Abstract
This retrospective study used data from patients treated for uterine fibroids with ultrasound-guided high-intensity focused ultrasound (USgHIFU) from April 2015 to April 2019. One hundred and seven patients with solitary fibroids were divided into two groups: (1) the L group with larger fibroids (≥10 cm) and (2) the S group with smaller fibroids (<10 cm). Using magnetic resonance imaging (MRI), we examined the efficacy of high-intensity focused ultrasound (HIFU) ablation by comparing uterine and fibroid volumes before and three months after the procedure. The three-month follow-up clinical visit used a visual analog scale and a uterine fibroid symptom health-related quality of life questionnaire to evaluate clinical symptoms. Both the L and S groups had significant reduction in uterine and fibroid volumes, but the rate was significantly higher in the S group (p < 0.05). Both groups also had improvements in clinical symptoms, but there was no statistical difference. USgHIFU reduced the size of both large and small fibroids but was most effective on fibroids smaller than 10 cm. Both the L and S groups had improved dysmenorrhea symptoms and quality of life.
1. Introduction
Benign uterine fibroid tumors are common, with an incidence of 20%–40% among women of reproductive age [Citation1]. In 10%–20% of women, uterine fibroids affect the quality of life with adverse symptoms such as heavy and prolonged menstrual bleeding, severe pain, urinary frequency and constipation [Citation2]. Surgical interventions such as myomectomy or hysterectomy, performed conventionally or laparoscopically, are the most common treatments for uterine fibroids. However, surgery on large fibroids (>10 cm) can cause significant blood loss, leading surgeons to choose conventional surgery for these myomas [Citation3]. High-intensity focused ultrasound (HIFU) has been shown to be a successful non-surgical treatment for fibroids in many studies over the last 15 years. The correlation between fibroid volume and ablation efficiency has been documented by previous studies and they pointed out that HIFU ablation efficiency was easier to achieve in uterine fibroids with a larger size [Citation4,Citation5]. However, the largest fibroid in Fan’s studies was 10.4 cm and few previous studies have focused on using this technique on larger fibroids (>10 cm). There were no existing data for the efficacy of HIFU on large fibroids, especially those larger than 10 cm. In this study, we investigated whether USgHIFU can be safely and effectively used for large uterine fibroids.
2. Objective
This study explored USgHIFU ablation as an effective treatment for solitary fibroids greater or less than 10 cm.
3. Methods
3.1. Patients
We retrospectively collected data from patients who had received USgHIFU treatment for uterine fibroids at the Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, from April 2015 to April 2019. This study was approved by the ethics committee of our hospital (approval IRB number: KMUHIRB-E(II)-20200382). The requirement for informed consent was waived, because the study was retrospective and patient confidentiality was maintained.
All patients underwent pelvic contrast-enhanced magnetic resonance imaging (MRI) before and three months after treatment. We counted the number of fibroids each patient had and identified the fibroids’ volume, location and T2 signal intensity. We initially looked at 397 patients but excluded those with multiple fibroids or adenomyosis. The remaining 107 patients were divided into two groups based on the measurement of their fibroids in the longitudinal, anteroposterior or transverse diameter: (1) L group, fibroids with a diameter ≥10 cm (31 patients) and (2) S group, fibroids with a diameter <10 cm (76 patients). The baseline characteristics of the patients showed no significant differences in age or body mass index (BMI).
3.2. Usghifu ablation
Treatment was performed using the Haifu Model JC Focused Ultrasound Tumor Therapeutic System (Haifu Medical Technology Co., Chongqing, China). Patients were carefully placed in a prone position with the abdominal wall immersed in degassed water. Conscious sedation and analgesia with fentanyl were administered during the procedure. To shrink blood vessels surrounding the fibroids, an infusion of 80 U oxytocin was administered before surgery. The ultrasound ablated the fibroid plane by plane, focusing on a single ‘ablation spot’ at a time. The procedure was terminated when a gray-scale change indicated that the treated lesion had been ablated. The hyperechoic change (hyperechoic region) is a sign that the treated lesion has been ablated, and it indicates a quick response to HIFU [Citation6]. The treatment status, treatment effect and complications were recorded.
3.3. Data collection
The uterine and fibroid volumes were calculated by an ellipsoid formula: 0.5233 × longitudinal diameter × anteroposterior diameter × transverse diameter. Volume changes of the uterus and fibroid lesions were calculated with MRI images before and three months after treatment. The percentage reduction in fibroid volume was calculated as percent reduction = ([V0 − V1]/V0) × 100%. V0 = fibroid volume at baseline, and V1 = fibroid volume at three months after treatment.
