Abstract
Objective
To explore a new high-intensity focused ultrasound (HIFU) sonication strategy for cesarean scar pregnancy (CSP) and to compare the clinical effectiveness and safety of this new HIFU sonication strategy with the conventional HIFU sonication strategy followed by ultrasound-guided dilation and curettage (USg-D&C) for CSP.
Materials and methods
91 patients with CSP treated by HIFU and USg-D&C in People’s Hospital of Deyang City between January 2017 and December 2019 were retrospectively reviewed in this study. Based on the HIFU sonication strategy, patients were divided to two groups: 44 patients were exposed to ‘C-shape’ sonication layer by layer around the implantation location of the pregnancy sac (control group), while the other 47 patients were exposed to ‘I-shape’ sonication layer by layer only on the deep part which close to the bladder of the implantation location of the pregnancy sac (experimental group). The differences in clinical efficacy between the two groups were analyzed. Baseline characteristics, technical parameters of HIFU treatment and USg-D&C data were recorded. Adverse events were also recorded.
Results
No statistically significant difference was observed between the two groups in baseline characteristics including age, body mass index (BMI), menopause time, largest diameter of gestational sac, pretreatment serum β-hCG, thickness of gestational sac, embedding myometrium, previous cesarean sections and interval from last cesarean section (CS). The average treatment intensity in the experimental group was significantly lower than that in the control group (p < .05). The median sonication time, total energy used for HIFU ablation, and energy efficiency factor (EEF) in the experimental group were significantly lower than the control group (p < .05). No statistically significant difference was observed between the two groups in treatment power and treatment time (p > .05). Sciatic/buttock pain and postoperative lower abdominal pain in the control group were significantly stronger than that in the experimental group (p < .05). There were no statistically significant differences in post-HIFU vaginal bleeding and discharging, urinary tract irritation, the operation time of USg-D&C, the amount of vaginal bleeding during USg-D&C, and the time for serum β-hCG back to a normal level between the two groups (p > .05).
Conclusions
The ‘I-shape’ strategy of HIFU treatment for CSP was effective and safe, with shorter sonication time, less energy input and lower incidence of sonication-related pain occurred in postoperative lower abdominal and sciatic nerve/buttock.
Introduction
Cesarean scar pregnancy (CSP) is a rare ectopic pregnancy that refers to the implantation of a gestational sac within the scar of a previous cesarean delivery [Citation1]. Because the uterine scar lacks muscular tissue and is rich in fibrous tissue, the elasticity of myometrium in scar area is poor. CSP may cause life-threatening complications such as uterine rupture and massive hemorrhage. From 1980s, the cesarean section (CS) rate in China has remained high, which reached 54.9% in 2014. With the termination of ‘one-child’ policy in 2016, many women who had a history of cesarean section planned to be pregnant again, which made the incidence of CSP increased [Citation2]. The incidence of CSP reported several years ago accounted for 1.15% in women with CS history and 6.1% of ectopic pregnancy [Citation3,Citation4].
Currently, there is no standardized or unified treatment guideline for CSP. Drug therapy, dilation and curettage (D&C), uterine artery embolization (UAE), laparoscopic excision, hysteroscopic resection, and hysterectomy have been used in the management of CSP. High intensity focused ultrasound (HIFU) ablation, as a new therapeutic technique, is currently being applied to treat CSP in clinical practice. The principle of HIFU treatment of CSP is to damage and block the nourishing blood vessels of the gestational sac in the scar area, dissolve the adhesion between the gestational sac and the implanted scar, especially in the deep site of the scar which was the base of pregnancy sac implantation and usually with the most abundant blood supply, and at last achieve the purpose of reducing the amount of blood loss during ultrasound-guided D&C (USg-D&C). Zhu et al. conducted a comparative study on the efficacy and safety between HIFU and UAE in the treatment of CSP [Citation5–8], showing that HIFU combined with suction curettage has obvious advantages over UAE followed by suction curettage. However, since HIFU was a relatively new therapy for CSP, which has been applied in clinical practice for no more than two decades, there was no completely unified and standardized sonication strategy for the treatment process of HIFU. At present, a ‘C-shape’ spot-by-spot path is commonly used in HIFU treatment for CSP, in which the focus was moved in a pattern shaped like a ‘C’ around the embedding area of the gestational sac closed to the bladder wall from deep to superficial, and in some hospitals it would be combined with sonication on the embryo. Several subsequent reports suggested that the sonication time and the total energy of treatment were the main factors related to various complications [Citation9,Citation10], and excessive energy input may lead to serious adverse events, which indicated that it was necessary and meaningful to control energy input. In this paper, we explored a new sonication strategy with the purpose of optimizing the HIFU treatment for CSP, by reducing the sonication dot arrangement. We also tried to compare the effectiveness and safety of this new strategy with the conventional sonication strategy for CSP.
