Abstract
Background
Hyperthermia is a widely used adjunct treatment for different cancers including nasopharyngeal carcinoma (NPC). The protooncogene c-Myc is up-regulated in NPC and its expression is associated with poor prognosis.
Objective
We hypothesized that c-Myc constitutes an important hyperthermia treatment target, and we investigated its contribution to hyperthermia responses in NPC.
Methods
The growth of the human NPC cell lines CNE1 and CNE2 was analyzed using CCK-8 and clonogenicity assays after 43 °C hyperthermia, knockdown or overexpression of c-Myc. Flow cytometry measurements assessed cell cycle parameters and apoptosis, while levels of c-Myc together with key transcriptional targets were determined using qPCR and Western blotting. Parallel experiments were undertaken using NPC xenografts in nude mice and lastly, global transcriptomic changes were determined using ‘RNAseq’.
Results
Hyperthermia increased the ubiquitination and proteasomal destruction of c-Myc, causing a rapid decline in c-Myc protein levels in NPC cells. Similar to c-Myc knockdown, NPC cells treated with hyperthermia showed growth inhibition associated with the downregulation of c-Myc target genes. Moreover, low levels of c-Myc could be sustainably repressed in NPC cells through repeated hyperthermia treatments. Importantly, the key findings of growth inhibition and decreased c-Myc protein levels were reproduced in NPC tumor xenografts. Bioinformatic analyses showed that downregulation of c-Myc constituted a central node in the hyperthermia response of NPC cells.
Conclusion
Our study reveals that hyperthermia can readily destabilize c-Myc levels in NPC cells and inhibit tumor growth. This proposes new strategies for implementing hyperthermia to target c-Myc-driven cancers to improve therapeutic efficacy.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck malignancies and is considered an endemic disease in southern China and throughout Southeast Asia [Citation1]. When confined to the nasopharynx, early-stage NPCs are sensitive to radiotherapy, with intensity-modulated therapy generally achieving good therapeutic effects [Citation2]. However, a high proportion (30–40%) of NPC patients are diagnosed with locally advanced disease with invasion evident to nearby structures or to lymph nodes in the neck [Citation3]. Outcomes worsen according to stage with the five-year survival rate of patients with stage I and II disease being 72–90%, decreasing to 30–55% in stage III and IV cases [Citation4]. Radiation therapy combined with chemotherapy can significantly increase the response rates of NPC, but nonetheless, tumor recurrence and distant metastasis are still the main failure modes and important causes of disease-related deaths [Citation5–7]. Moreover, chemotherapy resistance and various adverse reactions to radiotherapy represent significant obstacles for treating patients with recurrent NPC. Thus, it remains necessary to find more effective treatments.
Hyperthermia is a therapeutic procedure used to treat cancer by raising the temperature of tumor tissues. In addition to traditional surgery, chemotherapy, radiation, and biological therapy, hyperthermia has become a crucial part of many multidisciplinary treatments [Citation8]. For some patients who are not suitable candidates for surgery, hyperthermia can be implemented as an alternative to improve quality of life [Citation9]. For example, the addition of hyperthermia to standard treatment regimens has been proven to be effective in different cancer types including cervical cancer, malignant melanoma, recurrent breast cancer, soft tissue sarcoma, and bladder cancer [Citation10–12]. Furthermore, hyperthermia combined with radiotherapy and chemotherapy can improve the local control of N2–N3 stage NPC with improvements in the overall survival of patients with advanced disease [Citation13,Citation14]. However, despite decades of use as an adjunctive treatment, the underlying molecular mechanisms invoked by hyperthermia are only beginning to be better understood.
Hyperthermia exerts changes in tumor physiology to promote the accumulation and distribution of small molecules and nano-drugs within tumors. It has also been shown to affect DNA damage repair following radiation, helping to improve the efficacy of radiotherapy [Citation15–17]. Moreover, hyperthermia can also be combined with immunotherapy to enhance immune cell infiltration and responsiveness through modifying the tumor microenvironment of solid tumors [Citation18,Citation19]. Other studies investigating the molecular basis of hyperthermia treatment have established important roles for key cancer molecules and their associated signaling pathways. For example, hyperthermia blocks the interaction between p53 and the human papillomavirus (HPV) E6 protein, promoting the restoration of p53 function including G2 cell cycle phase arrest and apoptosis of HPV-positive cervical cancer cells [Citation20]. Similarly, microwave-induced hyperthermia also promotes G2/M arrest and apoptosis of non-small cell lung cancer cells through the ATM pathway [Citation21]. Hyperthermia also destroys the stability of tumor fusion proteins including PML/RARα in acute promyelocytic leukemia, working synergistically to improve therapeutic efficacy in relapsed and refractory disease [Citation22]. Likewise, the increased activation of the AKT pathway in glioblastoma caused by radiotherapy can be reduced by adding hyperthermia before radiotherapy, thereby increasing the sensitivity of radiotherapy [Citation23]. Most recently, it was found that heat shock induces YAP dephosphorylation and Hippo pathway activation, with clear evidence showing that this response enables the survival of mouse B16 melanoma cells following hyperthermia [Citation24]. Thus, hyperthermia invokes a variety of different pathways that either allow for cell adaptation and survival or alternatively can induce cell death.
Previous studies have established that c-Myc expression is commonly up-regulated in NPC through gene amplification or by other mechanisms [Citation25–27] and its increased expression is associated with poor prognosis [Citation28,Citation29]. Inspired by these observations, we speculated that the effects of hyperthermia on NPC cells could be mediated through c-Myc. This protooncogene is a helix-loop-helix leucine zipper transcription factor arguably best known for its role in regulating cell proliferation where it elicits dual effects: acting on one hand to promote cell cycle progression by transactivating positive regulators such as CDC25A, CDK4 and cyclins A, D2, and E, while on the other hand, preventing growth arrest through inhibiting the expression of negative cell cycle regulators such as p21 and p27 [Citation30,Citation31]. In this study, we show that hyperthermia inhibits the expression of c-Myc in NPC cells both in vitro and in vivo with associated cell growth inhibition and the induction of apoptosis. We further establish that hyperthermia promotes the rapid turnover of c-Myc through increased ubiquitination and proteasomal degradation, and this effect can be sustained through repeated treatment cycles. Overall, our results promote the utility of hyperthermia as a treatment to effectively target c-Myc expression in nasopharyngeal carcinoma.
Materials and methods
Cell culture
The human nasopharyngeal carcinoma cell lines CNE1 and CNE2 were obtained from the ATCC. NPC cells were maintained in RPMI-1640 medium (SparkJade) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin, 100 mg/mL streptomycin while HEK293T cells were cultured with DMEM medium (SparkJade) with the same supplements. All cells were maintained at 37 °C in a humidified 5% CO2 atmosphere.
Hyperthermia
Heat treatments were conducted in a pre-equilibrated water bath at 43 °C. Cells were first changed to preheated medium before semi-submerging the cell culture dishes for 30–60 min as indicated.
Cell transfection
Negative control (si-NC) siRNAs and those targeting c-Myc were synthesized by Tong Yong (Shanghai, China). The human c-Myc siRNA sequences were as follows: 5′-GGAACAAGAAGAUGAGGAATT-3′ and 5′-UUCCUCAUCUUCUUGUUCCTT-3′. For overexpression, the human c-Myc cDNA was subcloned into pCDH (GenePharma, Shanghai, China) with empty pCDH vector used as the control. All transfections were conducted using the Lipo6000 reagent (Beyotime) according to the manufacturer’s instructions.
