Abstract
Objective
To evaluate the feasibility, efficiency, and safety of microwave ablation (MWA) for T1N0M0 papillary thyroid carcinoma (PTC) with capsular invasion (CI).
Methods
The data of 69 T1N0M0 PTC with CI underwent MWA from August 2015 to January 2020 were retrospectively analyzed. All PTC nodules were pathologically confirmed by fine needle aspiration (FNA). The extended ablation was performed in all cases, that is, the ablation zone completely covered the tumor and extended the tumor edge by at least 2 mm. The strategy of fluid isolation was successfully applied before and during ablation. The strategy of multiple point ablation was applied. After ablation, the changes in tumor size at different time points, local recurrence, new lesions, lymph node metastasis (LNM), and complications were evaluated and recorded. The technical feasibility, technical success rate, and safety were analyzed.
Results
Based on the contrast-enhanced ultrasound results, complete ablation has been achieved in all enrolled cases after ablation. The mean maximum tumor diameter and the mean volume of PTC nodules before ablation were 0.84 ± 0.39 cm (range, 0.3–2 cm) and 0.26 ± 0.35 ml (range, 0.01–1.72 ml) respectively. The mean follow-up time was 26 ± 10 months (range, 9–48 months). Nodules in 47 cases (68.1%) completely disappeared in the follow-up period. No local recurrence was detected. The incidence of new lesions and LNM was 4.3% (3/69) and 4.3% (3/69) respectively. Further ablations have been successfully employed for all of the new lesions and LMN. Light voice changes (2.9%, 2/69) were the only major complication, which was relieved within 6 months after MWA. The sizes of the ablation zone increased firstly within 6 months after MWA compared with the pretreatment tumor size (p < 0.05). Twelve months later, the sizes were smaller than those before MWA (p < 0.05 for all).
Conclusions
MWA is an effective, safe, and feasible method in treating T1N0M0 PTC with CI.
Background
The incidence of papillary thyroid carcinoma (PTC) has increased significantly in recent years, while disease-related mortality rates have not obviously changed [Citation1,Citation2]. Surgical resection is the classic treatment for PTC, while the specific method and scope of resection are distinct for different stages of PTC. PTC lesions extending to the thyroid capsule, perithyroidal soft tissue, or sternothyroid muscle are classified as a minimal extrathyroidal extension (mETE). The rate of extrathyroidal extension (ETE) in PTC is reported to be 22.7%, of which capsular invasion (CI) accounts for 4.7% [Citation3]. CI is defined as a PTC nodule penetrating into the thyroid capsule without attaching to surrounding tissue [Citation4]. The thyroid capsule is histologically defined as the connective tissue layer that is close to and envelops the thyroid gland. The thyroid capsule penetrates through the gland parenchyma, which includes blood vessels, lymphatics, and nerves, but does not include muscle components. The structure of the capsule provides an anatomical basis for the relatively higher incidence of lymph node metastasis (LNM) in PTC with CI. Previous studies have shown that CI is an independent risk factor for LNM in PTC patients [Citation5,Citation6].
However, CI in PTC is not the key factor that triggers lymph node metastasis. Based on the eighth edition of the American Joint Committee on Cancer (AJCC), mETE has excluded from tumor-node-metastasis (TNM) pathologic staging for pT3 disease in well-differentiated thyroid cancers [Citation7,Citation8]. This exclusion could prevent overtreatment with more aggressive therapies and reduce related complications. Therefore, it remains controversial how to properly treat PTC with CI.
Ultrasound (US)-guided microwave ablation (MWA) has been reported to be a reliable minimally invasive technique for effectively and safely treating PTC [Citation9–13]. Promising results have been achieved with MWA for T1aN0M0, T1bN0M0 and T2N0M0 PTC [Citation14–18]. To date, no study has been conducted on the specific application of MWA for PTC with CI. The purpose of this study was to evaluate the feasibility, efficacy and safety of ultrasound-guided MWA for the treatment of PTC with CI.
Materials and methods
Patients
This retrospective study was approved by the institutional review board of China-Japan Friendship Hospital. Written informed consent was obtained from each patient before the ablation procedure. All patients consented to publish their examination results and radiological images anonymously.