Materials and methods
This research was approved by the ethics committee at People’s Hospital of Deyang City (the approved protocol number: 2021-04-038-K01). The requirement for informed consent was waived.
Patients
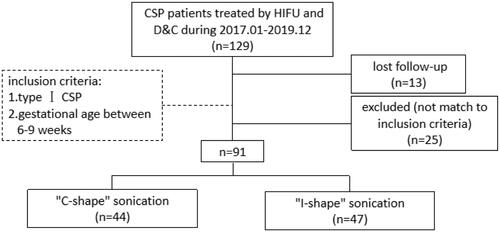
As shown in , among the 129 patients with CSP treated by HIFU combined with USg-D&C in People’s Hospital of Deyang City between January 2017 and December 2019, 13 patients were lost to follow-up and 25 patients were excluded for not matching to the inclusion criteria. A retrospective analysis was performed on data collected from the rest 91 patients. Based on the different sonication strategy of HIFU treatment, patients were divided into two groups: 44 patients in C-shape group (control group) and 47 patients in I-shape group (experimental group). Detailed sonication strategy was illustrated in the part of ‘Ultrasound-guided HIFU ablation’.
Figure 1. Flow chart of different HIFU sonication strategies of CSP patients treated with USgHIFU and USg-D&C.
The inclusion criteria consisted of: (1) a history of amenorrhea, and increased serum β-hCG level; (2) diagnosis of CSP confirmed by transvaginal ultrasound or pelvic magnetic diagnostic imaging (MRI); (3) the bowel could be push away from the acoustic pathway; (4) type I CSP (endogenic type), with progression to the cervicoisthmic space or uterine cavity [Citation11]; (5) gestational age was between 6 and 9 weeks; (6) The previous cesarean section was performed by transverse incision, with no history of myomectomy.
Exclusion criteria included:(1) patients with a history of connective tissue diseases (CTDs), abdominal liposuction or lower abdominal radiotherapy history; (2) trophoblastic disease or cervical pregnancy; (3) bowel in the acoustic pathway or pregnant sac was too small to display by trans-abdominal ultrasound; (4) type II CSP (exogenic type), with deep invasion of scar defect with progression toward the bladder and abdominal cavity [Citation11]; (5) gestational age < 6 weeks or > 9 weeks.
Pre-HIFU treatment preparation
The patients were requested to have a three-day bowel preparation, including a low-fiber diet, bland diet of semi-liquid and liquid food, for bowel safety. Polyethylene glycol (PEG) was taken orally the night before treatment day, and fasting followed by 1–3 times of enema performed on the morning of treatment day. Besides, the skin from the umbilicus to the upper margin of the pubic symphysis was required to be prepared, including shaving the hair, degreasing with 75% ethanol and degassing with degassed water. A catheter was inserted into the bladder to control its volume.
Ultrasound-guided HIFU ablation
HIFU ablation was performed by JC200 focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology Co. Ltd, Chongqing, China), and a B mode probe (MyLab 70, Esaote, Genova, Italy) which provided real-time sonographic monitoring.
Patients were positioned prone on the HIFU table of the system with the abdominal wall immersed in degassed water. To enable patients have a better experience during the treatment, the treatment was conducted under conscious sedation with fentanyl (1.0 μg/kg), followed by midazolam hydrochloride (20.0 μg/kg) every 5 min after fentanyl injection. The conscious sedation medicines were given every 20–40 min to keep a score of 4 or less on the Visual Analogue Scale/Score (VAS) for pain. The sagittal ultrasound scanning mode was chosen for both pretreatment planning and sonication. The focus was adjusted around embedding area of the gestational sac and the bowel was pushed away from the acoustic pathway by a water balloon under ultrasonic monitoring.