Based on the MRI images, the combined T2-weighted imaging (T2WI) of the lesions was compared to normal myometrial signal intensity. The lesions were labeled as hypointense when the signal intensity was lower than that of the myometrium, isointense when the signal was comparable to the myometrium and hyperintense when the signal was higher than the myometrium.
For the evaluation of HIFU ablation, the non-perfusion volume (NPV) was assessed according to T1-enhanced MRI images three months after treatment and calculated by the ellipsoid formula. Non-perfusion volume rate (NPVR) was defined as the NPV divided by the uterine fibroid volume: NPVR = NPV/V1 × 100%. The NPVR is related to symptom relief and can represent the HIFU ablation rate [Citation7]. The energy efficiency factor (EEF) was defined as the acoustic energy delivered for ablating 1 mm3 of the fibroid: EEF (J/mm3) = ηtotal energy delivery/NPV, where η was the HIFU transducer focus factor (which was 0.7 in this study). The EEF reflects the energy deposition efficiency of the HIFU. NPV, EEF and the time of the first hyperechoic region occurrence reflect the ablative efficiency of HIFU treatment in our study.
Symptom improvement was evaluated during clinical visits. Dysmenorrhea was evaluated by the visual analog scale (VAS) [Citation8]. The uterine fibroid symptom and quality of life questionnaire (UFS-QOL) [Citation9] was used before and three months after HIFU treatment. The UFS-QOL consists of an eight-item symptom severity scale and 29 health-related quality of life (HRQL) items comprising six domains: concern, activities, energy/mood, control, self-consciousness and sexual function. All items are scored on a five-point Likert scale, ranging from ‘not at all’ to ‘a very great deal’. Symptom severity and HRQL subscale scores are summed and evaluated on a 100-point scale. A higher UFS-QOL indicates more adverse symptoms and less favorable HRQL.
3.4. Statistical analysis
The data in normal distribution were reported as means ± SD. The IBMM SPSS statistic 20, paired sample t-test or chi-square test was used for comparison between the two groups. Differences were considered statistically significant at p < 0.05.
4. Results
4.1. Baseline characteristics
Patients had similar ages and BMI. Thirty-one patients had fibroids greater than 10 cm (L group), and 76 patients had fibroids less than 10 cm (S group). A large percentage (93.6%) of patients in the L group had an anteverted uterus, which could be caused by large and heavy fibroids. According to the FIGO staging system established by the International Federation of Gynecology and Obstetrics, 74.2% of the fibroids in the L group were of the intramural type (FIGO 3 and FIGO 4 on a scale of 0–8); the fibroids in the S group were more evenly distributed in the uterus. There was no statistical difference between the two groups in the location of the fibroids on the anterior, posterior, fundal or lateral surfaces of the uterus.
4.2. Effect of HIFU treatment
Before HIFU treatment, the mean diameter of the fibroid lesions was 13.0 ± 2.4 cm (range: 10 cm–17.8 cm) in the L group and 6.7 ± 1.8 cm (range: 3.1 cm–9.9 cm) in the S group. The mean volume of the fibroid lesions was 728.4 ± 342.5 cm3 in the L group and 139.6 ± 96.7 cm3 in the S group, and the mean uterine size was 1003.8 ± 417.6 cm3 and 324.0 ± 162.6 cm3, respectively. Fibroids greater than 10 cm had a higher rate of hyperintense signal intensity on T2WI (48.4% vs. 26.3%) and a lower rate of hypointense signal intensity (38.7% vs. 55.3%), but this did not reach statistical significance (P = 0.087) ().
Table 1. Baseline characteristics of solitary fibroid patients.
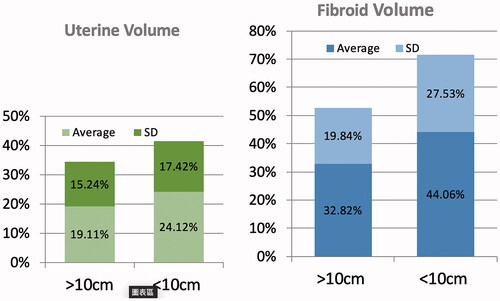
Comparing pretreatment and three-month data showed the reduction rate of fibroid volume to be 32.8% ± 19.8% in the L group and 44.1% ± 27.5% in the S group, which were both statistically significant. The reduction rate in the uterine volume was 19.1% ± 15.2% in the L group and 24.1% ± 17.4% in the S group; both reduction rates were also significant (). The S group had significantly more shrinkage in fibroid lesion volumes (p < 0.05) ().
