Abstract
Objective
To evaluate the potential risk factors influencing cure rate of ultrasound-guided microwave ablation (MWA) for primary hyperparathyroidism (PHPT).
Materials and methods
Seventy five patients (25 males and 50 females; mean age, 56.80 ± 12.34; age range, 26–85) with PHPT undergoing MWA under ultrasound guidance were enrolled between May 2017 and December 2020. The cure rate and complications were evaluated after treatment. The potential factors influencing cure rate of ultrasound-guided MWA for PHPT were analyzed by univariate and multivariate binary logistic regression.
Results
Fifty six of 75 patients had normal PTH and serum calcium levels after at least 6 months after one session MWA, and the cure rate was 74.7% (56/75). 6 uncured patients received the second session MWA during follow-up, and the cure rate achieved 81.3% (61/75) after the second session MWA. Voice changes occurred in 4 patients (5.33%) and recovered within 3 months after ablation without special treatment. Nodule volume was the independent risk factor associated with cure in PHPT patients undergoing MWA, whether after one session (p = 0.0224; odds ratio, 0.67) or the second session MWA (p = 0.0408; odds ratio, 0.74). The cutoff value for nodule volume in predicting the cure was 0.96 cm3 (one session: sensitivity, 76.8%; specificity, 73.7%; the second session: sensitivity, 72.1%; specificity, 71.4%).
Conclusion
In conclusion, parathyroid nodule volume was the independent risk factor associated with cure in PHPT patients undergoing MWA.
Introduction
Primary hyperparathyroidism(PHPT) is usually due to adenoma or hyperplasia of one or more parathyroid glands, which can lead to bone loss, kidney stones and other symptoms [Citation1,Citation2]. Parathyroidectomy is the main treatment for PHPT [Citation3,Citation4]. However, some patients may refuse surgery because of unsuitable physical conditions or fear of scar formation. Percutaneous ethanol injection (PEI) has been determined to be an alternative therapeutic approach in small number of cases of PHPT. However, it needs to repeat the procedure several times and the long-term efficacy is uncertain [Citation5]. Recently, some thermal ablation techniques, including high intensity focused ultrasound (HIFU), radiofrequency ablation (RFA), microwave ablation (MWA)and laser ablation (LA) have been used as an alternative to surgery for PHPT, with the technical success rate or cure rate of 81–92% [Citation6–11]. MWA, in particular, has high thermal efficiency and is increasingly used in the treatment of PHPT. Wei et al. reported a multicenter study of MWA for PHPT and the effective rate was 89.9% [Citation12]. However, compared with the highest cure rate of 99.4% of surgical resection, the cure rate of MWA is slightly inferior to that of surgical resection [Citation13]. Therefore, the purpose of the study was to evaluate the potential risk factors influencing cure rate of ultrasound-guided MWA for PHPT.
Materials and methods
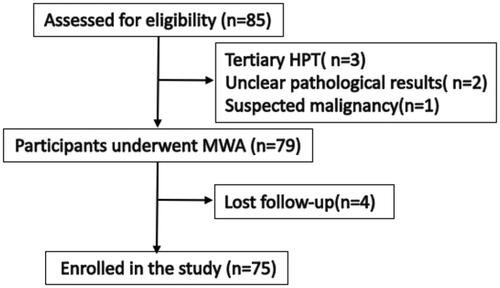
This clinical application was approved by medical ethics committee of Chinese PLA General Hospital (S2017-058-03). The study was approved by our Institutional Review Board. The data are anonymous, and the requirement for informed consent was therefore waived. From May 2017 to December 2020, data pertaining to patients who had underwent MWA for PHPT under ultrasound guidance in our department were retrospectively analyzed. The clinical symptoms, side effects and complications were recorded as well. Biochemical diagnosis and localization studies were based on the recommendations proposed by the International Workshop on Primary Hyperparathyroidism [Citation3]. US-guided core needle biopsy (CNB) was performed for all parathyroid nodules to obtain the pathology results to exclude parathyroid cancer. In order to reduce the occurrence of bleeding, hemostatic drugs were given intravenously before puncture to prevent bleeding. Parathyroid lesions were localized by ultrasound and 99mTc sestamibi (MIBI). Laryngoscopy was performed to rule out recurrent laryngeal nerve (RLN) impairment in all patients before MWA. The inclusion criteria based on the guidelines from the 4th International Workshop were as follows: (1) Patients with symptomatic PHPT. (2). Patients with asymptomatic PHPT combined with any one of the following conditions: (a) hypercalcemia, serum calcium >0.25 mmol/L above normal reference range; (b) A glomerular filtration rate of less than 60 ml per minute, renal stones; (c) for patients younger than 50 years of age, and for men and perimenopausal or postmenopausal women 50 years of age or older who have T scores of −2.5 or lower at a central bone densitometry site or in the distal third of the radius. (3) Ultrasound can display the parathyroid lesion and have a safe needle approach. (4) Prothrombin time does not exceed the normal limit of 5 s, prothrombin activity >50%, platelet count >50 × 109/L. The exclusion criteria were as follows: (1) secondary or tertiary hyperparathyroidism; (2) patients with a severe hypertension or cardiac insufficiency that refractory to management by medication; (3) Patients with unclear pathological results or pathological findings of non-parathyroid gland lesions, and suspected malignancy. A flowchart of patient selection is displayed in . Between May 2017 and December 2020, a total of 85 PHPT patients who underwent MWA were reviewed to assess for eligibility, and 75 patients were enrolled in the study. Vitamin D deficiency patients started taking vitamin D after MWA at a dose of 800–1600 IU/day.