The inclusion criteria of the present study were as follows: (1) patients with T1N0M0 PTC confirmed by fine-needle aspiration (FNA); (2) unifocal PTC with CI on US examination; (3) no LNM or distant metastasis on imaging examination; and (4) follow-up of >9 months after MWA. The definition of CI is that the PTC nodule penetrates into the thyroid capsule without attaching to the surrounding tissue, and the signs of CI in the US mainly refer to the discontinuity liner hyperecho of thyroid capsule closing to the PTC tumor.
Pretreatment assessments
All patients underwent laboratory examinations, neck and chest computed tomography (CT), US and FNA before ablation. Laboratory examinations mainly included blood routine examination, coagulation, and thyroid function tests. Neck and chest CT scans were conducted to detect LNM or distant metastasis. The ultrasound scanner GE LOGIQ E9 (GE Healthcare, USA) was used for US examination and guidance. Cytological pathology analysis and BRAF V600E mutation test were both conducted. Three orthogonal diameters of all PTC nodules, location and invaded capsule were recorded in US image. The tumor volume was calculated with the following formula: V = πabc/6 (V as volume, a as the largest diameter, and b and c as the other two perpendicular diameters) [Citation14].
According to the adjacent structure, the thyroid capsule was divided into anterior, medial, lateral, and posterior capsules on the transverse ultrasonographic views. The anterior capsule refers to the capsule close to the anterior cervical muscle, the medial capsule close to the trachea, the lateral capsule close to the carotid sheath, and the posterior capsule close to the retropharyngeal space. The exact position of all PTC nodules and their relationships to the thyroid capsule were reviewed by three doctors retrospectively on saved US and CT images.
MWA procedure
MWA was performed by radiologists with more than 3 years of experience in MWA treatment for thyroid nodules. Patients were placed in the supine position and the neck was extended. Intravenous access was obtained via a median cubital vein. The US contrast agent SonoVue (sulfur hexafluoride microbubbles; Bracco, Milan, Italy) was applied with a recommended 0.015 ml/kg dose in the present study. After a bolus intravenous injection of SonoVue via a median cubital vein, a flush of 10 ml normal saline was followed [Citation14]. After routine sterilization and spread towels, local anesthesia was applied with one percent lidocaine before puncture. An 18-gauge needle for hydrodissection was inserted under US guidance, and the needle tip was placed between the thyroid capsule corresponding to the PTC nodule and surrounding critical structure. Then the normal saline was injected to keep a distance for hydrodissection at least 5 mm to avoid thermal damage. After the isolation band expanded to at least 5 mm, an internally cooled MWA antenna (17 G) with a 0.3 cm tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China; or KY-2000, Kangyou Medical, Nanjing, China) was inserted into the PTC nodule under US guidance. The multi-point ablation strategy was employed. That is, in one point ablation, a fixed applicator ablation was performed with the power of 30 W and radiation time of 15–20 s, then the antenna tip was moved to the next point guided by ultrasound and performed the next ablation with the same radiation time. The following ablation was performed until the overlap ablation zone covered the whole nodule. The normal saline was continuously injected during ablation as isolating fluid to keep the thickness of the isolation band to prevent thermal damage. When the hyperechoic ablation zone covered the whole PTC, the ablation was terminated. After MWA, contrast-enhanced ultrasound (CEUS) was conducted to confirm a complete ablation, that is, the ablation zone completely covered the tumor and extended the tumor edge by at least 2 mm (). If there was nodular enhancement inside the ablation zone or the margin of the extended ablation zone was less than 2 mm, additional ablation should be performed immediately. If the patient complained of intolerable pain during MWA, the procedure would be temporarily suspended and further local anesthesia was added. The procedure would be continued after the pain was significantly relieved. Because of pre-operative education, most patients could keep lying still and cooperate well with the procedure without sedation. Sedation was only performed in a few anxious patients during the MWA procedure. Before and after ablation, vocal cord function was assessed by US and laryngoscope. During the procedure, the assessment of vocal cord function was only conducted by the US in case of recurrent laryngeal nerve (RLN) heat injury-related symptoms encountered, which include abnormal pronunciation or uncomfortable breathing [Citation15]. Patients remained under observation for 2 h for potential complications.