Table 2. Changes in the size of uterus and solitary fibroid lesion.
The L group had a significantly longer total treatment time (the time from the first to last ablation spot), longer sonication time (ultrasound pulse times), larger total energy delivery and larger ablation volume (). There was no difference between the L and S groups in the percentage of hyperechoic region occurrence during treatment (86.5% vs. 82.89%). However, there was a significantly shorter time between ablation of the first spot and the occurrence of the hyperechoic region in the larger fibroids in group L. The NPV ratio three months after treatment was 53.46% ± 33.18% in the L group and 51.32% ± 33.69% in the S group, and there was no statistical difference. The EEF was significantly lower in the L group (4.6 ± 9.4 J/mm3) vs. the S group (14.0 ± 19.6 J/mm3), indicating that large fibroids were easier to ablate (). If we further analyze the ablation efficiency according to T2 signal intensity in both groups, there is a significant higher percentage of NPVR in L group with T2 signal hypointensity comparing to hyperintensity. ().
Table 3. Treatment status of solitary fibroid patients.
Table 4. Treatment results of solitary fibroid patients: hyperechoic region, NPVR, EEF.
Table 5. Treatment results of solitary fibroids ≥ 10 cm with different MRI T2 intensity.
Table 6. Treatment results of solitary fibroids <10 cm with different MRI T2 intensity.
Both groups had a large improvement in clinical symptoms. At three months after HIFU treatment, the VAS score had decreased by 61.4% ± 32.5% in the L group and 63.2% ± 42.2% in the S group. The UFS-QOL score decreased by 28.6% ± 19.5% (from 75.4 ± 23.3 to 53.4 ± 21.9) in the L group and by 35.6% ± 39.5% (from 72.8 ± 30.1 to 51.7 ± 23.5) in the S group ().
Table 7. Symptomatic improvement: baseline and 3 months after treatment.
Table 8. Symptomatic improvement: reduction rate of VAS and UFS-QOL.
5. Discussion
Many factors may affect the efficiency of HIFU ablation in the treatment of uterine fibroids. The correlation between fibroid volume and ablation efficiency has been documented by Cheng et al., who studied uterine fibroids in 43 patients treated with USgHIFU from April 2012 to June 2014 and classified the fibroid size into four categories: <3 cm, 3–5 cm, 5–7 cm and >7 cm. The study reported that fibroids with a size of 3–5 cm were most effectively ablated [Citation10]. Fan et al. studied 346 patients with uterine fibroids treated with USgHIFU and found that the fibroid volume was negatively correlated with EEF, so large fibroids are more easily ablated [Citation5]. Gong et al. studied the factors that influenced HIFU ablative efficiency and stated that gray-scale change during treatment indicates a quick response to HIFU. Based on these studies, gray-scale changes occurred in many lesions [Citation7]. However, there were no existing data for the efficacy of HIFU on large fibroids, especially those larger than 10 cm, and the correlation between ablation efficiency and the reduction rate had not been proven.
In our study, two factors affected the ablative efficiency of HIFU treatment: EEF and the time of hyperechoic region occurrence. The EEF was significantly lower in the L group, and the time between ablation of the first spot and the occurrence of the hyperechoic region was also shorter. Based on both the EEF and hyperechoic region occurrence, large fibroids had a quicker response to HIFU and were easier to ablate [Citation7]. This phenomenon may be explained by the ‘damage–damage interference effect’: the expansion of the necrotic area and the ascent of temperature on the focal point will dynamically influence the acoustic environment of the surrounding tissue and contribute to the ultrasonic energy deposition [Citation11].
All fibroids showed significant volume reduction three months after treatment, indicating that HIFU treatment was effective even in large myomas. However, volume reduction was higher in the S group, and we have four possible reasons for this occurrence.
First, after fibroid tissue is ablated, it is absorbed by the surrounding normal tissue and blood supply; a greater distance between the necrosis center and surrounding normal tissue means a slower absorption rate.
Second, the myometrium around smaller fibroids is thicker and will more easily absorb fibroids. Larger fibroids usually distend and thin the myometrium more severely, which may make ablated myoma absorption slower.