Microwave equipment and ablation technique
All treatments were performed in our institution and were carried out under US guidance with the patients under local anesthesia in the operating room. The MW unit (ky-2000; Kangyou Medical, Nanjing, China) consists of an MW generator, a flexible low-loss coaxial cable, and a cooled-shaft antenna. The generator is capable of generating 1–100 W of power in pulse or continuous form at 2450 MHz. The microwave antenna is a 16 gauge (tip length 3 mm) coated with polytetrafluoroethylene to prevent adhesion. One of the authors (Liu FY, with 10 years of MWA experience in thyroid and parathyroid nodules) performed MWA on patients. After injecting local anesthesia with 1% lidocaine (Synera, Salt Lake City, Utah) at the puncture site, in order to separate the important structures (such as carotid artery, trachea, esophagus, nerve, etc.) around parathyroid nodule, normal saline was injected continuously to form a 5 mm liquid isolation area [Citation11,Citation12]. Under ultrasound guidance, the antenna was inserted into the target parathyroid gland, by avoiding the blood vessels on the puncture path and around the parathyroid lesion (e.g., carotid artery, thyroid artery, etc.). Before each ablation, ultrasound was used to determine the position of the needle tip in the gland, and then ‘moving-shot’ or fixed ablation technology was used to ablate parathyroid lesions according to the size of the lesions. In general, moving-shot technology was used for lesions with a diameter of more than 1 cm, and fixed ablation technology was used for lesions with a diameter of less than 1 cm. The power for ablation was 20–30 W for each microwave application. When the gland was covered with transient hyperechogenicity, the ablation was terminated. After ablation, contrast-enhanced ultrasound (CEUS) (SonoVue; Bracco, Milan, Italy) was used to assess the extent of the ablation area. The ablation is considered complete if the ablated glands are not enhanced. After MWA, mild compression was applied to the site of the needle insertion for 20 min.
Therapeutic efficacy assessment and follow-up
After 2 h, 1 day, 3 days, 7 days, 1 month, 3 months and 6 months after MWA and then at 6-month intervals, all patients underwent laboratory tests, US or CEUS, and the clinical symptoms, side effects and complications were recorded. Cure rate was defined as the proportion of patients whose PTH and calcium levels returned to normal 6 months after MWA. During the follow-up, if the patient found recurrent lesions and had further treatment again, the follow-up was recalculated. The follow-up period was 6–36 (median, 18) months.
Statistical analysis
Statistical analyses were performed using the SPSS software (version 23.0), Empower (R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA), and R (http://www.R-project.org) and the continuous data were expressed as means ± standard deviations (SD) where data followed a normal distribution, or medians and interquartile range (IQR) where they did not. Hormone level change at different visit time analysis used a mixed model of longitudinal regression for repeated measures with patients identification as random effect and visit time as fixed effect. Seven related risk factors, including gender, age, body mass index (BMI), preoperative PTH level, alkaline phosphatase (ALP) level, nodule’s volume and 25-hydroxyvitamin D3 were analyzed using multivariable logistic regression model method. Receiver operating characteristic (ROC) curves were constructed, and the area under the ROC curve (AUC) was calculated by using the trapezoidal rule. Optimal cutoff values for risk factors were selected to maximize sensitivity, specificity. P value for trend was calculated from the logistic regression models. All of the statistical tests were two-sided, and significance was set at p < 0.05.