Cell proliferation
Cell proliferation was assessed using CCK-8 and colony formation assays. For CCK-8 assays, 96 well plates were seeded with 4000 cells/well and after the specified times, 10 µl of CCK-8 solution (SparkJade, CT0001-B) was added per well and the plates further incubated at 37 °C for 1 h. OD values at 450 nm were measured using a spectrophotometer (ThermoFisher Varioskan LUX) and the values normalized for analysis. For colony formation assays, six-well plates were seeded with 1500 cells per well and then incubated until the number of cells in single colonies equaled or exceeded 50 cells. The wells were then washed twice with cold PBS, fixed 15 min in 4% formaldehyde, and stained 10 min with 0.1% crystal violet. Thereafter, the wells were imaged, and the number of total colonies counted. When studying the effect of heat treatment on cell proliferation, cells were heat treated every two days.
Antibodies
The following antibodies and dilutions used in the study were: CDK1 (1:1000; Huaan, ET1605-54), CDK4 (1:1000; Proteintech,11026-1-AP), PCNA (1:1000; Proteintech, 10205-2-AP), GAPDH (1:1000; Proteintech, 10494-1-AP), p27 (1:1000; Huaan, ET1608-61), Ubiquitin (1:1000; Huaan, ET1609-21), c-Myc (1:1000; Proteintech, 10828-1-AP), Goat anti-Rabbit HRP (1:5000; Proteintech, SA00001-2).
Western blot
Cell monolayers were washed twice with cold PBS before direct lysis with 2 × SDS loading buffer. The lysates were then boiled for 15 min before protein separation using 7.5–12% SDS-PAGE gels and transfer to nitrocellulose membranes. Protein loading and transfer to the membranes was first checked with Ponceau S staining before incubation of the membranes with blocking solution (Ncmbio Blot Blocking Buffer, P30500) for 20 min at room temperature. After overnight incubation at 4 °C with the designated primary antibodies, the membranes were washed three times with TBST for 10 min and then incubated with secondary antibodies in TBST for 1 h at room temperature. After further washing, the antibody decorated bands were visualized washed using a chemiluminescent HRP substrate (Yamei ECL PicoLight Substrate, SQ202-1) and images recorded using a ChemiDoc MP system (BioRad).
RNA extraction and real-time PCR
Total RNA was extracted from trypsinized cell suspensions using the Trizol reagent (SparkJade) according to the manufacturer’s instructions. After quantitation using a NanoDrop spectrophotometer (Thermo scientific), 1 μg of total RNA was used as template for reverse transcription according to the PrimeScript RT kit (Takara, RR037A). The cDNA was then diluted and used as a template for quantitative PCR (qPCR) reactions using the 2 × SYBR Green qPCR Mix (Spark Jade, AH0104-B). Reactions were performed on the Step OnePlus real-time PCR system (Applied Biosystems, USA) and the relative expression levels of the target gene mRNA determined against the GAPDH housekeeping gene control using the 2–ΔΔCt method. The primer sequences are as follows:
GAPDH F:GGAGCGAGATCCCTCCAAAAT; R:GGCTGTTGTCATACTTCTCATGG;
PCNA F:CCTGCTGGGATATTAGCTCCA; R: CAGCGGTAGGTGTCGAAGC;
CDK1 F:AAACTACAGGTCAAGTGGTAGCC; R:TCCTGCATAAGCACATCCTGA;
CDK4 F:ATGGCTACCTCTCGATATGAGC; R:CATTGGGGACTCTCACACTCT;
c-Myc F:GGCTCCTGGCAAAAGGTCA; R:CTGCGTAGTTGTGCTGATGT;
Flow cytometry
Cell cycle parameters and apoptosis were measured using the CyclePlus kit (KeyGEN BioTECH, KGA512) and the Annexin V-FITC/PI Apoptosis Detection Kit (KeyGEN BioTECH, KGA103) according to the manufacturer's protocols. Cells were heat-treated at 43 °C for 30 min and recovered at 37 °C for 24 h. Briefly, the adherent cells were harvested using trypsin without EDTA and pooled with the culture supernatant and centrifuged before resuspending the cells in 500μl binding buffer. After adding Annexin-V-FITC and PI, the cell suspension was incubated in the dark for 15 min at RT before data acquisition for apoptosis using a BD FACSAriaTM III Cell Sorter (BD Biosciences). For the assessment of cell cycle, cells were heat-treated at 43 °C for 30 min and allowed to recover at 37 °C for 8 h. The harvested cells were fixed overnight at 4 °C using 70% ice-cold alcohol before the addition of 500 µl PI/RNase Staining Buffer solution for 30 min at RT. Data analysis was performed using FlowJo V10 software to analyze apoptosis, while Modfit software was used to estimate cell cycle parameters.
Animal model
Female BALB/c nude mice were purchased from Jiangsu GemPharmatech (China) and housed under SPF conditions. After acclimatization, 1 × 107 CNE2 were injected subcutaneously into the dorsal flanks of the mice. When tumor volumes reached an appropriate size of 0.2 cm3 at day 14, hyperthermia treatments were performed every three days. The mice were first anesthetized with 4% Chloral hydrate (10 µl/g) before immersion of the tumor site in a 43 °C water bath for 30 min. The hyperthermia group was heat-treated a total of four times and the experiment concluded on day 26 when the mice were humanely sacrificed, and the tumors excised, measured and weighed. Tumors were then divided, and portions frozen for later biochemical analysis or fixed in 4% formaldehyde for histopathology. All animal experiments were performed in accordance with the protocol approved by the Institutional Animal Care under the study approval number ZZU-LAC20210326 following the guidelines provided by the Animal Care Program. No experiment in this study produced a tumor burden that exceeded the 1.5 cm limit of a maximum diameter of single tumors.
Immunohistochemistry and TUNEL staining
After fixation, subcutaneous tumor samples were dehydrated and embedded in paraffin. Sections were cut (4 μm), deparaffinized and hydrated before being subjected to antigen heat repair. Thereafter, immunohistochemical staining was performed with the indicated antibodies (c-Myc, Ki67, PCNA) using the 2-step plus Poly-HRP Anti Rabbit IgG Detection System (E-IR-R215, elabscience) according to the manufacturer's instructions. Alternatively, TUNEL staining was used to reveal apoptotic cells. Briefly, after the deparaffinization and rehydration steps, the sections were treated with Proteinase K before were processing the tissue sections according to CoraLite@488 TUNEL Apoptosis Detection Kit (PF00006). Then, the sections were counterstained with DAPI before recording the results using an epifluorescence microscope.
