Abstract
Objective
To investigate the survival benefit of thermal ablation (TA) plus chemotherapy for Stage-IV nonsmall cell lung cancer (NSCLC).
Methods
From the Surveillance, Epidemiology and End Results (SEER) database, data of Stage-IV NSCLC patients receiving different treatment modalities (TA plus chemotherapy vs. chemotherapy) from 2004 to 2016 were retrospectively analyzed using propensity-score matching (PSM) for covariates. Kaplan–Meier curves and the log-rank test for intergroup comparison of overall survival (OS) and lung cancer-specific survival (LCSS) and subgroup analyses in the PSM cohort evaluated possible survival benefits. Cox proportional risk models evaluated independent prognostic factors.
Results
Among 52,574 patients, 152 received TA plus chemotherapy. After PSM, the TA plus chemotherapy and chemotherapy groups included 150 and 445 patients, respectively. Compared to the chemotherapy group, the TA plus chemotherapy group had better OS (p = 0.042) and LCSS (p = 0.031), especially in patients aged 70 and older in age-stratified subgroup analysis; no statistically significant beneficial trend was noted for patients younger than 70 years. Subgroup analysis by tumor size showed superior OS and LCSS with TA plus chemotherapy than chemotherapy for tumors ≤3.0 cm; however, no significant difference was found in subgroups with larger tumors. Multivariate analysis showed that TA plus chemotherapy was an independent prognostic factor for OS and LCSS (hazard ratio 0.70 [95% confidence interval 0.59–0.84] and 0.70 [0.58–0.84], respectively; p < 0.001).
Conclusion
TA plus chemotherapy is a potential treatment option for Stage-IV NSCLC, especially for patients aged 70 or older with tumor size ≤3 cm.
Introduction
Lung cancer is a highly invasive and metastatic malignancy, with one of the highest incidences and cancer-related mortality rates worldwide. The 2020 GLOBOCAN statistics indicate that there are 2,206,771 new cases and 1,796,144 deaths due to lung cancer, which accounts for 18.0% of the global cancer mortality rate [Citation1]. Nonsmall cell lung cancer (NSCLC) is the major pathological subtype, accounting for 80–85% of all lung cancer cases [Citation2]. The prognosis of NSCLC patients remains dismal due to the advanced stage at diagnosis that precludes surgical treatment [Citation3]. For patients without sensitive gene mutations or low programmed death-1/ligand-1 (PD-1/L1) expression, chemotherapy-oriented comprehensive treatment is the mainstay of treatment for advanced NSCLC [Citation4], despite the unsatisfactory therapeutic effects. Therefore, new therapeutic approaches to reduce tumor burden and prolong patient survival are urgently needed.
A locoregional method characterized by safety, effectiveness, and reproducibility, thermal ablation (TA) provides a viable alternative option to stereotactic body radiation therapy (SBRT) and surgery that has been widely used for the treatment of malignant lung tumors [Citation5–8]. Currently, thermal ablation has shown potential therapeutic promise for early-stage NSCLC [Citation6–12], but the efficacy of thermal ablation for advanced NSCLC has not been elucidated. Goldberg et al [Citation13]. reported that thermal ablation enhanced NSCLC sensitivity to chemotherapy. Li et al [Citation14]. revealed that thermal ablation plus chemotherapy could significantly improve progression-free survival (PFS) for patients with advanced lung adenocarcinoma, although the trend toward a benefit in overall survival (OS) was not statistically significant. Thus, thermal ablation potentially provides a novel therapeutic option for patients with advanced NSCLC.
Although several studies [Citation15–18] have shown the potential effectiveness of thermal ablation plus chemotherapy for patients with advanced NSCLC, it remained unknown which patients may preferentially benefit from the combination therapy. Therefore, we undertook the present study to investigate (1) whether thermal ablation combined with chemotherapy, compared with chemotherapy, could improve the overall prognosis of patients with stage-IV NSCLC, (2) which of these patients might be suitable for such combination therapy in the future.