Results
Patients
Seventy five PHPT patients (25 males and 50 females; mean age, 56.80 ± 12.34; age range, 26–85) with 84 nodules were enrolled in the study. The baseline characteristics of the patients enrolled are summarized in .
Table 1. Baseline demographics and medical characteristics of patients with PHPT.
Efficacy outcome
All patients underwent MWA successfully and well tolerated (). Overall cure rate was 74.7% (56/75) after one session MWA. The comparison of relevant clinical parameters between the cured cases and the uncured cases is shown in . Compared with cured patients, uncured patients had higher preoperative PTH level, larger nodule volume and maximum diameter. Among the 19 patients who were not cured, six patients had a second session MWA due to recurrence or new lesion. Five patients had local recurrence, which was confirmed by CEUS at the 3rd (two patients), 6th (two patients) and 12th (one patient) month after one session MWA. One patient had a new hyperplastic parathyroid nodule in the contralateral neck of the ablation lesion 18 months after one session MWA. After the second session MWA, five of them achieved cure standard 6 months later, and one had normal calcium level with elevated PTH level. No recurrence or new parathyroid lesions were found in the remaining 13 cases, so no further treatment was taken. After the second session MWA, the cure rate achieved 81.3% (61/75).
Figure 2. Female, 49 years old, diagnosed as primary hyperparathyroidism. (PTH: 137 pg/ml, serum calcium: 2.74 mmol/L, serum phosphorus: 0.80 mmol/L). (A) Contrast-enhanced ultrasonography showed that a hypoechoic nodule behind the left thyroid lobe was highly enhanced in arterial phase; (B) MIBI showed radioactive concentration in the lower left lobe of thyroid and revealed parathyroid adenoma; (C) Microwave antenna was placed into the nodule under US guidance after hydrodissection was performed; (D) After ablation, contrast-enhanced ultrasonography showed the nodule no enhancement.
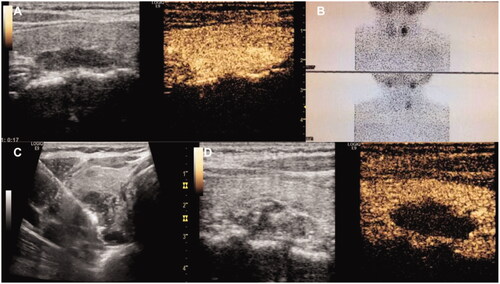
Table 2. Baseline characteristic between curd group and non-cured group.
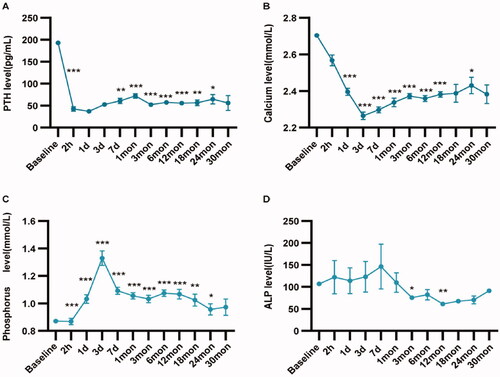
During the follow-up, serum PTH, calcium and ALP decreased significantly, while serum phosphorus increased after MWA. The PTH level decreased significantly 2 h after MWA, reached the lowest level one day after MWA, then rebounded slightly, and became stable three month after MWA. Compared with the change of PTH, the changes of serum calcium and phosphorus levels were a little slower. The change trend of serum PTH, calcium, phosphorus and ALP before and after MWA is shown in and Supplemental Table 1.
Figure 3. The change trend of serum PTH, calcium, phosphorus and ALP before and after MWA for the patients with primary hyperparathyroidism. (A) The change trend of serum PTH; (B) The change trend of serum calcium; (C) The change trend of serum phosphorus; (D) The change trend of ALP. *p < 0.05; **p < 0.01; ***p < 0.001.
Side effects and complications
The main complication observed was voice change. Voice change occurred in 4 patients (5.33%) and recovered within 1-3 months after MWA without special treatment.