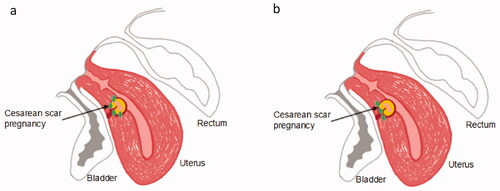
Point-by-point scan mode was used, and power was set between 300 and 400 W. In the control group, the focus was put around the base of the gestational sac embedding area (not the part of sac intruding into the uterine cavity) and treated from deep to superficial, from the head side to the foot side and then back to the head side, which were just like the ‘C’ glyph, as shown in [Citation12]. In the experimental group, the focus was only put in a line in the base of the embedding area of the gestational sac from deep to superficial, which were like the ‘I’ glyph, as shown in [Citation12].
Figure 2. Sonication points distribution. (a) Sonication points (green dots) around the embedding area of the gestational sac were just like the ‘C’ glyph; (b) Sonication points (green dots) in a line in the base of the embedding area of the gestational sac were like the ‘I’ glyph.
Vital signs of patients were closely monitored during the procedure, and adverse events were handled in time. Criteria for terminating HIFU treatment included: (1) great scale change or significantly general scale change was observed through real-time ultrasound monitoring; (2) Color Doppler showed that the blood flow grading of the embedding area was reduced to grade 0 or grade 1 (Adler grading). Contrast-enhancing ultrasound with micro-bubble agent (Sonovue, Bracco, Milan, Italy) was performed pre- and post-HIFU to evaluate the blood supply changes in the embedding area.
HIFU treatment parameters, including the average treatment power, sonication time (the total time of ultrasound sonication), treatment time, the intensity of treatment sonication energy and energy-efficiency factor (EEF = sonication energy/the treated volume) were recorded.
Ultrasound-guided dilatation and curettage (USg-D&C)
The patients underwent USg-D&C 24–72 h after HIFU ablation. The procedure was performed under general anesthesia. Patients were placed in the lithotomy position and transabdominal ultrasound imaging (BELSON 760 K, Belson Imaging Technology Co. Ltd, Wuxi, China) was used to determine uterine morphology and gestational sac location, and also to guide the procedure. The depth of the uterus was measured before suction curettage. A 7.0 mm suction cannula was placed with the suction orifice facing the gestational sac carefully under the guidance of ultrasound. The ablated trophoblasts and decidual tissues were detached by the cannula with a vacuum pressure of 400 Pa and the residue was gently scraped. Then, ten units of oxytocin was injected into the cervix at the 12 o’clock position. If there was still active bleeding, Foley catheter balloon injected with water would be used for hemostasis by compression. The amount of bleeding was measured by the suction cup connected with the negative pressure aspirator during the operation.
Adverse events observation and follow up
Complications during and after HIFU treatment were closely observed and recorded, including lower abdominal pain, sciatic/buttock pain, vaginal secretion, hematuresis, skin injury and nerve injury. Serum β-hCG was monitored in the outpatient department of obstetrics every week until it returned to normal level.
Statistical analysis
SPSS software (SPSS 25.0, IBM Company, Chicago, IL) was used for statistical analysis. A one-sample Kolmogorov-Smirnov test was used to obtain statistics of the probability distribution for quantitative data. All normally distributed data are reported as mean ± SD. Non-normally distributed data were reported as median and inter-quartile range (P25, P75). The t-test was employed for quantitative data that conformed to normal distribution, while the the Mann-Whitney U test was employed for non-normally distributed data. For categorical data, a constituent ratio was reported, and the Chi square test was used for inter-group analysis. A p < .05 indicated a significant difference.