RNAseq and bioinformatic analyses
Total RNA was prepared as described above and commercially sequenced by Shanghai Sinomics Corporation (Shanghai, China). Paired-end libraries were synthesized by using the TruSeq™ RNA Sample Preparation Kit (Illumina, USA) following TruSeq™ RNA Sample Preparation Guide. Clustering was generated by cBot with the library diluted to 10 pM and then were sequenced on the Illumina NovaSeq 6000 (Illumina, USA). Differential regulated genes were assigned by applying thresholds of log fold change ≥2 or ≤0.5 and presented as a Volcano plot. To assess for functional associations, we uploaded the differentially regulated gene data to the STRING database (http://www.string-db.org/) to create a protein–protein interaction network based on differentially expressed gene cutoffs of logFC ≥ 1.5 and logFC ≤ 1.5 with p < 0.05. The output was combined in Cytoscape software (version 3.6.1) to illustrate the protein–protein interaction network. The hub genes, which were defined as genes that play essential roles in the network, were distinguished according to the cutoff criteria of degree calculated by cytoHubba in Cytoscape. On this basis, we screened for the top 10 genes with the CytoHubba plugin of Cytoscape by using topological analysis methods including Edge Percolated Component, Maximum Neighborhood Component, Degree, Closeness, and EcCentricity. Lastly, we downloaded the c-Myc target gene set from GSEA (http://www.gsea-msigdb.org/) and determined which target genes overlapped with the genes differentially altered by hyperthermia. We then similarly constructed a PPI network based on the c-Myc target genes identified using this approach.
Statistical analysis
p Values were determined using two-sided unpaired t-tests using Microsoft Excel. Pearson correlation coefficients were used to determine the strength of association between two variables. All data were expressed as mean ± s.d. with differences of p < 0.05 considered statistically significant.
Results
c-Myc is highly expressed in NPC and promotes cell proliferation
We first evaluated the relative expression levels of c-Myc in ex vivo NPC tissues in comparison to levels in para-cancerous NP tissues. Interrogating three independent datasets from the Gene Expression Omnibus (GEO) resource representing a total of nasopharyngeal carcinoma patients (GSE12452, GSE53819 and GSE61218) showed that c-Myc expression was significantly increased in NPC versus normal tissues (Supplementary Figure S1(A)–(C)). In addition, dividing the cases by disease stage we found that c-Myc expression was increased in all stages with the overall highest expression in stage III cases, suggesting a relationship between c-Myc expression and NPC progression (Supplementary Figure S1(D)). Moreover, we observed strong correlations between c-Myc and these target genes known to drive cell progression (Supplementary Figure S2).
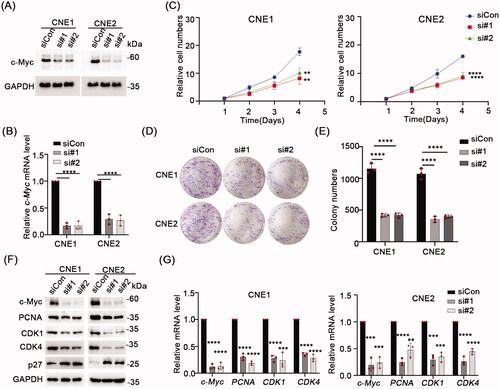
Next, to establish that c-Myc is essential for NPC cell growth, we used siRNA to knockdown c-Myc expression in the NPC-derived cell lines, CNE1 and CNE2. Relative to the control siRNA (siCon) treatment, Western blot and qPCR assays verified that c-Myc was significantly depleted at the protein and mRNA levels, respectively, using two independent siRNAs targeting c-Myc (). Further analysis of cell proliferation assays using CCK-8 assays demonstrated that c-Myc depletion significantly inhibited cell proliferation in both CNE1 and CNE2 cells after 72 h (p < 0.05) (). Similarly, colony formation assays revealed that c-Myc knockdown decreased the number of colonies formed by CNE1 and CNE2 cells relative to the siCon treatment (). In accordance, Western blot analysis showed the levels of the cell proliferation markers PCNA, CDK1 and CDK4 were all decreased while correspondingly, the levels of p27, a negative regulator of cell proliferation were upregulated (). Consistently, qPCR data showed the mRNA levels of PCNA, CDK1 and CDK4 were down-regulated ().
Figure 1. Knock down c-Myc inhibits NPC cells proliferation. (A,B) The knockdown efficiency of c-Myc confirmed using Western blot and qRT-PCR. (C) CCK-8 assay in CNE1 and CNE2 cells transfected with negative control (siNC) or c-Myc siRNA and cultured for the indicated times. (D,E) Colony formation assay showed reduced colony number of CNE1 and CNE2 cells after silence of c-Myc. F) Western blot analysis of the indicated proteins in CNE1 and CNE2 cells transfected with negative control (siNC) or c-Myc siRNA. GAPDH served as loading control. (G) mRNA levels expression of c-Myc, PCNA, CDK1 and CDK4 was evaluated by qRT-PCR. GAPDH served as reference gene. Data shown as mean ± sd.
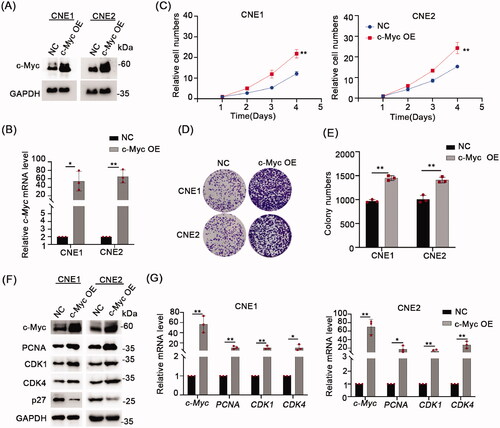
To further clarify the effect of c-Myc on NPC cell growth, we overexpressed c-Myc in NPC cell lines using lentiviral-mediated transduction. The results of Western blotting and qPCR assays confirm that c-Myc protein and mRNA levels were substantially increased in both CNE1 and CNE2 cell lines (). Assessing the effects of c-Myc overexpression using CCK-8 and colony formation assays showed that c-Myc significantly promoted cell proliferation in NPC cells (). In parallel, we conducted analyses of cell proliferation markers, showing the protein levels of PCNA, CDK1 and CDK4 were increased while p27 levels were down-regulated after overexpression of c-Myc (). Consistently, qPCR analysis revealed the mRNA levels of PCNA, CDK1 and CDK4 were up-regulated in the c-Myc overexpressing NPC cells ().
Figure 2. Overexpression c-Myc inhibits NPC cells proliferation. (A) Western blot analyses were used to analyze c-Myc protein expression in CNE1 and CNE2 cells stablely expressing c-Myc. (B) qRT-PCR was used to analyze c-Myc mRNA level in CNE1 and CNE2 cells stably expressing c-Myc. (C) CCK-8 assay indicated that c-Myc overexpression significantly increased cell proliferation ability in CNE1 and CNE2 cells. (D,E) c-Myc overexpression promoted colony formation ability in CNE1 and CNE2 cells stably expressing c-Myc. (F) Western blot analysis of the indicated proteins in CNE1 and CNE2 cells stably expressing c-Myc. GAPDH served as loading control. G, mRNA levels of c-Myc, PCNA, CDK1 and CDK4 was evaluated by qRT-PCR in CNE1 and CNE2 cells stably expressing c-Myc. Data shown as mean ± sd.
Collectively these data establish that c-Myc is commonly upregulated in clinical NPC tissues with confirmation using in vitro analyses showing c-Myc promotes NPC cell proliferation.