Postablation assessment and follow-up
The patients were followed up every 3 months within one year after MWA, and then every 6 months. Clinical evaluations, ultrasound and laboratory tests were performed at each follow-up. Three orthogonal diameters of the ablation zone were evaluated by ultrasound, and the tumor volumes and volume reduction ratio (VRR) were also calculated. The VRR = (initial volume – final volume)/initial volume [Citation14]. Due to extended ablation, the sizes of the ablation zone are generally larger than those of the original tumor at the first several follow-ups [Citation19]. Patients received thorax and chest CT every year to screen for metastasis. CEUS and FNA would be performed in patients with suspected local recurrence, new tumor or LNM.
Technical feasibility was defined as successfully targeting the PTC nodule and performing ablation according to the preoperative plan. Technical success was defined as achieving complete ablation confirmed by CEUS at the end of every procedure [Citation19].
Statistical methods
Statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY, USA). Data were presented as mean ± standard deviation (SD), and the median and 25–75% interquartile range (IQR) were used if data did not fit a normal distribution. The changes in tumor size after MWA were compared by the Wilcoxon signed-rank test. The Chi-Square test, independent t-test and Wilcoxon signed-rank test were used to assess the heterogeneity of demographic characteristics between groups with or without tumor progression. Statistical tests were two-sided, and P values less than 0.05 were considered to indicate statistical significance.
Results
Demographic and clinical characteristics
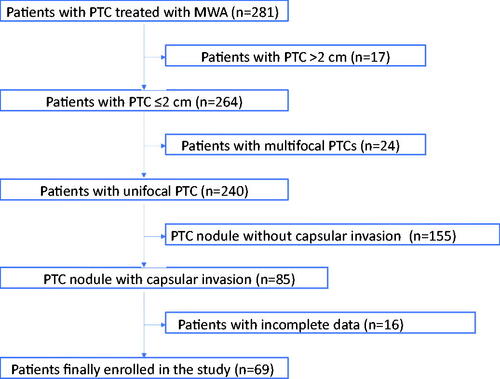
From August 2015 to January 2020, a total of 69 PTC patients with CI treated with US-guided MWA were enrolled in the present study (), which included eighteen men and fifty-one women. The mean age is 45.55 ± 11.82 years (range, 26–74 years). The demographic characteristics of enrolled patients are summarized in . All enrolled 69 cases were ineligible for or refused surgery. Among them, fifty-eight patients refused surgery due to cosmetic reasons and worried about surgery-related complications, while eleven patients were ineligible for surgery because of high risk of general anesthesia: poor pulmonary function with chronic obstructive pulmonary disease (n = 8), coronary heart disease with unstable angina (n = 3). The mean maximum tumor diameter of PTC was 0.84 ± 0.39 cm (range, 0.3–2 cm), and the mean volume of target lesion was 0.26 ± 0.35 ml (range, 0.01–1.72 ml) (, ). 15 nodules invaded the anterior thyroid capsule, 10 nodules invaded the lateral thyroid capsule, 7 nodules invaded the medial thyroid capsule, 12 nodules invaded the posterior thyroid capsule, 12 nodules invaded the anterior and medial thyroid capsule, 6 nodules invaded the anterior and lateral thyroid capsule, 5 nodules invaded the lateral and posterior thyroid capsule, 2 nodules invaded the anterior, lateral and posterior thyroid capsule. 54 nodules belonged to the T1a subgroup, and 15 nodules belonged to the T1b subgroup. 59 (85.5%) cases were tested positive for the BRAF V600E mutation, 5 (7.2%) cases were negative, and 5 (7.2%) cases did not undergo the test. The mean follow-up time after MWA was 26 ± 10 months (range, 9–48 months), and 69, 65, 58, 44, 29, 22, 9, 2 patients were followed up for more than 9, 12, 18, 24, 30, 36, 42 and 48 months, respectively. The mean volume of isolation fluid was 54 ± 17 ml (range, 48–75 ml).
Figure 1. (A) 42-year-old woman with papillary thyroid cancer with capsular invasion (CI) was treated with microwave ablation (MWA). (A) pre-MWA, ultrasound(US) showed a hypoechoic target tumor (arrows) with CI (triangles); (B) before MWA, contrast-enhanced US (CEUS) showed a hypo-enhancement pattern in the artery phase (arrows); (C) the hydrodissection technique (triangles) was used to protect the surrounding structures (arrows); (D) US showed a hyperechoic pattern in the tumor (arrows) during ablation; (E) post-MWA, CEUS showed no enhancement (arrows) in the tumor area; and (F) on one-month post-MWA, US showed a hypoechoic ablation zone (arrows).