Patients and methods
Data source
This retrospective study involved an analysis of data from the Surveillance, Epidemiology and End Results (SEER) database, a population-based program sponsored by the National Cancer Institute, which collects cancer information from 18 areas of the United States and covers 35% of the US population [Citation19]. Access to the database for the purpose of this study was approved (reference number 14524-Nov2020 by SEER∗Stat 8.3.8.).
Study population
We limited the study cohort to adult patients (age >18 years), with histologically confirmed stage IV NSCLC diagnosed between 2004 and 2016, who were treated with chemotherapy alone and/or radiotherapy or chemotherapy in combination with thermal ablation (including laser ablation, cryotherapy, electrocautery, and fulguration). Thermal ablation of only the primary lesion was performed. Patients with a diagnosis at autopsy or death, more than one primary cancer, unknown or incomplete survival data, and missing follow-up information were excluded, as were patients with other surgical procedures beyond thermal ablation. A flow diagram was presented in Figure S1. Data on demographics, including age, sex, race and marital status, were obtained. Tumor characteristics included the primary tumor site, laterality, grade, histologic type, tumor size and nodal stage. Details of therapeutic interventions included data on radiotherapy, chemotherapy and thermal ablation. Based on the different treatment modalities, participants were divided into two groups: thermal ablation plus chemotherapy or chemotherapy. TNM staging was based on the criteria described in the 8th Edition American Joint Committee on Cancer (AJCC) Staging Manual.
Outcomes
The primary endpoints were OS and lung cancer-specific survival (LCSS); OS was defined as the duration from the diagnosis of NSCLC to death from any cause, and patients who were alive were censored at the last follow-up. LCSS was defined as the time to death from lung cancer, and patients who were alive at the time of the last data entry or those who died from any cause other than lung cancer were censored [Citation20].
Propensity score matching
To equalize baseline characteristics between the groups, propensity score matching (PSM) was performed using 1:3 nearest neighbor matching with a caliper of 0.05 to obtain a matched pair [Citation8,Citation21]. Propensity scores were calculated using a logistic regression model that comprised 11 variables (age, laterality, primary tumor site, marital status, sex, race, grade, histologic type, tumor size, nodal stage and radiotherapy). Balances in characteristics before and after PSM were evaluated using the paired t-test or the non-parametric Wilcoxon signed rank test for continuous variables and McNemar’s test for categorical variables.
Ethical statement
Data in the SEER database are freely available and constitute de-identified information, which poses no privacy or confidentiality risks for individual participants. Therefore, this study was exempted from the need for institutional review board approval by the ethics committee of the hospital and was completed in accordance with the National Cancer Institute SEER user agreement.
Statistical analysis
All statistical analyses were performed using SPSS version 26 (IBM Corp, Armonk, NY), GraphPad Prism 8.0.2 (GraphPad Prism Inc. La Jolla, CA), and R version 3.5.0 (http://www.R-project.org). Categorical variables are described as frequency (percentage). PSM was performed to minimize the effect of potential confounders. Unadjusted OS and LCSS curves were estimated using the Kaplan–Meier method, and the intergroup differences were compared with the log-rank test. Subgroup analyses were conducted in the PSM cohort to identify the subgroups that might benefit from thermal ablation plus chemotherapy. Cox proportional hazards models were used to identify factors that were associated with OS or LCSS. Variables with p < 0.05 in the univariable analysis were included in the multivariable analysis. p < 0.05 was considered statistically significant.
Results
Baseline characteristics
In total, 52,574 NSCLC patients were enrolled, and 152 received thermal ablation combined with chemotherapy. The demographics and clinical characteristics of the participants are summarized in . In the study cohort, 34,796 (66.18%) and 17,778 (38.82%) patients were <70 or ≥70 years old. Patients were predominantly male (54.67%), white (77.86%) and married (57.37%). Subgroup analysis showed that there were 15,147, 17,087, 11,139 and 9201 patients with tumor sizes 0–3.0, 3.1–5.0, 5.1–7.0, and >7.0 cm, respectively. The median follow-up duration was 55 months. During the follow-up period, 45,287 patients died, including 42,975 deaths due to NSCLC. The 1-, 2- and 3-year OS and LCSS of the entire study cohort were 37.46%, 17.71% and 9.77% and 39.25%, 19.25% and 11.04%, respectively. Figure S2 provides a representative sample of the typical imaging characteristics of patient with thermal ablation plus chemotherapy.