Results
Baseline characteristics of CSP patients
As shown in , the median age of CSP patients in the control group and the experimental group were 31.50 years and 33.00 years respectively. The median BMI was 21.33 kg/m2 in control group and 22.19 kg/m2 in experimental group. The median duration of amenorrhea in both groups was 48 days. The median serum β-hCG before treatment was 41,885.55 mIU/mL in the control group and 27,913.20 mIU/mL in the experimental group. The median thickness of gestational sac embedding myometrium was 4.0 mm and the median number of previous cesarean sections was 1 in both groups. The median interval from the last cesarean section was 5.50 years in the control group and 5.00 years in the experimental group. There was no statistically significant difference in baseline characteristics between the two groups (p > .05).
Table 1. Comparison of baseline characteristics of CSP patients treated with HIFU in different strategies.
HIFU ablation results and safety evaluation
All patients received only one session of HIFU treatment. As shown in , the median treatment power was 400 W in both groups. The median sonication time in the experimental group was 265.00 s, which was significantly lower than that in the control group (380.00 s, p < .05). The median treatment time of the experimental group was 42.00 min, and the difference was not statistically significant compared with that of the control group (45.50 min, p > .05). The average treatment intensity in the control group was 467.98 ± 103.54 s/h, while that in the experimental group was 370.55 ± 97.34 s/h, which was significantly lower than that in the control group (p < .05). Median total energy input in the two groups were 152.00 kJ and 106.00 kJ respectively. In addition, the median EEF in the two groups were 53.67 J/mm3 and 25.38 J/mm3 respectively, with a lower number in the experimental group than in the control group (p < .05).
Table 2. Comparison of HIFU treatment results for CSP patients treated with HIFU in different strategies.
Complications and adverse events
All the adverse effects occurred were classified as SIR grade A (), no complications scaled at SIR grade B or above were observed in the two groups [Citation13]. The adverse effects observed most frequently were sciatic/buttock pain and lower abdominal pain that often disappeared one day after treatment. The incidence of sciatic/buttock pain in two groups was 22.72% (10/44) and 6.38% (3/47) respectively, with a lower number in the experimental group than in the control group (χ2=4.777, p = .029). However, the incidence of lower abdominal pain was 43.18% (19/44) in the control group, which was significantly higher than that in the experimental group of 14.89% (7/47). The difference was statistically significant (χ2 = 8.911, p = .003). A small amount of vaginal bleeding or discharging was observed in both groups, which did not require special treatment. The incidence of hematuresis was 6.82% (3/44) in the control group and 2.13% (1/47) in the experimental group, which indwelling catheterization was required until the urine was clear. There was no severe HIFU-related complication reported, such as bowel injury, skin burn or nerve injury.
Table 3. Comparison of adverse events during HIFU treatment between the patients treated with different strategies.
Suction curettage results
Patients in both groups received ultrasound-guided suction curettage 24–72 h after HIFU treatment. All the patients successfully finished the procedure, and there was no significant difference in the operation time between the two groups (p > .05, ). And the amount of blood loss during the procedure was 10–100 mL. The median blood loss was 15.0 (10.0, 20.0) (range 10–100) mL in the control group, 20.0 (10.0, 20.0) (range 10–100) mL in the experimental group, with no statistical significance (p > .05, ).
Table 4. Comparison of USg-D&C treatment results for CSP patients treated with HIFU in different strategies.
Follow up of β-hCG and reintervention
There was no statistical difference between the two groups in serum β-hCG 1-day post-curettage (p > .05, ), which was 17,230.05 (9619.00, 25,850.60) mIU/mL in the control group and 12,818.00 (7070.20, 18,701.80) mIU/mL in the experimental group. The median time for serum β-hCG returning to normal level in the two groups was 27.0 days and 23.0 days respectively. There was also no statistical difference observed between the two groups (p > .05, ).
There was no significant difference in the need of retreatment after HIFU and suction curettage (p > .05, ) between the two groups (5 cases in the control group and no cases in the experimental group). Among the 5 patients in the control group, three women were found for unsatisfied decrease of β-hCG during the follow up, and then treated with methotrexate (MTX) + calcium folinate (MTX 50 mg on day 1, 3, 5 and 7, and calcium folinate 5 mg on day 2, 4, 6 and 8), achieving satisfactory results. The other two patients in the control group had a large amount of vaginal bleeding (100 mL and 200 mL, respectively) about 2 h after USg-D&C. The oxytocin was used to keep uterus contraction and reduce the vaginal bleeding.