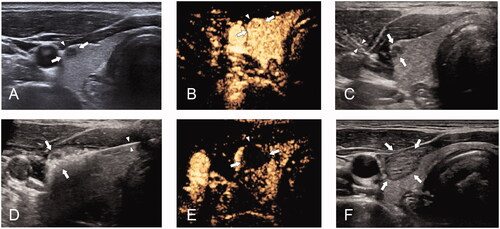
Table 1. Demographic characteristics of PTC with CI included in this study (n = 69).
Table 2. Tumor size (maximum diameter and volume) before MWA and at each follow-up time-point after MWA.
Technical feasibility and technical success
All of the PTC nodules have been radically ablated according to the protocol in one session. The mean ablation time was 125 ± 71 s (51–382 s). According to the CEUS results after ablation, complete ablation was achieved in all cases. So, both the technical feasibility and technical success rate were 100%.
Changes in tumor size after ablation
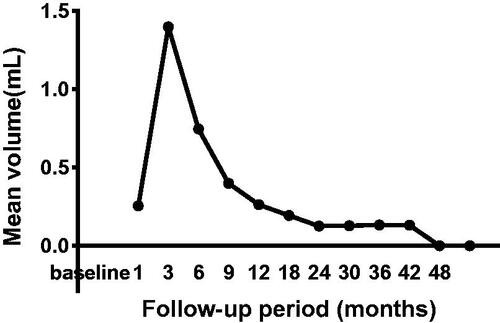
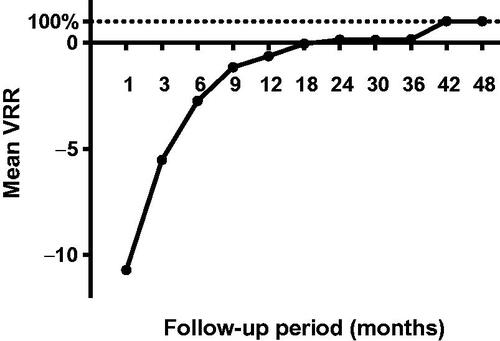
At the end of follow-up, nodules in 47 cases (68.1%) completely disappeared, in which 2 nodules disappeared at the 6th month after ablation, 13 at the 9th month, 20 at the 12th month, 5 at the 18th month, 3 at the 24th month, 4 at the 30th month. There were significant differences regarding the maximum diameters and mean tumor volume between the tumor pre ablation and the ablation zone in each follow-up within 30 months (p < 0.05) (, ). Due to expanding ablation, the sizes of the ablation zone were larger than those of the original tumor at the 1-, 3- and 6-month follow-ups (p < 0.01 for all). However, the sizes of the ablation zone after 12 months were smaller than those of the original tumor before MWA (p < 0.05 for all). The mean tumor volume decreased from 0.26 ± 0.35 ml (range, 0.01–1.72 ml) before ablation to 0.13 ± 0.47 (range, 0.01–2.12 ml) at the 36 months follow-up (, ). There were significant differences in the VRR between every 2 follow-up periods before 36 months (p < 0.05) ().
Disease progression after ablation
During the follow-up period, there was no local recurrence encountered. Both new lesion and lymph node metastasis were detected in one patient (1/69, 1.4%), while new lesions were detected in two patients (2/69, 2.9%), and lymph node metastasis occurred in two patients (2/69, 2.9%). All five patients with tumor progression had T1a PTC nodules, and the original nodules invaded the anterior capsule. Two new PTC nodules were detected in the contralateral thyroid lobe 24 months after ablation. The third new PTC nodule in the ipsilateral thyroid lobe occurred in one patient 36 months after ablation. Metastatic lymph nodes were detected ipsilaterally in zone III in one patient 12 months after ablation, and contralaterally in zone III and VI in two patients 24 months after ablation. Further ablations were employed to successfully inactivate these new PTC nodules and metastatic lymph nodes. After the second ablation, no tumor progression occurred in the follow-up period. According to the result of univariate analysis, there was no parameter screening out as risk factor to tumor progression, which includes sex, age, tumor stage, tumor size, BRAF mutation, the position of invaded thyroid capsule and ablation time ().
Table 3. Demographic characteristics of cases with or without tumor progression in the present study.