Table 1. Baseline characteristics in the unmatched cohort and the matched cohort, n (%).
Significant differences in age (p = 0.003), primary tumor site (p < 0.001), histological grade (p < 0.001), histological type (p < 0.001) and radiotherapy (p < 0.001) were noted between the chemotherapy and thermal ablation plus chemotherapy groups. Specifically, patients who received thermal ablation plus chemotherapy were younger, had a higher proportion of squamous cell carcinoma and had an associated higher histological grade and were more frequently treated with radiotherapy. Thus, we conducted 1:3 PSM analysis, and 150 and 445 patients were included in the thermal ablation plus chemotherapy and chemotherapy groups, respectively. In the final analysis model, both groups showed a good match with regard to all baseline characteristics.
Survival analysis
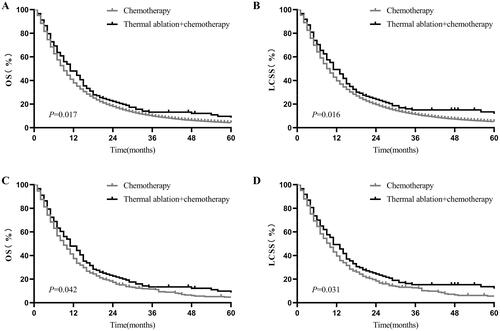
Both before and after PSM, the Kaplan–Meier curve showed that patients who received thermal ablation plus chemotherapy had better OS and LCSS. Before PSM, the estimated OS at 1 and 2 years for thermal ablation plus chemotherapy versus chemotherapy was 46.07% versus 37.43% and 22.10% versus 17.70% (p = 0.017, ), whereas the LCSS at 1 and 2 years was 47.27% versus 39.22% and 22.43% versus 17.07% (p = 0.016, ), respectively. Similar survival results were obtained after PSM. The 1- and 2-year OS of the thermal ablation plus chemotherapy and chemotherapy groups was 46.66% versus 37.14% and 22.43% versus 17.07%, respectively (p = 0.042, ). The 1- and 2-year LCSS of the thermal ablation plus chemotherapy and chemotherapy groups was 47.99% versus 39.18% and 24.20% versus 18.16%, respectively (p = 0.031, ).
Figure 1. Kaplan–Meier curves of stage-IV NSCLC. (A) thermal ablation plus chemotherapy versus chemotherapy for OS before PSM; (B) thermal ablation plus chemotherapy versus chemotherapy for LCSS before PSM; (C) thermal ablation plus chemotherapy versus chemotherapy for OS after PSM; (D) thermal ablation plus chemotherapy versus chemotherapy for LCSS after PSM. NSCLC: nonsmall cell lung cancer; OS: overall survival; LCSS: lung cancer-specific survival; PSM: propensity score matching.
Post-PSM age-stratified subgroup analysis
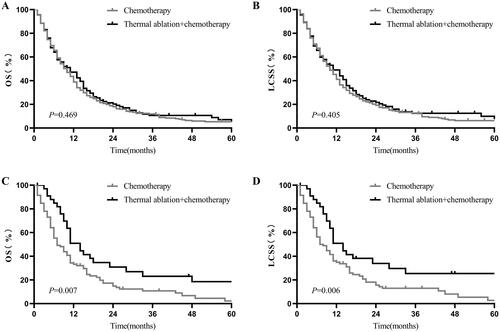
In patients who were younger than 70 years, the 1- and 2-year OS of the thermal ablation plus chemotherapy and chemotherapy groups was 43% vs. 38.4% and 20.07% vs. 17.79%, respectively (p = 0.469, ). The 1- and 2-year LCSS of the thermal ablation plus chemotherapy and chemotherapy groups was 44.62% versus 40.6% and 21.46% versus 19.01%, respectively (p = 0.405, ). In patients aged 70 or older, the 1- and 2-year OS of the thermal ablation plus chemotherapy and chemotherapy groups was 49.91% versus 33.22% and 30.85% versus 14.8%, respectively (p = 0.007, ). The 1- and 2-year LCSS of the thermal ablation plus chemotherapy and chemotherapy groups was 50.91% versus 34.87% and 33.94% versus 15.54%, respectively (p = 0.006, ).