Discussion
HIFU has become a new option in the treatment of CSP, as the pretreatment of the ultrasound-guided dilatation and curettage. For CSP ablation, the purpose of using HIFU before USg-D&C was to block or reduce the blood supply of the pregnancy sac in the embedding area in the CS scar of uterus, while not to get the non-perfused volume (NPV) of the entire embedding area. The effectiveness of HIFU has been widely recognized, with the advantages of noninvasiveness, no bleeding, no radiation, quick recovery et al. However, little attention has been paid to the risks that HIFU energy makes in treatment. To find the balance between maximize the therapeutic effect and minimize the risk, clinical practitioners should pay attention to the energy input during the HIFU treatment.
At the beginning of the twenty-first century, Wang et al. [Citation14] defined the biological focal region (BFR), which was the individual ellipsoid-shaped coagulative necrosis produced by the US energy deposition of a single dot. The size of BFR is affected by acoustic intensity, exposure time, sonication depth, the tissue structure and its functional status, as well as energy. Adequate energy is needed to achieve a satisfactory ablation rate. Wang and his colleagues also proposed the concept of energy efficiency factor (EEF), which refers to the energy that required to ablate tissue per unit volume [Citation14]. Previously, the calculation of EEF was reported to be based on the total sonication energy and NPV [Citation15]. Since there was no need to get the NPV of the entire embedding area for HIFU ablation of CSP, NPV was not applicable in CSP ablation. Instead, in this study, the treated volume automatically recorded by the JC200 system software was employed to get the EEF. Zhang et al. [Citation16] found the relationship between the size of the gestational sac and the energy used for ablation. They believed that the larger gestational sac was, the higher power would be required, the longer treatment time and sonication time would be demanded, and the more energy would be needed. Therefore, sufficient energy is the guarantee of therapeutic effect of HIFU to treat CSP. In this study, we used ‘C-shape’ sonication strategy in the early stage, but we found that during the USg-D&C, the scratched pregnancy sac tissue had showed obvious characteristics of coagulant necrosis, and some of the debris had showed black carbonization. This clinical finding made us consider that maybe the sonication time was too long, and the energy input was too high. Thus, the sonication mode was changed into ‘I-shape’, which only put focus in a line in the base of the embedding area of the gestational sac. It was found that the sonication time, energy input and EEF were all reduced when the ‘I-shape’ sonication was used, comparing those in the ‘C-shape’ strategy, and the effectiveness was equivalent, with significantly reduced incidence of sciatic/buttock pain and lower abdominal pain. It demonstrated that appropriate adjustment of the sonication strategy by reducing the distribution of sonication dots could not only achieve the same satisfactory effect, but also reduce the energy input, which may further reduce the risk of adverse events or complications.
The previous studies on the adverse events of HIFU in the treatment of uterine fibroids found that fascial swelling was positively associated with sonication time and therapeutic energy [Citation10]. The accumulation of energy input can cause pelvic fascial swelling, and with the increase of energy input, the degree of edema increased. Cun et al. [Citation17] found that, in both univariate analysis and multivariate analysis, the total energy and EEF possessed significant correlations with sacrum MR signal changes which indicated sacral injury. They also found that the total energy was significantly correlated with thermal injury of abdominal wall. Liu et al. [Citation18] reported that the total energy, sonication time, treatment time and EEF for fibroid ablation were significantly higher in patients with adhesions than that in patients without adhesions. Thus, the input of energy has risks, and as the energy accumulates, the risk increases. In this study, when compared with the ‘C-shape’, the range of treatment and the number of treatment dots reduced in the energy input mode of the ‘I-shape’, so as to reduce the total sonication time and thus reduce the EEF. The duration of treatment was not significantly shortened, but the treatment intensity was reduced. Although the energy input was less in ‘I-shape’ sonication strategy than in ‘C-shape’, it did not increase the risk of blood loss during USg-D&C, and did not increase the time for β-hCG falling to normal level. Therefore, it could be concluded that there was no difference in the treatment effect between the two groups, with the incidence of sciatic/buttock pain and lower abdominal pain after HIFU treatment reducing in the ‘I-shape’ group.