Complications
Slight voice changes were encountered in two cases as the only major complication (2/69,2.9%). Among them, one patient has a PTC nodule invaded posterior capsule, the other invaded the anterior capsule and medial capsule. The symptoms were relieved at 4-month and 6-month without any specific therapy. No serious hematomas or skin burns occurred.
Discussion
Considerable controversy exists focusing on the determination of the optimal clinical treatment for low-risk small PTC with minimal ETE. Total thyroidectomy is the traditional treatment option for PTC with ETE [Citation20]. A recent study showed that hemithyroidectomy can also be recommended for T1aN0M0 PTC with minimal ETE and considered for T1bN0M0 PTC with minimal ETE [Citation21]. Therefore, local treatment may be effective for PTC with minimal ETE. Recently, the application of thermal ablation has achieved promising results in PTC treatment [Citation22]. According to the 2021 European guidelines, thermal ablation could be considered in patients with low-risk PTMC and radioiodine-refractory metastases not amenable to surgery [Citation23,Citation24]. However, according to the 2019 edition of the “Expert Consensus Workshop Report: Guidelines for Thermal Ablation of Thyroid Tumours,” PTC nodules with CI are excluded from this therapeutic approach [Citation25].
In the present study, radical ablation was applied to all patients according to the protocol and no local recurrence was detected during the follow-up period. Both the technical feasibility and the success rate were 100%. These results indicate that MWA could achieve a promising outcome in PTC with CI. During a mean follow-up period of 26 ± 10 months (range, 9–48 months), 47 ablation zone (68.1%) completely disappeared. The present disappearance rate is comparable to the reported disappearance rate of 69% (466/673) observed in the T1a group in a previous study [Citation16]. No local recurrence was observed during follow-up in the present study, which is similar to the results of previous studies in which the local recurrence rate varied from below 0.5% to 0.85% after MWA [Citation26].
In our experience, the isolation technique used during ablation is a key factor to ensure safely and effectively expanding ablation for PTC nodules with CI. In the present study, the rate of new lesions was 4.3%, which is comparable with 4.2% in the previous study after ablation, but higher than 1% occurred in remnant thyroid after limited thyroidectomy [Citation26,Citation27]. However, the relatively lower rate of new lesions that occurred in remnant thyroid may be attributed to the partial removal of thyroid tissue. The rate of LNM was 4.3%, which was higher than 0.6–2.0% in a previous study on thermal ablation and 1.6% after initial surgery [Citation26,Citation27]. The CI maybe account for the relatively higher incidence of LNM after ablation. However, both the new lesions and the metastatic lymph nodes have been successfully ablated by MWA, and there was no further tumor progression observed during the final follow-up assessment. No distant metastasis was observed during follow-up in the present study, which is comparable to the rate of 0.2% after initial surgery [Citation27]. Therefore, the present results indicate that MWA is a potentially feasible and effective modality for T1N0M0 PTC nodules with CI, especially when surgery is not feasible or is refused by the patient.
In the present study, light voice changes occurred in two patients as an exclusive complication (2.9%, 2/69), which is lower than the reported overall complication rate of 7.1% after surgery and 6.0% post-MWA [Citation22,Citation28]. Furthermore, patients recovered without developing permanent hoarseness. No patients experienced hypothyroidism in the present study, which indicates that MWA has no influence on thyroid function, and the result is consistent with previously reported results [Citation14,Citation17,Citation22,Citation24]. Therefore, the results of the present study indicate that MWA is a safe treatment option for T1N0M0 PTC with CI.
In the present study, the lack of local recurrence and relatively low complication rate post-ablation may be attributed to a few innovations in the strategy. Generally, when the PTC nodule invades the capsule, it is difficult to perform extended ablation and simultaneously ensure safety. In the present study, the invaded thyroid capsule was successfully separated from the surrounding critical structures through the continuous injection of normal saline. The invaded anterior capsule was separated from the anterior cervical muscle, the medial capsule from the trachea, the lateral capsule from the carotid sheath, and the posterior capsule from the retropharyngeal space. This strategy could ensure complete ablation and simultaneously protect surrounding structures from heat injury. Especially when the medial capsule is invaded, with continuous injection of normal saline and successful separation of the tumor from the trachea and RLN, the surrounding critical structures are pushed away from the ablation zone during the whole ablation process, which can effectively prevent heat damage.
This is a retrospective study, which may have resulted in selection bias. Additionally, the number of patients enrolled in the present study was limited. Therefore, further studies involving more patients with long-term follow-up could definitely clarify the efficacy of MWA therapy for PTC with CI.