Figure 2. Kaplan–Meier curves of stage-IV NSCLC. A: thermal ablation plus chemotherapy versus chemotherapy for OS of patients aged <70 years before PSM; B: thermal ablation plus chemotherapy versus chemotherapy for LCSS of patients aged <70 years before PSM; C: thermal ablation plus chemotherapy versus chemotherapy for OS of patients aged ?70 years after PSM; D: thermal ablation plus chemotherapy vs. chemotherapy for LCSS of patients aged ?70 years after PSM. NSCLC: nonsmall cell lung cancer, OS: overall survival, LCSS: lung cancer-specific survival, PSM: propensity score matching.
Post-PSM subgroup analysis stratified by tumor size
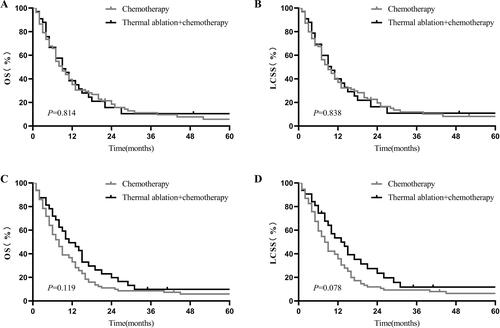
In patients with tumor size ≤3.0 cm, thermal ablation plus chemotherapy induced significantly more favorable OS (1-year OS: 54.17% vs. 37.31%, 2-year OS: 38.95% vs. 17.23%, p = 0.009; ) and LCSS (1-year LCSS: 53.72% vs. 38%, 2-year LCSS: 21.46% vs. 19.01%, p = 0.004; ). However, there was no statistically significant intergroup difference in survival between the thermal ablation plus chemotherapy and chemotherapy groups for tumor sizes of 3.1–5.0 cm (1-year OS: 47.15% vs. 42.12%, p = 0.815, ; 1-year LCSS: 45.15% vs. 44.75%, p = 0.985, ); 5.1–7.0 cm (1-year OS: 36.97% vs. 35.08%, p = 0.814, ; 1-year LCSS: 38.36% vs. 37.04%, p = 0.838, ); and >7.0 cm (1-year OS: 45.66% vs. 32.11%, p = 0.119, ; 1-year LCSS: 48.35% vs. 34.67%, p = 0.078, ).
Figure 3. Kaplan–Meier curves of stage-IV NSCLC. (A) thermal ablation plus chemotherapy versus chemotherapy for OS of patients with tumor size 3 cm before PSM; (B) thermal ablation plus chemotherapy versus chemotherapy for LCSS of patients with tumor size 3 cm before PSM; (C) thermal ablation plus chemotherapy versus chemotherapy for OS of patients with tumor sizes of 3.1–5.0 cm after PSM; (D) thermal ablation plus chemotherapy versus chemotherapy for LCSS of patients with tumor sizes of 3.1–5.0 cm after PSM. NSCLC: nonsmall cell lung cancer; OS: overall survival; LCSS: lung cancer-specific survival; PSM: propensity score matching.
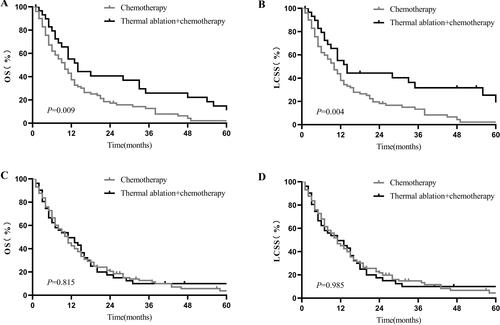
Figure 4. Kaplan–Meier curves of stage-IV NSCLC. (A) thermal ablation plus chemotherapy versus chemotherapy for OS of patients with tumor sizes of 5.1–7.0 cm before PSM; (B) thermal ablation plus chemotherapy versus chemotherapy for LCSS of patients with tumor sizes of 5.1–7.0 cm before PSM; (C) thermal ablation plus chemotherapy versus chemotherapy for OS of patients with tumor size >7.0 cm after PSM; (D) thermal ablation plus chemotherapy versus chemotherapy for LCSS of patients with tumor size >7.0 cm after PSM. NSCLC: nonsmall cell lung cancer; OS: overall survival; LCSS: lung cancer-specific survival; PSM: propensity score matching.
