al. A comparison of the pregnancy outcomes between ultrasound-guided high-intensity focused ultrasound ablation and laparoscopic myomectomy for uterine fibroids: a comparative study. Int J Hyperthermia. 2020; 37(1):617–623.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364.
- Yu SCH, Cheung ECW, Leung VYF, et al. Oxytocin-Augmented and Non-Sedating High-Intensity-Focused ultrasound (HIFU) for uterine fibroids showed promising outcome as compared to HIFU alone or uterine artery embolization. Ultrasound Med Biol. 2019;45(12):3207–3213.
- Liu X, Tang J, Luo Y, et al. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG. 2020;127(11):1422–1428.
- Jiang Z, Li Q, Li W, et al. A comparative analysis of pregnancy outcomes of patients with uterine fibroids after high intensity focused ultrasound ablation and laparoscopic myomectomy: a retrospective study. Int J Hyperthermia. 2021;38(1):79–84.
- Borah BJ, Yao X, Laughlin-Tommaso SK, et al. Comparative effectiveness of uterine leiomyoma procedures using a large insurance claims database. Obstet Gynecol. 2017;130(5):1047–1056.
- AbdElmagied AM, Vaughan LE, Weaver AL, et al. Fibroid interventions: reducing symptoms today and tomorrow: extending generalizability by using a comprehensive cohort design with a randomized controlled trial. Am J Obstet Gynecol. 2016; 215(3):338.e1–338.e18.
- Qu K, Mao S, Li J, et al. The impact of ultrasound-guided high-intensity focused ultrasound for uterine fibroids on ovarian reserve. Int J Hyperthermia. 2020; 37(1):399–1403.
- Ciebiera M, Łoziński T. The role of magnetic resonance-guided focused ultrasound in fertility-sparing treatment of uterine fibroids-current perspectives. Ecancermedicalscience. 2020;14:1034.
- Ravina JH, Merland JJ, Herbreteau D, et al. Preoperative embolization of uterine fibroma. preliminary results (10 cases). Presse Med. 1994;23(33):1540.
- Gabriel-Cox K, Jacobson GF, Armstrong MA, et al. Predictors of hysterectomy after uterine artery embolization for leiomyoma. Am J Obstet Gynecol. 2007;196(6):e1–6.
- Schirf BE, Vogelzang RL, Chrisman HB. editors. Complications of uterine fibroid embolization. Seminars in interventional radiology. New York (NY): Thieme Medical Publishers; 2006. p. 333.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016;206:172–176.
- Arthur R, Kachura J, Liu G, et al. Laparoscopic myomectomy versus uterine artery embolization: long-term impact on markers of ovarian reserve. J Obstet Gynaecol Can. 2014;36(3):240–247.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no.96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112:387–400.
- Dariushnia SR, Nikolic B, Stokes LS, et al. Quality improvement guidelines for uterine artery embolization for symptomatic leiomyomata. J Vasc Interv Radiol. 2014;25(11):1737–1747.
- Ghanaati H, Sanaati M, Shakiba M, et al. Pregnancy and its outcomes in patients after uterine fibroid embolization: a systematic review and Meta-Analysis. Cardiovasc Intervent Radiol. 2020;43(8):1122–1133.
- Liu L, Wang T, Lei B. Uterine artery embolization compared with high-intensity focused ultrasound ablation for the treatment of symptomatic uterine myomas: a systematic review and Meta-analysis. J Minim Invasive Gynecol. 2021;28(2):218–227.