Hyperthermia inhibits NPC cell growth through targeting c-Myc
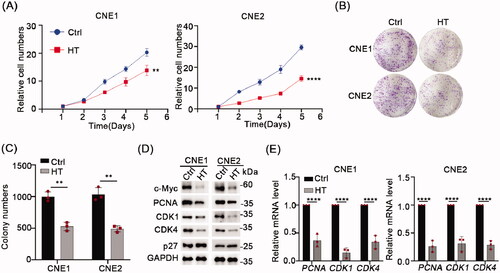
As described in the Introduction, hyperthermia has been used as an adjunct therapy with radiotherapy and/or chemotherapy to successfully treat NPC. Since c-Myc is involved in the progression of NPC and can promote cell proliferation, we hypothesized that hyperthermia may target c-Myc to inhibit NPC cell growth. To test our hypothesis, we mimicked in vivo hyperthermia conditions by subjecting NPC cell lines to 43 °C temperatures daily over two days before assessing the effects of hyperthermia on cell proliferation. Indeed, using CCK-8 and colony formation assays we found that heat treatment significantly inhibited cell growth in both the CNE1 and CNE2 cell lines (). Remarkably, performing Western blot analysis of NPC cells 24 h after heat treatment showed that c-Myc protein levels were substantially downregulated (). Moreover, there were accompanying changes in the cell proliferation markers (PCNA, CDK1, CDK4, p27) similar to the outcomes observed when c-Myc was knocked down. There were corresponding reductions in the level of PCNA, CDK1, CDK4 mRNAs (). Together these proposed that hyperthermia may inhibit NPC cell growth via effects on targeting and downregulating c-Myc.
Figure 3. Hyperthermia can inhibit NPC cells growth and degrade c-Myc. (A) Effect of 43 °C heat treatment on cell proliferation of CNE1 and CNE2 cells was measured using CCK-8 assays. The cells were heat treated every two days. (B) Representative pictures of colony formation assays in CNE1 and CNE2 cells. The cells were heat treated every two days. (C) Colony number counts compared between the Control and Heat treatment groups (HT). (D) Protein levels of c-Myc, PCNA, CDK1, CDK4 and p27 were evaluated by Western blot after 43 °C heat treatment for 30 min and recovery for 24 h at 37 °C. (E) mRNA levels of c-Myc, PCNA, CDK1, and CDK4 evaluated by qRT-PCR as per D. Data shown as mean ± s.d.
Hyperthermia blocks the cell cycle and induces apoptosis
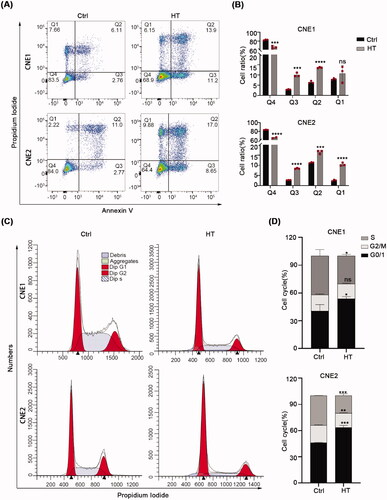
Next, we further explored the mechanisms whereby hyperthermia inhibits cell growth, particularly to distinguish whether hyperthermia only affects the cell cycle or also induces non-programmed or programmed cell death. Toward this, we performed flow cytometric analyses based on the annexin-V/PI assay capable of distinguishing between healthy, apoptotic and necrotic cells. In parallel, we also measured cell cycle phases. After treating CNE1 and CNE2 cells with hyperthermia, we observed the percentage of healthy cells (annexin V-/PI-) was reduced between ∼15 and 20% of the control cells. The differences were due to an increased number of early and late apoptotic cells (annexin V+/PI − and annexin V+/PI + cells, respectively) and to a lesser extent an increased number of necrotic (annexin V−/PI+) cells (). Moreover, after heat treatment, the proportion of cells in the G0/G1 phase increased, while the proportion of cells in S phase decreased (). Together these data suggest that heat treatment primarily inhibits NPC cell growth through a cell cycle block at the G0/G1 and through promoting apoptosis.
Figure 4. Hyperthermia blocks the cell cycle and induces apoptosis. (A,B) Apoptosis levels in CNE1 and CNE2 cells after 43 °C heat treatment for 30 min and recovery for 24h at 37 °C measured by flow cytometry using the Annexin V/PI dual-parameter method. (C) DNA content of CNE1 and CNE2 cells evaluated by flow cytometry using PI staining. Cells were heat-treated at 43 °C for 30 min and allowed to recover for 8 h at 37 °C before analysis. (D) Statistical data shows the different ratio of cell cycle phase in CNE1 and CNE2 cells. The data are displayed as mean ± s.d. **p < 0.01 ****p < 0.0001, compared with NC. NC: negative control; HT: hyperthermia.
Hyperthermia downregulates c-Myc via the proteasomal pathway
Our preceding experiments indicated a single heat treatment produced a sustained reduction in c-Myc protein levels after 24 h. To gain temporal insights into the effects of hyperthermia, we analyzed c-Myc proteins levels in NPC cell lines at shorter time points, immediately preparing samples after 43 °C heat treatment for 15, 30 and 60 min, respectively. Surprisingly, Western blotting analysis revealed there was a strong reduction in c-Myc protein levels after 30 min heat treatment () although this treatment did not affect the levels of c-Myc mRNA (). Instructively, the protein levels of PCNA, CDK1 or CDK4, were not substantially diminished under the same conditions (Supplementary Figure S3), implying that hyperthermia rapidly destabilizes c-Myc protein expression in NPC cells.
Figure 5. Hyperthermia induces c-Myc degradation through the proteasomal pathway. (A) CNE1 and CNE2 cells were subjected to 43 °C heat treatment for 15 min, 30 min or 60 min, and cell lysates immediately collected for Western blot detection of c-Myc levels. (B) CNE1 and CNE2 cells were subjected to 43 °C heat treatment for 30 min and cells immediately collected for qRT-PCR analysis to detect c-Myc mRNA levels. (C) c-Myc protein level recovery after hyperthermia. CNE1 and CNE2 cells were heat treated at 43 °C for 30 min and allowed to recover at 37 °C for the indicated times before Western blotting analysis. (D) c-Myc can be kept at a relatively low levels following daily hyperthermia. CNE1 and CNE2 cells were heat treated at 43 °C for 30 min every day and allowed to recover at 37 °C for the indicated times. (E) Hyperthermia-induced degradation of c-Myc can be rescued by MG132 but not CQ. CNE1 and CNE2 cells were treated with 10μM MG132 for 4h or 10μM CQ for 24h before heat treatment. Cells were collected immediately after heat treatment at 43 °C for 30 minutes. (F) Hyperthermia-induced degradation of exogenous c-Myc can be rescued by MG132. HEK293T cells were transfected with the pCDH-c-Myc vector before treatment with 10μM MG132 for 4h or 10μM CQ for 24h before heat treatment at 43 °C for 30 minutes. Cells were immediately collected for analysis by Western blot. (G) Hyperthermia increases the ubiquitination levels of c-Myc. Cells were collected immediately after heat treatment at 43 °C for 30 minutes. GAPDH served as loading control.
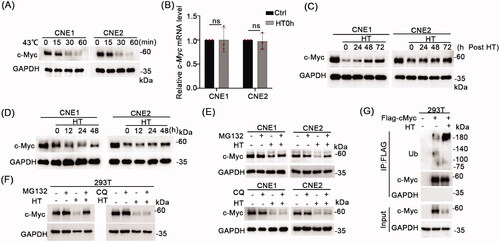
We then extended our experiments to reveal how quickly c-Myc levels would recover following hyperthermia. Postheat treatment analysis by Western blot showed that the reductions in c-Myc of protein levels were gradually reversed when the cells were shifted to 37 °C but did not fully recover to pretreatment levels, even at 72 h (). Moreover, we showed that c-Myc can be kept at a relatively low levels with repeated daily hyperthermia treatments (). Thus, hyperthermia can produce sustainable decreases in c-Myc protein levels.