Univariable and multivariable analyses
Univariable analysis including all patients in this study cohort showed that age, sex, race, primary tumor site, laterality, histological grade, histological type, tumor size, nodal stage, radiotherapy and treatment modalities were relevant variables that affected OS and LCSS. Factors with p < 0.05 in the univariable analysis were included in the multivariable analysis, and the results revealed that age, sex, race, primary tumor site, histological grade, histological type, tumor size, nodal stage, radiotherapy and treatment modalities were independent prognostic factors for OS and LCSS. Compared with chemotherapy, thermal ablation plus chemotherapy could decrease the mortality risk in patients with advanced NSCLC by 30% and the risk of cancer-related mortality by 30% ( and ).
Table 2. Univariable and multivariable analysis for OS in the whole cohort.
Table 3. Univariable and multivariable analysis for LCSS in the whole cohort.
Discussion
NSCLC is the predominant type of lung cancer. However, more than 50% of patients present with advanced-stage disease, thereby missing the optimal therapeutic window for curative resection [Citation3,Citation22]. The prognosis of these patients is extremely poor, with a 5-year survival rate of approximately 15%. For patients with inoperable advanced NSCLC, platinum-based doublet chemotherapy is the first-line treatment. However, besides the serious adverse effects of chemotherapy and increased chemoresistance of tumors, the posttreatment prognosis of these patients remains poor [Citation22]. Extending the treatment period does not confer survival benefits and results in increased treatment-related adverse effects after four to six cycles of chemotherapy [Citation23]. Therefore, more effective and less invasive strategies for advanced NSCLC remain a widespread necessity. During the last few years, new techniques of minimally invasive or noninvasive therapy have increasingly gained attention. As a precise, localized and minimally invasive technique, thermal ablation uses thermal energy to cause irreversible damage to or coagulative necrosis of tumor cells [Citation3,Citation5,Citation24]. Therefore, the present study evaluated the clinical efficacy of combination therapy with thermal ablation and chemotherapy in advanced NSCLC and gathered information about which patients might preferentially benefit from the combination therapy.
thermal ablation, including radiofrequency ablation (RFA), cryoablation (CRA), and microwave ablation (MWA), has become a hotspot in the treatment of malignant tumors of the liver, kidneys and lungs [Citation5,Citation25–27]. In early 2005, Calogero Camma et al. [Citation28] conducted a prospective study of 202 hepatocellular carcinoma patients (tumor size ≤5 cm) with well-compensated cirrhosis after RFA and showed that the OS at 12, 24 and 30 months was 80%, 67% and 57%, respectively. Moreover, they concluded that complete response after RFA can significantly improve the OS of hepatocellular carcinoma patients with well-compensated cirrhosis. This raises the question of the application of thermal ablation in lung cancer. Kwan et al. [Citation10] demonstrated no significant differences, after PSM, in OS (p = 0.695) or LCSS (p = 0.819) among older patients with early-stage NSCLC who underwent sub-lobar resection vs. thermal ablation. A later study [Citation29] of the same populations by the researchers further revealed that thermal ablation resulted in significantly lower treatment-related costs and cumulative medical costs at 1, 3 and 12 months after treatment compared with sub-lobar resection. This suggested that thermal ablation is undoubtedly a more optimal option because the two treatments confer the same survival benefit. Dupuy et al. [Citation30] showed that OS at 1 and 2 years is 86.3% and 69.8%, respectively, for stage IA NSCLC in medically inoperable patients after thermal ablation, and serious complications were rare. Consequently, the application of thermal ablation has good efficacy and safety and confers less expense in the treatment of early-stage NSCLC. This raises a question about the application of thermal ablation plus chemotherapy to improve OS for patients with advanced-stage NSCLC.