There were few literatures mentioned and analyzed the energy delivery. In this study, the sonication time of ‘C-shape’ group of 456 (140–1300) s was shorter than that in a previous study of 590 (100–2150) s [Citation8], but it was similar to 400 (281–600) s recently reported by Liu et al. [Citation19]. Since the treatment power was 350–400 W in all these studies, the total energy given in this study was reduced when compared with the previous studies and was consistent with the recent literature. The sonication time of ‘I-shape’ group was 265 (50–815) s, which was even less than that in the ‘C-shape’ group. With the number of CSP patients treated by HIFU and related reports increased, the experience of applying HIFU treatment for CSP was accumulated, it was possible for researchers to summarize the relationship between the energy input and treatment effect, which helped to shorten the sonication time and reduce the unnecessary energy.
In this paper, the energy input of HIFU treatment for CSP was further discussed by comparing two different sonication strategies. The ‘C-shape’ modality employed in the control group was a common sonication strategy used in many hospitals, in which the focus surrounded the base of the embedding area of gestational sac like an arc. The ‘I-shape’ modality employed in the experimental group was a new sonication strategy first used in our hospital, in which the focus was placed in the deep of implantation position at the scar. Since the base of gestational sac implantation has the most enriched blood supply and the adhesion between the scar and the sac is much tighter than the other part of embedding area, it was well accepted that the target area of CSP during HIFU treatment was the base of implantation, which was the main area causing the suction difficulty, having a high risk of massive blood loss during USg-D&C, leaving debris of gestational sac and leading to persistent post-suction vaginal bleeding and an unsatisfactory decrease of β-hCG. In addition, the rest part of CSP, which intruded into the uterine cavity, was left untreated, because this part was relatively easy to be removed by suction without heavy bleeding. The ‘I-shape’ strategy of intensively depositing energy in the base of gestational sac implantation was to block the blood supply to the maximum extent and to reduce the chance of unsatisfactory suction and β-hCG decrease. In this study, it was shown that during HIFU treatment, since the exposure dots in the experimental group was aimed at only the base of embedding area, the energy input was more precise than that in the control group (). Because the energy input was focused on the base of gestational sac implantation close to the serosa, to avoid the serosa damage, the pace of treatment was relatively slow, which made the treatment intensity low. Although the sonication time, treatment intensity, total energy and EEF in the experimental group were significantly lower than in the control group, there was no statistical difference of the amount of blood loss during suction curettage and of the time for β-hCG back to normal level, indicating that the ‘I-shape’ sonication strategy could achieve the same therapeutic effect with no increase of bleeding risk in suction curettage by reducing the energy input.
From the literature, it was shown that HIFU treatment for CSP was safe. It was reported that a small number of CSP patients had hematuria and bladder irritation happened after HIFU treatment, the incidence rate was 0.2–1.8%, and all patients had recovered soon after taking some hemostatics and anti-inflammatory drugs [Citation20,Citation21]. From a recent meta-analysis report, it was demonstrated that adverse events rate of HIFU treatment for CSP differed from 1.11% to 45.88% in different studies [Citation5]. In our study, the common adverse events included sciatic/buttock pain, lower abdominal pain and vaginal discharging, which were in accordance with previous studies [Citation5,Citation8]. The incidence of sciatic/buttock pain and lower abdominal pain in the experimental group was significantly lower than that in the control group, which was consistent with previous studies [Citation8]. The incidence of complications rose with the increase of sonication time and energy delivery [Citation22,Citation23]. In the experimental group, the energy was put in the base of implantation, which was close to the serosa and bladder wall, but no increase in the incidence of hematuresis occurred. So, it did not increase the risk of bladder wall injury. The numbers of cases with vaginal bleeding and the need for re-intervention (hemostatics or MTX or suction curettage) in the control group were more than that in experimental group, demonstrating that the reduce of energy input in the ‘I-shape’ group may help to reduce the incidence of such adverse events or complications. However, differences in case numbers of such adverse events between two groups were not tested with statistical significance, it was probably in part because of the relatively small sample size. In the future, a prospective study with large case number is required to further evaluate the relationship between energy input of this new sonication strategy (I-shape) and effectiveness of HIFU treatment for CSP, and to further observe and validate the adverse events rate, which may be conducive to a better and more sound plan of energy distribution for maximizing the effect and minimizing the risk when treating CSP patients by HIFU.