Next, to clarify the mechanisms responsible for the degradation of c-Myc, we assessed the effects of inhibitors of the proteasomal and lysosomal degradation pathways. NPC cell lines were pretreated with the proteasomal inhibitor (MG132) or lysosome inhibitor (chloroquine phosphate, CQ) prior to 43 °C heat treatment. Notably, we found that the levels of c-Myc were rescued by MG132 treatment but not CQ (), indicated that hyperthermia induced c-Myc degradation via the proteasomal pathway. Consistently, the same effects were observed in HEK293T cells expressing exogenous c-Myc (). To extend this analysis, we determined if hyperthermia could alter the levels of c-Myc ubiquitination that would be expected to increase when proteins are targeted for proteasomal degradation. As expected, hyperthermia decreased the levels of total c-Myc protein in HEK293T cells expressing ectopic c-Myc. Nonetheless, immunoprecipitation experiments in these cells showed the levels of polyubiquitinated c-Myc were substantially enhanced by hyperthermia ().
Therefore, hyperthermia acts to decrease the stability of c-Myc through inducing polyubiquitination and proteasomal degradation.
Hyperthermia suppresses NPC tumor growth in vivo
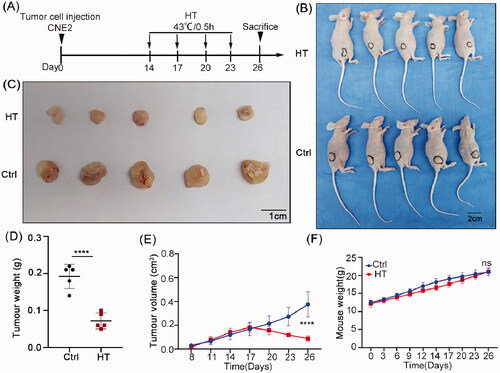
To ensure the relevance of our in vitro data to bona fide hyperthermia treatments, we sought to substantiate our findings using xenografts in nude mice. The NPC cell line CNE2 was injected subcutaneously into the dorsal flanks of nude mice. Tumors became palpable 5–7 days after inoculation and thereafter, mice were randomly assigned to control or hyperthermia treatment groups. Beginning 2 weeks after implantation, the mice were treated with control or hyperthermia treatments every 3 days for a total of four times (). At the conclusion of the experiment, analysis of tumor volumes and weights showed there were significant reductions in tumor growth in the hyperthermia group compared to the control group (). During the experiment, we observed no significant behavioral changes in the hyperthermia treatment group animals and a small lag in body weight gain was recovered by the end of the experiment (). Importantly, the skin area exposed to heat treatment displayed a temporary flush which returned to a normal skin color after about 1 h and the major internal organs of the mice appeared normal at necroscopy. Together, this indicated that the hyperthermia regimen used was well tolerated and safe.
Figure 6. Hyperthermia suppresses NPC xenograft growth in nude mice. (A) Treatment schedule. Mice were injected subcutaneously with CNE2 cells and allowed to establish xenografts for two weeks before subjecting the treatment group mice to immersion in a 43 °C water bath for 30 min on days 14, 17, 20 and 23. (B) Whole body images of the mice on day 26 showing hyperthermia treatment resulted in smaller tumors than the control group. (C) Images of the excised tumors after sacrifice on day 26. (D) Final tumor weight comparisons. (E) Tumor growth size was monitored throughout the experiment. (F) Total body weight monitoring throughout the experiment. Data are presented as mean ± s.d.; n = 5 tumors for each group. ****p < 0.0001.
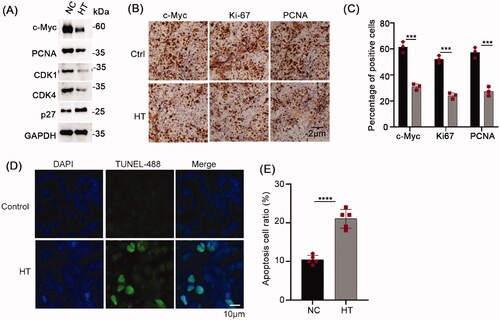
The tumor tissues were subsequently divided to conduct biochemical and histopathological analyses by Western blot and immunohistochemistry, respectively. Consistently, the expression of c-Myc in the hyperthermia group was significantly reduced with accompanying reductions in the protein expression of the proliferation markers PCNA, CDK1, and CDK4 with noticeably increased levels of p27 (). Additionally, the results of the immunochemical staining showed overall reductions in the percentages of NPC cells staining for c-Myc after heat treatment along with the cell proliferation markers Ki-67 and PCNA (). Moreover, using the TUNEL assay which indicates apoptosis, we found significant increases in apoptotic cell numbers in tumors subjected to hyperthermia (). Together these results establish that the reductions in c-Myc and associated inhibition of NPC cell proliferation caused by hyperthermia in vitro could be readily reproduced in vivo.
Figure 7. Hyperthermia inhibits NPC proliferation and promotes apoptosis in vivo. (A) Protein levels of c-Myc, PCNA, CDK1 and CDK4 in the xenografted CNE2 tumors subjected to control or hyperthermia treatments assayed using Western blot. (B) Representative immunohistochemical staining of c-Myc, Ki-67 and PCNA levels in CNE2 tumors. (B) Quantitative assessment of tumor cell positivity of the markers in C. (D) Representative images of TUNEL assays conducted on CNE2 tumor sections. Apoptotic cells are stained green with cell nuclei shown in blue. (E) Quantitative assessment of tumor cell positivity of TUNEL staining from D. Data are presented as mean ± s.d.; ***p < 0.001 and ****p < 0.0001.
Hyperthermia-induced changes in the NPC cell transcriptome
Our biochemical data indicated that inhibit of NPC cell growth by hyperthermia involved transcriptional inhibition of selected proliferative genes. To understand the changes more comprehensively, we performed comparative transcriptomic analysis of CNE2 cells after heat treatment. Using thresholds of fold change ≥2 or ≤0.5 we identified 567 genes that were significantly upregulated in response to hyperthermia with another 486 genes being downregulated (). To help rationalize these data, we then constructed protein–protein interaction (PPI) networks using STRING/CytoHubba analysis (). This analysis produced a PPI network showing the significant relationships between target genes with the size of node reflecting more known interactions. Notable central nodes in this map included c-Myc, JUN and CXCL8. Further ranking analysis of the top 10 genes according to five CytoHubba ranking characteristics showed all three genes predominantly featured in the top three positions (). Alternatively, we used the intersection between known c-Myc target genes and those differentially regulated by hyperthermia to construct a c-Myc target PPI network ().
Figure 8. Hyperthermia-induced changes in the NPC cell transcriptome. (A) Volcano plot based on RNA sequencing of CNE2 cells comparing heat treatment versus control treatment samples. Upregulated DEGs (Log2 Fold Change ≥2, p value < 0.05) are colored red; Downregulated DEGs (Log2 Fold Change ≤−2, p value < 0.05) are colored blue. n = 3 biologically independent samples were analyzed for each condition. (B) PPI network analysis based on RNA sequencing analysis in A. Red nodes represent upregulated genes and blue nodes represent downregulated genes. |log2FC| ≥1.5 and p < 0.05 were set as cutoff criteria. (C) Interaction networks between c-Myc and target DEGs. Red nodes represent upregulated genes and blue nodes represent downregulated genes.