Our study revealed that there was a significant difference in age, primary site, histology grade, pathological type, and radiotherapy in the two groups before PSM; however, after PSM, no statistically significant difference in baseline characteristics was observed. Thus, the results indicated that thermal ablation plus chemotherapy could indeed improve survival of patients with advanced NSCLC after eliminating the influence of confounding factors. Compared with the chemotherapy group, thermal ablation plus chemotherapy reduced the mortality risk and cancer-related mortality risk by 30%. The results are consistent with a multicenter, randomized and controlled phase-III trial [Citation2] conducted at 14 sites in China that enrolled 293 patients with advanced NSCLC and randomized them into two groups. The thermal ablation plus chemotherapy group had a longer median PFS (10.3 months vs. 4.9 months, p < 0.001) and median OS (median OS not reached vs. 12.4 months, p < 0.001) than the chemotherapy group. Another retrospective study [Citation14] showed that the MWA plus chemotherapy group had a longer median PFS (8.029 months vs. 4.654 months, p < 0.01) than the chemotherapy group in advanced lung adenocarcinoma. The above-mentioned results have been validated in some other retrospective studies [Citation15–18].
In addition, when conducting subgroup analyses by age, we found that thermal ablation plus chemotherapy can improve the OS and LCSS at 1 and 2 years in patients aged ≥70 years with advanced NSCLC. Although there was a survival benefit for NSCLC patients younger than 70 years, the difference was not statistically significant. It remains unclear why patients with advanced NSCLC aged ≥70 years can benefit from thermal ablation plus chemotherapy. Several factors may contribute to the outcome. Older patients may have complicated clinical conditions due to cardiopulmonary insufficiency or other systemic diseases; therefore, along with the same chemotherapy, additional survival benefits can accrue to them by thermal ablation – a localized treatment that reduces damage to surrounding normal tissues and older patients are able to better tolerate it [Citation18]. However, specific details regarding thermal ablation or chemotherapy were unavailable in our study. Further prospective studies are needed to explore the influence of age on thermal ablation and therapeutic option by different age segments. When the tumor size was analyzed in subgroups, we found that for patients whose tumor size ranged from 0 to 3 cm, the 1-year and 2-year OS and LCSS were longer in the thermal ablation plus chemotherapy group than in the chemotherapy group; however, statistically significant differences were not found in other subgroups with tumor sizes bigger than 3 cm. Schoellnast et al. [Citation31] reported that the post-RFA tumor local progression time among NSCLC patients with tumor size <3 cm was 24 months whereas that among patients with tumors ≥3 cm was 8 months (p = 0.07); this showed that, as a localized therapy, thermal ablation cannot completely destroy larger tumors, which leads to tumor recurrence. Thus, tumor size needs to be taken into consideration for subsequent clinical decisions when recommending thermal ablation plus chemotherapy.
Several factors may lead to better survival benefits from thermal ablation plus chemotherapy. Firstly, thermal ablation can lead to irreversible damage to tumor cells and upgrade some steps in the tumor immunity cycle, such as tumor-antigen release, antigenic presentation of dendritic cells and T-cell infiltration, which can enhance the anti-tumor immune function [Citation5,Citation32]. Secondly, hypoxic tumors was typically less sensitive to chemotherapy [Citation33], and thermal ablation can rapidly increase the core temperature of the tumor, induce coagulative necrosis and apoptotic destruction of the centrally located hypoxic cells [Citation34]. Thirdly, thermal ablation can increase the permeability of the tumor cell membrane, which can enable the delivery of chemotherapeutic drugs to the tumor center more effectively to enhance the effect of chemotherapy [Citation13]. Finally, thermal ablation can attenuate the tumor burden as a localized treatment while chemotherapy can eliminate peripheral tumor cells and subclinical micrometastases to reduce recurrence and metastases as a systemic treatment [Citation35]. Therefore, the two treatments complement each other to maximize their anticancer effects.