The side effects and mild complications observed included pain, perioral and limb numbness and fever. The pain score (NRS) of patients during ablation was 1–4 (median, 2). All patients had no obvious pain after MWA. Mild transient hypocalcemia accompanied by perioral and limb numbness occurred in 13 cases (17.33%) within 1–3 days after ablation, and the symptoms gradually disappeared within one week after oral administration of calcitriol and calcium. Two patients (2.67%) had fever with body temperature lower than 38 °C the first day after MWA, and the temperature was normal 2 days later without special treatment. No other side effects and complications occurred.
Risk factors analyses of cure after one session MWA
By univariate analysis, nodule volume (p = 0.002; odds ratio, 0.62) and pre-ablation PTH level (p = 0.007; odds ratio, 0.19) were statistically significant risk factors related to cure in PHPT patients undergoing one session MWA. By multivariate analysis, nodule volume (p = 0.0097; odds ratio, 0.59) was the independent risk factor associated with cure in PHPT patients undergoing one session MWA (). On ROC curves analysis, we found that nodule volume had an AUC of 0.742 and the cutoff value best predicting cure rate was 0.96 cm3 (sensitivity = 76.8%; specificity =73.9%) ().
Figure 4. The ROC curve of volume of nodules for predicting non cure in patients with primary hyperparathyroidism undergoing one session MWA (AUC, 0.74; sensitivity = 76.8%; specificity =73.9%).
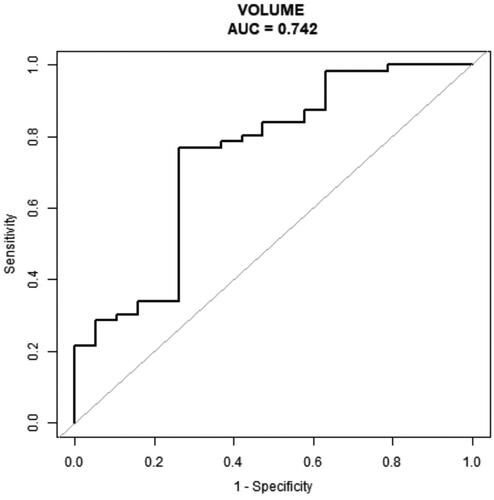
Table 3. Univariable and multivariable analyses of prognostic factors for cure after one session MWA.
The relationship between nodule volume and risk of non-cured was further explored in three logistic regression models. The total population was divided into four groups according to volume of parathyroid nodules. Compared with the reference group of patients in the lowest quartile of volume (<0.26 cm3), patients in quartile 3 (0.50–1.64 cm3) and quartile 4 (≥1.64 cm3) had a substantially higher risk of non-cured (). After adjusting age, sex, PTH level and phosphorus level, patients in quartiles 3 and 4 still had a higher risk of developing non-cured. Therefore, there was a tendency toward an increased risk of cure failure with increasing nodule volume.
Table 4. Trend in prognostic factors associated with therapeutic outcomes.
Risk factors analyses of cure after two sessions MWA
By univariate analysis, nodule volume (p = 0.0070; odds ratio, 0.72) and pre-ablation PTH level (p = 0.029; odds ratio, 0.22) were still statistically significant risk factors related to cure in PHPT patients undergoing MWA after the second session MWA.
By multivariate analysis, nodule volume (p = 0.0408; odds ratio, 0.74) was the independent risk factor associated with cure in PHPT patients undergoing MWA after the second session MWA as well (). On ROC curves analysis, we found that nodule volume had an AUC of 0.719 and the cutoff value best predicting cure rate was 0.96 cm3 (sensitivity = 72.1%; specificity =71.4%) as well ().
Figure 5. The ROC curve of volume of nodules for predicting non cure in patients with primary hyperparathyroidism undergoing two sessions MWA (AUC, 0.72; sensitivity = 72.1%; specificity =71.4%).
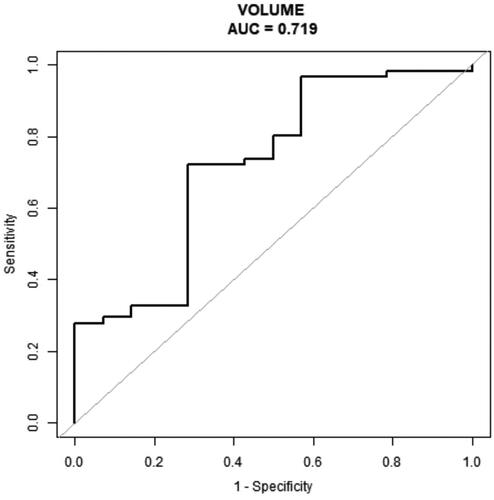
Table 5. Univariable and multivariable analyses of prognostic factors for cure after the second MWA.