Conclusions
In conclusion, this preliminary study demonstrated that MWA could be a feasible, safe, and effective treatment option for T1N0M0 PTCs with CI for patients who refuse or are not eligible for surgery.
Ethics approval and consent to participate
Our retrospective study was approved by the institutional review board of our hospital. Written informed consent was obtained from each patient before the ablation procedure.
Consent for publication
The patients consented to publish their examination results and radiological images anonymously. Written informed consent was waived.
Author contributions
WJ, ZZL, WY and YMA have reviewed the ultrasound images and did the main measurement. WY, PLL, LY and LNC have made substantial contributions to study design and revised the manuscript critically; WJ drafted the article critically and contributed substantially to data collection and data analysis. All authors have provided final approval of the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Mendelsohn AH, Elashoff DA, Abemayor E, et al. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136(11):1055–1061.
- Bortz MD, Kuchta K, Winchester DJ, et al. Extrathyroidal extension predicts negative clinical outcomes in papillary thyroid cancer. Surgery. 2021;169(1):2–6.
- Kim JW, Roh JL, Gong G, et al. Extent of extrathyroidal extension as a significant predictor of nodal metastasis and extranodal extension in patients with pillary thyroid carcinoma. Ann Surg Oncol. 2017;24(2):460–468.
- Kuo EJ, Thi WJ, Zheng F, et al. Individualizing surgery in papillary thyroid carcinoma based on a detailed sonographic assessment of extrathyroidal extension. Thyroid. 2017;27(12):1544–1549.
- Youngwirth LM, Adam MA, Scheri RP, et al. Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid. 2017;27(5):626–631.
- Tuttle RW, Haugen B, Perrier ND. Updated American Joint Committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017;27(6):751–756.
- American Joint Committee on Cancer, et al. Thyroid. In: Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, (eds). AJCC cancer staging manual. 6th edn. New York: Springer. 2002. p 77–87.
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol. 2014;81(Suppl 1):1–122.
- Cui T, Jin C, Jiao D, et al. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: a systematic review and meta-analysis. Eur J Radiol. 2019;118:58–64.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-A pilot study. Radiology. 2017;282(2):576–584. Feb
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab. 2020;1;105(6):dgaa128.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64(1):109–117.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581.
- Cao XJ, Zhao ZL, Wei Y, et al. Microwave ablation for papillary thyroid cancer located in the thyroid isthmus: a preliminary study. Int J Hyperthermia. 2021;38(1):114–119.
- Cao XJ, Wang SR, Che Y, et al. Efficacy and safety of thermal ablation for treatment of solitary T1N0M0 papillary thyroid carcinoma: a multicenter retrospective study. Radiology. 2021;300(1):209–216.
- Cao XJ, Yu MA, Zhu YL, et al. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: a multicenter retrospective study. Int J Hyperthermia. 2021;38(1):916–922.
- Xiao J, Zhang Y, Zhang M, et al. Ultrasonography-guided radiofrequency ablation for the treatment of T2N0M0 papillary thyroid carcinoma: a preliminary study. Int J Hyperthermia. 2021;38(1):402–408.
- Wu J, Zhao ZL, Cao XJ, et al. A feasibility study of microwave ablation for papillary thyroid cancer close to the thyroid capsule. Int J Hyperthermia. 2021;38(1):1217–1224.
- Mitchell AL, Gandhi A, Scott-Coombes D, et al. Management of thyroid cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S150–S160.
- Ji YB, Song CM, Kim D, et al. Efficacy of hemithyroidectomy in papillary thyroid carcinoma with minimal extrathyroidal extension. Eur Arch Otorhinolaryngol. 2019;276(12):3435–3442.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Mauri G, Hegedus L, Cazzato RL, et al. Minimally invasive treatment procedures have come of age for thyroid malignancy: the 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Cardiovasc Intervent Radiol. 2021;44(9):1481–1484.
- Mauri G, Hegedus L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197.
- Xu D, Ge M, Yang A, et al. Expert consensus workshop report: guidelines for thermal ablation of thyroid tumors (2019 edition). J Cancer Res Ther. 2020;16(5):960–966.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Ito Y, Masuoka H, Fukushima M, et al. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg. 2010;34(6):1285–1290.
- Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–276.