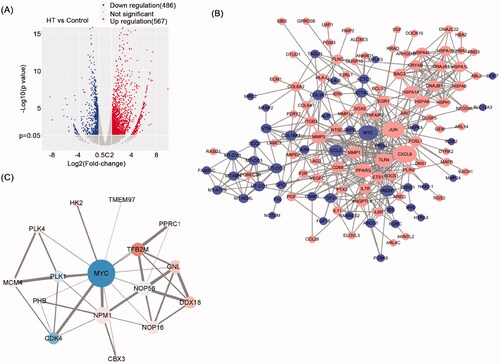
Table 1. Rank method in CytoHubba.
Discussion
Quite remarkably, c-Myc has been implicated in the transcriptional regulation of up to 15% of total cellular genes [Citation32]. Therefore, not surprisingly, c-Myc’s roles extend to an exhaustive list of physiological and pathological processes well beyond cellular growth control [Citation33,Citation34]. Notably, constitutive activation and/or overexpression of c-Myc occurs in over 70% of human cancers and frequent connections have been made to virtually all hallmark processes linked to tumorigenesis [Citation35–39]. Indeed, decades of research have provided little doubt as to the potential of targeting its expression and activity. Indeed, this tenet was reproduced here in our study where we showed that the levels of c-Myc were tightly associated with their tumorigenic potential of NPC cell lines, consistent with prior clinical evidence [Citation28,Citation29]. However, quietening oncogenic signaling through targeting transcription factors such c-Myc has proven challenging.
As different technologies have developed, so have different approaches to inhibit c-Myc. Some have considered c-Myc to be undruggable by means of small molecules but efforts in this space have persisted [Citation40–42]. Otherwise, antisense oligonucleotides [Citation43–45] or other RNAi technologies [Citation46] to inhibit c-Myc expression appear the most prevalent approaches reported. Others have leveraged knowledge that c-Myc transcriptional activity requires heterodimerization with its co-factor Max, and for example, peptides that inhibit this interaction have been pursued [Citation47]. In a similar manner, a dominant negative 90 amino acid miniprotein named omoMyc that alters the dimerization specificity of c-Myc has been developed [Citation48]. Considering the efforts made toward different targeting strategies and delivery systems, it is intriguing that a noninvasive approach using hyperthermia proved sufficient to destabilize c-Myc expression in NPC cells.
One of the biophysical effects of hyperthermia is to cause protein aggregates within cells which in turn acts to promote protein turnover. Indeed, this mechanism was disclosed as the basis for the desensitizing cancer cells to death receptor signaling through TRAIL and Mapatumumab, the latter a monoclonal antibody that simulates the effects of TRAIL by activating death receptor 4 [Citation49–51]. In these studies, hyperthermia increased the ubiquitination and proteasome-mediated destruction of c-FLIPL, relieving its inhibition of death receptor-mediated apoptosis. Likewise, DNA damage responses of cancer cells following hyperthermia were also compromised by rapid downregulation of BRCA2 via protein aggregation and proteasomal clearance [Citation52]. We similarly found that hyperthermia treatment in NPC cells increased the ubiquitination of c-Myc and reduced its total cellular levels. Thus, in a similar manner, it would be envisaged that destabilizing c-Myc by hyperthermia could also be exploited in combinatorial approaches to sensitize cancer cells to a range of treatments. In this regard it has been shown that c-Myc plays an important role in both the chemoresistance and radioresistance of NPC [Citation53,Citation54]. For instance, c-Myc was shown to bind to the CHK1/2 promoter in nasopharyngeal carcinoma to regulate DNA damage checkpoint responses and radiation resistance [Citation55]. Thus, the expression of c-Myc can be viewed as highly relevant to treatment responses in NPC. Nonetheless, hyperthermia can also be considered an effective treatment in its own right.
As essentially a form of heat-induced stress, hyperthermia elicits a range of responses, with a balance maintained between cell death and survival. Overwhelming heat approaching or above 50 °C causes necrotic cell death in cultured cells while more typical temperatures aligned with clinical hyperthermia treatments result in apoptotic cell death [Citation56,Citation57]. Apoptosis induction is considered the endpoint response for unresolved stress associated with effects on different physiological processes. These include but are not limited to unfolded protein responses (UPR) associated with endoplasmic reticulum stress [Citation58,Citation59] along with increases in reactive oxygen species (ROS) causing oxidative stress [Citation60]. For instance, excessive ROS levels which can be increased by hyperthermia [Citation61], can overcome the antioxidant defenses of cells, destabilizing the mitochondrial respiratory chain with resulting loss of mitochondrial membrane potential and the induction of apoptosis [Citation62]. Here, we established the effects of hyperthermia of NPC growth resulted from a combination of apoptosis together with inhibition of the cell cycle, the latter confirmed by changes in the levels of key cell cycle regulatory proteins. The percentage of necrotic NPC cells also increased but appeared to play a minor role in the overall effects of hyperthermia.
On the other hand, one of the known limitations of hyperthermia concerns thermotolerance, the physiological response that cells mount to overcome heat stress to survive [Citation63]. Central to thermotolerance is the upregulation of the heat shock transcription factor 1 (HSF1) which drives the expression of key heat shock proteins including HSP27, HSP70 and HSP90 [Citation64,Citation65]. Heat shock proteins fulfill an essential role in the quality control of protein maturation, promoting the refolding of misfolded proteins and also eliminating damaged proteins through the proteasomal system [Citation66]. Nonetheless, we showed that c-Myc levels could be sustainably reduced in NPC cells with repeated hyperthermia treatments, both in vitro and as xenografts. At face value this suggests that thermotolerance is not problematic from the perspective of reducing c-Myc expression. This conceivably may result from the elevated proteasomal activity associated with cancer cells [Citation67–69], thus pushing the balance toward c-Myc degradation. Moreover, hyperthermia effects are closely linked with temperature and time [Citation70], making it also possible that the treatment regimens used here could minimize the activation of thermotolerance mechanisms. However, these postulates need to be more formally investigated.
A final consideration of our study concerns how to gauge the relative importance of c-Myc to the effects of hyperthermia on NPC cells. However, given that both hyperthermia and c-Myc have broad actions, this is notionally a difficult question. Certainly, c-Myc was shown to be highly thermosensitive in comparison to its target genes PCNA, CDK1 and CDK4, the latter also declining after hyperthermia but in association with decreased mRNA levels. We further addressed this point through transcriptomic analyses, showing there was an approximately equal number of genes with either decreased or increased expression following hyperthermia, respectively. Using an unbiased approach to construct a PPI network promoted c-Myc as a central node of the hyperthermia response in NPC cells, along with JUN and the C-X-C motif ligand 8 (CXCL8). We then compared the gene expression changes to the classical set of c-Myc target genes where only a relative few were featured in the PPI analysis. Of these, the strongest links were between c-Myc expression and downregulation of the Polo-like kinase (PLK1) and upregulation of NPM1. The relationship between c-Myc and PLK1 is intriguing: a positive feedforward loop occurs where c-Myc drives PLK1 transcription while PLK1 itself acts to increase the stability of c-Myc [Citation71,Citation72]. A similar scenario occurs with N-MYC where PLK1-mediates phosphorylation targets the E3 ubiquitin ligase FBW7 which protects N-MYC from degradation [Citation73]. Thus, it is plausible that the hyperthermia-induced reduction in c-Myc protein levels also reflects the consequences of reductions of PLK1 levels. Similarly, c-Myc is known to transactivate NPM1 which encodes a nucleolar phosphoprotein involved in RNA maturation and DNA repair [Citation74]. However, the fact that NPM1 levels are increased in conjunction with reductions in c-Myc may suggest that alternative responses are invoked. From a broader perspective, it is also intriguing to consider the relationship between cancer stem cells and hyperthermia, the former being a minor subset of tumor cells believed to be the main cause of tumor recurrence [Citation75]. Notably, c-Myc is closely related to tumor cell stemness and hyperthermia may inhibit tumor progression by targeting stem cells [Citation55,Citation76]. Therefore, it could also be speculated that our study provides a theoretical basis for hyperthermia inhibition of tumor cell stemness. In any event, these data centrally position c-Myc as an important component of the hyperthermia response of NPC cells and support our view that hyperthermia can be used to rationally target c-Myc, especially to improve the therapeutic efficacy of other treatments.