Discussion
Thermal ablation, including RFA, MWA, LA and HIFU, has been an effective treatment for PHPT, but only a few small samples have been reported [Citation6–11]. A meta-analysis based on five articles included only 84 PHPT patients treated by thermal ablation, and the results also confirmed its efficacy [Citation14]. Compared with RFA, LA and HIFU, MWA has higher thermal efficiency. Theoretically, MWA has greater application potential in the treatment of PHPT. In our study, the cure rates of PHPT treated with MWA after one session and the second session were 74.7 and 81.3%, respectively, slightly lower than the previously reported surgical resection [Citation13]. Our results showed that nodule volume was an independent risk factor associated with cure after one session or the second session of MWA, and the cutoff value was 0.96 cm3. Local tissue coagulation necrosis caused by MWA or other thermal ablation was different from surgical resection of abnormal parathyroid glands. Moving-shot or fixed multipoint superposition ablation technology was used in the ablation process. Especially for large lesions, the superposition of multipoint thermal field was easy to cause the omission in three-dimensional space, resulting in residual. On the other hand, the hyperecho generated by gasification of ablation area affected the clear display of the non-ablation area, which could also lead to incomplete ablation. Therefore, theoretically, the larger the volume of parathyroid adenoma, the more likely it was to lead to incomplete ablation. Wei et al. reported that incomplete ablation was one of the key factors leading to operative failure [Citation15]. Therefore, how to improve the ablation scheme for the large lesions to achieve complete necrosis is very important.
High preoperative PTH was reported as a risk factor associated with cure rate for patients who underwent surgical resection [Citation16]. Pre-ablation PTH level was related to cure in PHPT patients undergoing MWA by univariate analysis in our study, while it was not by multivariate analysis. Deficiency of vitamin D and small parathyroid nodules (maximum diameter <0.6 cm) were reported as the key factors leading to ablation failure as well [Citation15,Citation17]. However, in our study, vitamin D deficiency was not associated with the cure rate, possibly because all vitamin D deficiency patients were given timely vitamin D supplementation after ablation. Effective calcium and vitamin D supplementation was a very important factor to improve the cure after ablation, especially for patients with preoperative vitamin D deficiency [Citation18,Citation19]. The accuracy of localization diagnosis may be one of the factors affecting the efficacy of MWA for PHPT with small parathyroid nodules. The lesion location of PHPT for MWA was determined only by imaging, but not by neck exploration like surgery. Multiple parathyroid hyperplasia or adenoma may lead to missed diagnosis of focal localization. The sensitivity of MIBI localization for solitary adenomas was reported between 54 and 96% [Citation20,Citation21]. These results were partially dependent on the examiners and were influenced by double adenomas, thyroid nodules, cervical lymphadenopathy and thyroiditis [Citation22,Citation23]. In addition, the prevalence of ectopic hyperparathyroidism (mainly adenomas) was 6.3% to 16%, which was challenging in localization [Citation24]. The sensitivity of ultrasound and CEUS to the localization of parathyroid lesions was over 90% in experienced operators, but there were limitations in the localization of retrosternal ectopic lesions [Citation25]. Therefore, the localization of parathyroid gland before operation needs to combine with a variety of imaging examinations to give full play to the advantages of a variety of images, including MIBI, contrast-enhanced CT and MRI if necessary, multi-modal image fusion can be used to improve the accuracy of parathyroid lesions location [Citation26]. Of course, multiple gland diseases (parathyroid hyperplasia or multiple parathyroid adenomas) accounted for no more than 5–7% of PHPT cases [Citation1,Citation3], but the non-cure rate of ablation was much higher than this proportion. The failure rate of parathyroidectomy was less than 5%, even in centers with good professional experiences, the failure rate was less than 1–2% [Citation13]. Therefore, the factor affecting the lower cure rate was more likely due to the incomplete ablation of parathyroid tissue, especially for large parathyroid nodules. Although most patients did not find evidence of local recurrence in follow-up imaging, as long as there was a small survival of abnormal glands, even if it did not occur in imaging, it was easy to lead to the increase of PTH.
This study had some limitations. First, as a retrospective study, there may be selection bias, so more prospective studies are needed; second, the follow-up time was only 6-36 (median 18) months, so longer follow-up period is required; third, this is a single center study, and multi-center clinical studies are very necessary.