Our study has some limitations. Firstly, as previously described, thermal ablation can enhance the body's immune function while destroying tumor cells. Thus, there is no doubt that the treatment has a synergistic effect with immunotherapy [Citation5,Citation36]. Currently, immunotherapy plus chemotherapy is the gold standard first-line therapy in Stage-IV NSCLC. However, the immunotherapy status of the two groups was not evaluated as a variable in our study due to the limitations of the SEER database. The number of participants who received immune checkpoint inhibitor therapy plus chemotherapy remains unknown, which can result in correlation errors. Furthermore, we could not investigate the theoretical benefit of thermal ablation in patients who received immunotherapy plus chemotherapy as the first-line therapy. Secondly, different ablation types based on different principles may have an influence on the prognosis. However, the SEER database fails to differentiate RFA, MWA and CRA. Therefore, we could not ascertain detailed information of the number of participants by each thermal ablation modality (RFA, MWA, CRA, etc.). Thirdly, our study has a retrospective design, which incurs certain selection and information biases. It is very important to know the tumor burden regarding the patients with metastasis. The location and number of metastases may have important implications for prognosis and treatment, and thereby influence survival. However, due to the limitations with regards to the data available in the SEER database, we could not ascertain the specific number of metastatic sites and identify the site of metastasis, which may result in bias. Fourthly, for the survival outcome, based on the LCSS and OS, only the follow-up time after the diagnosis, vital status and cause of death were ascertained from the SEER database. Information about tumor recurrence is not captured in the SEER database; therefore, disease-free survival, recurrence-free survival, PFS and distant PFS could not be analyzed. Moreover, information on the date of the first thermal ablation was unavailable in the SEER database. Finally, our study used the SEER database, which lacks information about the specific chemotherapy regimens and doses, and different regimens could also have an influence on the prognosis. Therefore, large-scale prospective studies are needed to validate the outcome that thermal ablation plus chemotherapy can improve the clinical effects of patients with stage IV NSCLC.
Conclusion
In summary, thermal ablation plus chemotherapy may be a potential treatment option for stage IV NSCLC patients, especially for those who are older than 70 years with tumor size of up to 3 cm.
Supplemental Material
Download PDF (771.9 KB)Acknowledgements
We would like to thank the native English-speaking scientists of Wordvice Company (The Aske Stables, Aske, Richmond, North Yorkshire, U.K.) for editing our manuscript.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
All the data that support the results of the current study are publicly available in the SEER database (https://seer.cancer.gov/).
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Wei Z, Yang X, Ye X, et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a multicenter, randomized, controlled, phase III clinical trial. Eur Radiol. 2020;30(5):2692–2702.
- Xu S, Qi J, Li B, et al. Survival prediction for non-small cell lung cancer patients treated with CT-guided microwave ablation: development of a prognostic nomogram. Int J Hyperthermia. 2021;38(1):640–649.
- Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171–210.
- Rangamuwa K, Leong T, Weeden C, et al. Thermal ablation in non-small cell lung cancer: a review of treatment modalities and the evidence for combination with immune checkpoint inhibitors. Transl Lung Cancer Res. 2021;10(6):2842–2857.
- Chan MV, Huo YR, Cao C, et al. Survival outcomes for surgical resection versus CT-guided percutaneous ablation for stage I non-small cell lung cancer (NSCLC): a systematic review and Meta-analysis. Eur Radiol. 2021;31(7):5421–5433.
- Li M, Xu X, Qin Y, et al. Radiofrequency ablation vs. stereotactic body radiotherapy for stage IA non-small cell lung cancer in nonsurgical patients. J Cancer. 2021;12(10):3057–3066.
- Zeng C, Lu J, Tian Y, et al. Thermal ablation versus wedge resection for stage I non-small cell lung cancer based on the eighth edition of the TNM classification: a population study of the US SEER database. Front Oncol. 2020;10:571684.
- Li M, Qin Y, Mei A, et al. Effectiveness of radiofrequency ablation therapy for patients with unresected stage IA non-small cell lung cancer. J Can Res Ther. 2020;16(5):1007–1013.
- Kwan SW, Mortell KE, Talenfeld AD, et al. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv Radiol. 2014;25(1):1–9.e1.