Lastly, we must consider the limitations of our study. Foremost, in clinical practice, hyperthermia is combined radiotherapy and chemotherapy while our study investigated the effects of hyperthermia alone. Traditional hyperthermia has some practical limitations such as accuracy and temperature uniformity although with the continuous technological developments, hyperthermia monotherapy may still have future potential. Arguably, a better understanding of the mechanism of action of hyperthermia such as conducted here is required to optimize its effects in clinical practice. Secondly, hyperthermia actions occur in the context of the tumor microenvironment where the immune system plays an active role in the outcome of anti-cancer treatments [Citation77]. Our study involved athymic nude mice which have defective adaptive immunity, so this model was unable to properly assess the contribution of a fully intact immune system to hyperthermia responses. Finally, we must consider that the therapeutic effects of hyperthermia are multifaceted and unlikely to be attributed solely to the actions of a single mediator such as c-Myc. However, the transcriptional repertoire of c-Myc is rather exceptional and indisputably important for most cancer cells. In this regard, the effects of hyperthermia on c-Myc cannot be readily ignored and further work delving further into this effect together with establishing its practical significance in clinical practice is warranted.
Supplemental Material
Download TIFF Image (2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5(5):423–428.
- Chang JT, See LC, Liao CT, et al. Locally recurrent nasopharyngeal carcinoma. Radiother Oncol. 2000;54(2):135–142.
- Bensouda Y, Kaikani W, Ahbeddou N, et al. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(2):79–85.
- Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet (London, England). 2016;387(10022):1012–1024.
- Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–668.
- Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and Meta-analysis. Psychother Psychosom. 2012;81(4):206–216.
- Guo Q, Pan J, Zong J, et al. Suggestions for lymph node classification of UICC/AJCC staging system: a retrospective study based on 1197 nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Medicine (Baltimore). 2015;94(20):e808.
- Qi D, Hu Y, Li J, et al. Hyperthermia induces apoptosis of 786-O cells through suppressing Ku80 expression. PloS One. 2015;10(4):e0122977.
- Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–1279.
- van der Zee J, González González D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch deep hyperthermia group. Lancet (London, England). 2000;355(9210):1119–1125.
- Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized High-Risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492.
- Colombo R, Salonia A, Leib Z, et al. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU International. 2011;107(6):912–918.
- Zheng N, Xu A, Lin X, et al. Whole-body hyperthermia combined with chemotherapy and intensity-modulated radiotherapy for treatment of advanced nasopharyngeal carcinoma: a retrospective study with propensity score matching. Int J Hyperthermia. 2021;38(1):1304–1312.
- Kang M, Liu WQ, Qin YT, et al. Long-term efficacy of microwave hyperthermia combined with chemoradiotherapy in treatment of nasopharyngeal carcinoma with cervical lymph node metastases. Asian Pacific Journal of Cancer Prevention: APJCP. 2013;14(12):7395–7400.
- Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753.
- Dunne M, Regenold M, Allen C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv Drug Deliv Rev. 2020;163–164:98–124.
- Oei AL, Vriend LE, Crezee J, et al. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 2015;10:165.
- Stephen ZR, Zhang M. Recent progress in the synergistic combination of Nanoparticle-Mediated hyperthermia and immunotherapy for treatment of cancer. Adv Healthc Mater. 2021;10(2):e2001415.
- Moy AJ, Tunnell JW. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv Drug Deliv Rev. 2017;114:175–183.
- Oei AL, van Leeuwen CM, ten Cate R, et al. Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res. 2015;75(23):5120–5129.
- Zhao YY, Wu Q, Wu ZB, et al. Microwave hyperthermia promotes caspase‑3-dependent apoptosis and induces G2/M checkpoint arrest via the ATM pathway in non‑small cell lung cancer cells. Int J Oncol. 2018;53(2):539–550.
- Maimaitiyiming Y, Wang QQ, Yang C, et al. Hyperthermia selectively destabilizes oncogenic fusion proteins. Blood Cancer Discov. 2021;2(4):388–401.
- Man J, Shoemake JD, Ma T, Rizzo AE, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769.
- Luo M, Meng Z, Moroishi T, et al. Heat stress activates Yap/TAZ to induce the heat shock transcriptome. Nat Cell Biol. 2020;22(12):1447–1459.
- Luo J, Xiao J, Tao Z, et al. Detection of c-myc gene expression in nasopharyngeal carcinoma by nonradioactive in situ hybridization and immunohistochemistry. Chin Med J (Engl). 1997;110(3):229–232.
- Fan CS, Wong N, Leung SF, To KF, et al. Frequent c-myc and int-2 overrepresentations in nasopharyngeal carcinoma. Hum Pathol. 2000;31(2):169–178.
- Zheng J, Li W, Huang R. [Studies on c-myc gene expression and p16 gene inactivation in nasopharyngeal carcinoma]. Zhonghua er bi Yan Hou ke za Zhi. 2000;35(6):464–468.
- Guo Z, Wang Y, Zhao Y, et al. A functional 5'-UTR polymorphism of MYC contributes to nasopharyngeal carcinoma susceptibility and chemoradiotherapy induced toxicities. J Cancer. 2019;10(1):147–155.
- Niu Z, Liu H, Zhou M, et al. Knockdown of c-Myc inhibits cell proliferation by negatively regulating the cdk/Rb/E2F pathway in nasopharyngeal carcinoma cells. Acta Biochim Biophys Sin (Shanghai). 2015;47(3):183–191.
- Yang W, Shen J, Wu M, et al. Repression of transcription of the p27(Kip1) cyclin-dependent kinase inhibitor gene by c-Myc. Oncogene. 2001;20(14):1688–1702.
- Bretones G, Delgado MD, León J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849(5):506–516.
- Gearhart J, Pashos EE, Prasad MK. Pluripotency redux-advances in stem-cell research. N Engl J Med. 2007;357(15):1469–1472.
- Carroll PA, Freie BW, Mathsyaraja H, et al. The MYC transcription factor network: balancing metabolism, proliferation and oncogenesis. Front Med. 2018;12(4):412–425.
- Dhanasekaran R, Deutzmann A, Mahauad-Fernandez WD, Hansen AS, et al. The MYC oncogene - the grand orchestrator of cancer growth and immune evasion. Nat Rev Clin Oncol. 2022;19(1):23–36.
- Cotterman R, Jin VX, Krig SR, et al. N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor. Cancer Res. 2008;68(23):9654–9662.