Third, the correlation between HIFU outcome and T2WI intensity has been well documented. In Fan’s study, the energy deposition efficiency of fibroids with hypointense T2WI was higher than those with isointense and hyperintense T2WI [Citation4,Citation5]. Similarly, in Cheng’s study, uterine fibroids were optimally ablated with low T2WI signal intensity [Citation7]. Undegenerated uterine fibroids usually have low T2WI signal intensities, but large fibroids are prone to degeneration and experience an increase in free-water content, which elevates the signal intensity and negatively affects ablation. Although not reaching statistical difference, it was shown in our study that L group patients had a higher percentage of hyperintense T2WI signals (48.4% vs. 26.3%). We further analyzed ablation efficiency according to the T2 signal intensity in both groups, and there was a significant higher percentage of NPVR in L group with hypointense T2WI; however, there was no difference in the S group. These data emphasized that the degeneration and increase in free-water content in large fibroids deteriorate the effect of ablation.
Fourth, it is more difficult to focus energy on a fibroid larger than 10 cm, which may increase the need for a higher energy dosage and prolonged sonication time. A longer ablation time may lead to patient complications including skin burns, thermal injury on the sciatic nerve, or damage to the intestines. The treatment may be terminated before complete ablation because of the patient’s discomfort. Large fibroids may be attached to the sacrum and surrounded by nerve tissue.
Both groups had a large improvement in clinical symptoms. There were equally significant decreases in VAS and UFS-QOL in both groups. It is interesting to note that even though fibroids larger than 10 cm had a lower reduction rate, larger original and post-treatment fibroid size compared to those smaller than 10 cm, the quality of life improved similarly for both groups. This may be explained by the reason that L group patients had fibroids >10 cm is that fibroids did not cause symptoms until they grew big enough, therefore an average reduction rate of 32.82% can significantly reduce bothersome symptoms even the remaining fibroids were sill bulky.
6. Limitations
Our study had limitations. First, due to the difficulty of accessing MRI equipment in our busy hospital, this imaging was arranged three months after treatment rather than one day after HIFU procedure. Therefore, in the immediate post-treatment time, the non-perfusion ratio could not be calculated. Instead, we used the NPV ratio three months after treatment and the time of the first hyperechoic region occurrence to represent the ablative efficiency in our study. Compared to immediate post-treatment NPV, the NPV three months later was due to reperfusion and partially absorbed ablated fibroid tissue. Second, this was a short-term outcome comparison, and long-term follow-up was not completed. Third, the treatments were performed by different doctors. The speed by which the ‘ablation spots’ could be performed might be different according to the operator’s experience, which may affect energy accumulation. Each operator’s subjective experience would influence when treatment was terminated because of gray-scale changes or the patient’s tolerance.
7. Conclusions
USgHIFU was an effective technique to reduce the size of both large and small fibroids but was more effective on fibroids less than 10 cm. Fibroid size may be a parameter to predict outcome in patients. Even though fibroids larger than 10 cm had a smaller volume reduction rate, clinical improvements from dysmenorrhea and quality of life showed equal improvement in patients with both large and small fibroids.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet Gynecol. 2013;121(3):654–673.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372(17):1646–1655.
- Tanos V, et al. Prevention and management of complications in laparoscopic myomectomy. Biomed Res Int. 2018;2018:8250952.
- Fan H-J, Cun J-P, Zhao W, et al. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35(1):534–540.
- Fan H-J, Zhang C, Lei H-T, et al. Ultrasound-guided high-intensity focused ultrasound in the treatment of uterine fibroids. Medicine. 2019;98(10):e14566.
- Fukuda H, Numata K, Nozaki A, et al. Hyperecho in ultrasound images during high-intensity focused ultrasound ablation for hepatocellular carcinomas. Eur J Radiol. 2011;80(3):e571–e575.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32(5):496–503.
- Burckhardt CS, Jones KD. Adult measures of pain: the McGill pain questionnaire (MPQ), rheumatoid arthritis pain scale (RAPS), Short-Form McGill pain questionnaire (SF-MPQ), verbal descriptive scale (VDS), visual analog scale (VAS), and west Haven-Yale multidisciplinary pain inventory (WHYMPI). Arthritis Care Res. 2003;49(S5):S96–S104.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Cheng H, Wang C, Tian J. Correlation between uterine fibroids with various magnetic resonance imaging features and therapeutic effects of high-intensity focused ultrasound ablation. Pak J Med Sci. 1969;31(4):869–873.
- Chen L, ter Haar G, Hill CR. Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol. 1997;23(6):921–931.