- Yao W, Lu M, Fan W, et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int J Hyperthermia. 2018;34(8):1329–1336.
- Liu B, Liu L, Hu M, et al. Percutaneous radiofrequency ablation for medically inoperable patients with clinical stage I non-small cell lung cancer. Thorac Cancer. 2015;6(3):327–333.
- Goldberg SN, Saldinger PF, Gazelle GS, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology. 2001;220(2):420–427.
- Li C, Wang J, Shao JB, et al. Microwave ablation combined with chemotherapy improved progression free survival of IV stage lung adenocarcinoma patients compared with chemotherapy alone. Thorac Cancer. 2019;10(7):1628–1635.
- Yu S, Wu ZZ, Si HT, et al. Short-term effect analysis of radiofrequency ablation combined chemotherapy on Middle and late period non-small cell lung cancer. Oncol Lett. 2016;12(6):4399–4402.
- Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32(2):464.
- Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38(1):135–142.
- Lee H, Jin GY, Han YM, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol. 2012;35(2):343–350.
- Shah S, Gosain R, Groman A, et al. Incidence and survival outcomes in patients with lung neuroendocrine neoplasms in the United States. Cancers (Basel). 2021;13(8):1753.
- Puijk RS, Ahmed M, Adam A, et al. Consensus guidelines for the definition of time-to-Event end points in image-guided tumor ablation: Results of the SIO and DATECAN initiative. Radiology. 2021;301(3):533–540.
- Zhao ZR, Situ DR, Lau R, et al. Comparison of segmentectomy and lobectomy in stage IA adenocarcinomas. J Thorac Oncol. 2017;12(5):890–896.
- Yi L, Fan J, Qian R, et al. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: a meta-analysis. Int J Cancer. 2019;145(1):284–294.
- Rossi A, Chiodini P, Sun JM, et al. Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2014;15(11):1254–1262.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- John JB, Anderson M, Dutton T, et al. Percutaneous microwave ablation of renal masses in a UK cohort. BJU Int. 2021;127(4):486–494.
- Ni Y, Xu H, Ye X. Image-guided percutaneous microwave ablation of early-stage non-small cell lung cancer. Asia‐Pac J Clin Oncol. 2020;16(6):320–325.
- Wang L, Xu J, Yu J, et al. Review of clinical tumor ablation advance in Asia. Int J Hyperthermia. 2021;38(1):1639–1649.
- Cammà C, Di Marco V, Orlando A, et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol. 2005;42(4):535–540.
- Kwan SW, Mortell KE, Hippe DS, et al. An economic analysis of sublobar resection versus thermal ablation for early-stage non-small-cell lung cancer. J Vasc Interv Radiol. 2014;25(10):1558–1564. quiz 1565.
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American college of surgeons oncology group Z4033 (alliance) trial. Cancer. 2015;121(19):3491–3498.
- Schoellnast H, Deodhar A, Hsu M, et al. Recurrent non-small cell lung cancer: evaluation of CT-guided radiofrequency ablation as salvage therapy. Acta Radiol. 2012;53(8):893–899.
- Chavez M, Silvestrini MT, Ingham ES, et al. Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics. 2018;8(13):3611–3628.
- Kim SW, Kim IK, Lee SH. Role of hyperoxic treatment in cancer. Exp Biol Med (Maywood). 2020;245(10):851–860.
- Li X, Zhao M, Wang J, et al. Percutaneous CT-guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non-small cell lung cancers. AJR Am J Roentgenol. 2013;201(6):1362–1367.
- Sun YH, Song PY, Guo Y, et al. Effects of microwave ablation or its combination with whole-body chemotherapy on serum vascular endothelial growth factor levels in patients with stage IIIB/IV NSCLC. Genet Mol Res. 2015;14(3):10015–10025.
- Leppelmann KS, Mooradian MJ, Ganguli S, et al. Thermal ablation, embolization, and selective internal radiation therapy combined with checkpoint inhibitor cancer immunotherapy: Safety analysis. J Vasc Interv Radiol. 2021;32(2):187–195.