In conclusion, parathyroid nodule volume was the independent risk factor associated with cure in PHPT patients undergoing MWA.
Supplemental Material
Download PDF (81.1 KB)Acknowledgments
We thank the site investigators, study coordinators and patients who participated in the trial. This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Baloch ZW, LiVolsi VA. Pathology of the parathyroid glands in hyperparathyroidism. Semin Diagn Pathol. 2013;30(3):165–177.
- Cetani F, Pardi E, Marcocci C. Parathyroid carcinoma. Front Horm Res. 2019;51:63–76.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569.
- Van Udelsman B, Udelsman R. Surgery in primary hyperparathyroidism: extensive personal experience. J Clin Densitom. 2013;16(1):54–59.
- Singh Ospina N, Thompson GB, Lee RA, et al. Safety and efficacy of percutaneous parathyroid ethanol ablation in patients with recurrent primary hyperparathyroidism and multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2015;100(1):E87–E90.
- Kim HS, Choi BH, Park JR, et al. Delayed surgery for parathyroid adenoma misdiagnosed as a thyroid nodule and treated with radiofrequency ablation. Endocrinol Metab. 2013;28(3):231–235.
- Appelbaum L, Goldberg SN, Ierace T, et al. US-guided laser treatment of parathyroid adenomas. Int J Hyperthermia. 2020;37(1):366–372.
- Sormaz IC, Poyanlı A, Açar S, et al. The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):835–840.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Wei Y, Peng CZ, Wang SR, et al. Effectiveness and safety of thermal ablation in the treatment of primary hyperparathyroidism: a multicenter study. J Clin Endocrinol Metab. 2021;106(9):2707–2717.
- Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253(3):585–591.
- Ye J, Huang W, Huang G, et al. Efficacy and safety of US-guided thermal ablation for primary hyperparathyroidism: a systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):245–253.
- Ying W, Zhen-Long Z, Xiao-Jing C, et al. A study on the causes of operative failures after microwave ablation for primary hyperparathyroidism. Eur Radiol. 2021;31(9):6522–6530.
- Carsello CB, Yen TWF, Wang TS, et al. Persistent elevation in serum parathyroid hormone levels in normocalcemic patients after parathyroidectomy: does it matter? Surgery. 2012;152(4):575–581.
- Stewart ZA, Blackford A, Somervell H, et al. 25-hydroxyvitamin D deficiency is a risk factor for symptoms of postoperative hypocalcemia and secondary hyperparathyroidism after minimally invasive parathyroidectomy. Surgery. 2005; 138(6):1018–1025.
- Norenstedt S, Pernow Y, Zedenius J, et al. Vitamin D supplementation after parathyroidectomy: effect on bone mineral density-a randomized double-blind study. J Bone Miner Res. 2014;29(4):960–967.
- Norenstedt S, Pernow Y, Brismar K, et al. Primary hyperparathyroidism and metabolic risk factors, impact of parathyroidectomy and vitamin D supplementation, and results of a randomized double-blind study. Eur J Endocrinol. 2013;169(6):795–804.
- Lavely WC, Goetze S, Friedman KP, et al. Comparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase (99m)Tc-sestamibi parathyroid scintigraphy. J Nucl Med. 2007;48(7):1084–1089.
- Nasiri S, Soroush A, Hashemi AP, et al. Parathyroid adenoma localization. Med J Islam Repub Iran. 2012;26(3):103–109.
- Erbil Y, Barbaros U, Yanik BT, et al. Impact of gland morphology and concomitant thyroid nodules on preoperative localization of parathyroid adenomas. Laryngoscope. 2006;116(4):580–585.
- Ruda JM, Hollenbeak CS, Stack BC. Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132(3):359–372.
- Theurer S, Siebolts U, Lorenz K, et al. Ektopes gewebe der schilddrüse und der nebenschilddrüsen [Ectopic tissue of the thyroid gland and the parathyroid glands]. Pathologe. 2018;39(5):379–389.
- Agha A, Hornung M, Schlitt HJ, et al. The role of contrast-enhancend ultrasonography (CEUS) in comparison with 99mTechnetium-sestamibi scintigraphy for localization diagnostic of primary hyperparathyroidism. Clin Hemorheol Microcirc. 2014;58(4):515–520.
- Insogna KL. Primary hyperparathyroidism. N Engl J Med. 2018;379(11):1050–1059.