- Deb-Basu D, Karlsson A, Li Q, et al. MYC can enforce cell cycle transit from G1 to S and G2 to S, but not mitotic cellular division, independent of p27-mediated inihibition of cyclin E/CDK2. Cell Cycle. 2006;5(12):1348–1355.
- Kim J, Woo AJ, Chu J, Snow JW, et al. A myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143(2):313–324.
- Dejure FR, Eilers M. MYC and tumor metabolism: chicken and egg. Embo J. 2017;36(23):3409–3420.
- Casey SC, Baylot V, Felsher DW. MYC: master regulator of immune privilege. Trends Immunol. 2017;38(4):298–305.
- Han H, Jain AD, Truica MI, et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36(5):483–497.e415.
- Huang MJ, Cheng YC, Liu CR, et al. A small-molecule c-Myc inhibitor, 10058-F4, induces cell-cycle arrest, apoptosis, and myeloid differentiation of human acute myeloid leukemia. Exp Hematol. 2006;34(11):1480–1489.
- Madden SK, de Araujo AD, Gerhardt M, et al. Taking the myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol Cancer. 2021;20(1):3.
- Skorski T, Perrotti D, Nieborowska-Skorska M, et al. Antileukemia effect of c-myc N3'->P5' phosphoramidate antisense oligonucleotides in vivo. Proc Natl Acad Sci USA. 1997;94(8):3966–3971.
- Ricker JL, Mata JE, Iversen PL, et al. Gattone VH: c-myc antisense oligonucleotide treatment ameliorates murine ARPKD. Kidney Int. 2002;61(1 Suppl):S125–S131.
- Dhanasekaran R, Park J, Yevtodiyenko A, et al. MYC ASO impedes tumorigenesis and elicits oncogene addiction in autochthonous transgenic mouse models of HCC and RCC. Mol Ther Nucleic Acids. 2020;21:850–859.
- Habib S, Ariatti M, Singh M. Anti-c-myc RNAi-Based onconanotherapeutics. Biomedicines. 2020;8(12):612.
- Bidwell GL, 3rd, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135(1):2–10.
- Massó-Vallés D, Soucek L. Blocking myc to treat cancer: Reflecting on two decades of omomyc. Cells. 2020;9(4):883.
- Song X, Kim SY, Zhou Z, et al. Hyperthermia enhances mapatumumab-induced apoptotic death through ubiquitin-mediated degradation of cellular FLIP(long) in human Colon cancer cells. Cell Death Dis. 2013;4(4):e577.
- Morlé A, Garrido C, Micheau O. Hyperthermia restores apoptosis induced by death receptors through aggregation-induced c-FLIP cytosolic depletion. Cell Death Dis. 2015;6(2):e1633.
- Song X, Kim HC, Kim SY, et al. Hyperthermia-enhanced TRAIL- and mapatumumab-induced apoptotic death is mediated through mitochondria in human Colon cancer cells. J Cell Biochem. 2012;113(5):1547–1558.
- van den Tempel N, Laffeber C, Odijk H, et al. The effect of thermal dose on hyperthermia-mediated inhibition of DNA repair through homologous recombination. Oncotarget. 2017;8(27):44593–44604.
- Zhong Q, Liu ZH, Lin ZR, et al. The RARS-MAD1L1 fusion gene induces cancer stem cell-like properties and therapeutic resistance in nasopharyngeal carcinoma. Clin Cancer Res. 2018;24(3):659–673.
- Deng X, Liu Z, Liu X, et al. miR-296-3p negatively regulated by nicotine stimulates cytoplasmic translocation of c-Myc via MK2 to suppress chemotherapy resistance. Mol Ther. 2018;26(4):1066–1081.
- Wang WJ, Wu SP, Liu JB, et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73(3):1219–1231.
- Song AS, Najjar AM, Diller KR. Thermally induced apoptosis, necrosis, and heat shock protein expression in 3D culture. J Biomech Eng. 2014;136(7).
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Wroblewski D, Jiang CC, Croft A, Farrelly ML, et al. OBATOCLAX and ABT-737 induce ER stress responses in human melanoma cells that limit induction of apoptosis. PLoS One. 2013;8(12):e84073.
- Oakes SA. Endoplasmic reticulum stress signaling in cancer cells. Am J Pathol. 2020;190(5):934–946.
- Tian J, Mo J, Xu L, et al. Scoulerine promotes cell viability reduction and apoptosis by activating ROS-dependent endoplasmic reticulum stress in colorectal cancer cells. Chem Biol Interact. 2020;327:109184.
- Ba MC, Long H, Wang S, et al. Hyperthermia enhances radiosensitivity of colorectal cancer cells through ROS inducing autophagic cell death. J Cell Biochem. 2018;119(4):3763–3774.
- Slimen IB, Najar T, Ghram A, et al. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30(7):513–523.
- Urano M. Kinetics of thermotolerance in normal and tumor tissues: a review. Cancer Res. 1986;46(2):474–482.
- Tabuchi Y, Kondo T. Targeting heat shock transcription factor 1 for novel hyperthermia therapy (review). Int J Mol Med. 2013;32(1):3–8.
- Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia. 1995;11(4):459–488.
- Kim HJ, Joo HJ, Kim YH, et al. Systemic analysis of heat shock response induced by heat shock and a proteasome inhibitor MG132. PLoS One. 2011;6(6):e20252.
- Arlt A, Bauer I, Schafmayer C, et al. Increased proteasome subunit protein expression and proteasome activity in Colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2). Oncogene. 2009;28(45):3983–3996.
- Soave CL, Guerin T, Liu J, et al. Targeting the ubiquitin-proteasome system for cancer treatment: discovering novel inhibitors from nature and drug repurposing. Cancer Metastasis Rev. 2017;36(4):717–736.
- Hui KF, Chiang AK. Combination of proteasome and class I HDAC inhibitors induces apoptosis of NPC cells through an HDAC6-independent ER stress-induced mechanism. Int J Cancer. 2014;135(12):2950–2961.
- Genet SC, Fujii Y, Maeda J, et al. Hyperthermia inhibits homologous recombination repair and sensitizes cells to ionizing radiation in a time- and temperature-dependent manner. J Cell Physiol. 2013;228(7):1473–1481.
- Ren Y, Bi C, Zhao X, et al. PLK1 stabilizes a MYC-dependent kinase network in aggressive B cell lymphomas. J Clin Invest. 2018;128(12):5517–5530.
- Mo H, He J, Yuan Z, et al. PLK1 contributes to autophagy by regulating MYC stabilization in osteosarcoma cells. Onco Targets Ther. 2019;12:7527–7536.
- Xiao D, Yue M, Su H, et al. Polo-like kinase-1 regulates myc stabilization and activates a feedforward circuit promoting tumor cell survival. Mol Cell. 2016;64(3):493–506.
- Zeller KI, Haggerty TJ, Barrett JF, et al. Characterization of nucleophosmin (B23) as a myc target by scanning chromatin immunoprecipitation. J Biol Chem. 2001;276(51):48285–48291.
- Krause M, Dubrovska A, Linge A, et al. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63–73.
- Oei AL, Vriend LEM, Krawczyk PM, et al. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;33(4):419–427.
- Li Z, Deng J, Sun J, et al. Hyperthermia targeting the tumor microenvironment facilitates immune checkpoint inhibitors. Front Immunol. 2020;11:595207.