In conclusion, treatment of CSP with HIFU could mainly and precisely targets at the embedding area of gestational sac implantation, without sonication around the whole gestational sac or even inside the gestational sac. The ‘I-shape’ strategy can reduce the sonication time, energy input and the rate of lower abdominal pain during HIFU treatment for CSP, with no increase of the risk of bladder injury and massive bleeding during curettage. This study provides a new clinical strategy for making HIFU treatment plans. However, the influencing factors related to the energy input of HIFU treatment for CSP are complicated and requires further explorations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol. 2003;21(3):220–227.
- Liu Y, Li G, Chen Y, et al. A descriptive analysis of the indications for caesarean section in mainland China. BMC Pregnancy Childbirth. 2014;14:410.
- Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2013;25(6):456–461.
- Seow K-M, Huang L-W, Lin Y-H, et al. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247–253.
- Xiao X, Feng Z, Li T, et al. Comparing the efficacy and safety of High-Intensity focused ultrasound and uterine artery embolization in caesarean scar pregnancy: a meta-analysis. Adv Ther. 2019;36(6):1314–1325.
- Xiao J, Shi Z, Zhou J, et al. Cesarean scar pregnancy: comparing the efficacy and tolerability of treatment with high-intensity focused ultrasound and uterine artery embolization. Ultrasound Med Biol. 2017;43(3):640–647.
- Chen L, Xiao S, Zhu X, et al. Analysis of the reproductive outcome of patients with cesarean scar pregnancy treated by high-intensity focused ultrasound and uterine artery embolization: a retrospective cohort study. J Minim Invasive Gynecol. 2019;26(5):883–890.
- Zhu X, Deng X, Xiao S, et al. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. Int J Hyperthermia. 2016;32(2):144–150.
- Yin N, Hu L, Xiao ZB, et al. Factors influencing thermal injury to skin and abdominal wall structures in HIFU ablation of uterine fibroids. Int J Hyperthermia. 2018;34(8):1298–1303.
- Zhang YJ, Xiao ZB, Lv FR, et al. MRI evaluation of endopelvic fascial swelling and analysis of influencing factors in patients with uterine fibroids after high-intensity focused ultrasound ablation. Int J Hyperthermia. 2020;37(1):175–181.
- Gonzalez N, Tulandi T. Cesarean scar pregnancy: a systematic review. J Minim Invasive Gynecol. 2017;24(5):731–738.
- Hoffman T, Lin J. Cesarean scar ectopic pregnancy: diagnosis with ultrasound. Clin Pract Cases Emerg Med. 2020;4(1):65–68.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:199–202.
- Wang Z, Bai J, Li F, et al. Study of a ‘biological focal region’ of high-intensity focused ultrasound. Ultrasound Med Biol. 2003;29(5):749–754.
- Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method . Korean J Radiol. 2018;19(4):724–732.
- Zhang Y, Zhang C, He J, et al. The impact of gestational sac size on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment for caesarean scar pregnancy. Int J Hyperthermia. 2018;35(1):291–297.
- Cun JP, Fan HJ, Zhao W, et al. Factors influencing MR changes associated with sacral injury after high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2019;36(1):21–28.
- Liu X, Dong X, Mu Y, et al. High-intensity focused ultrasound (HIFU) for the treatment of uterine fibroids: does HIFU significantly increase the risk of pelvic adhesions. Int J Hyperthermia. 2020;37(1):1027–1032.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35(1):56–61.
- Hong YF, Guo QW, Pu YJ, et al. Outcome of high-intensity focused ultrasound and uterine artery embolization in the treatment and management of cesarean scar pregnancy: a retrospective study. Medicine (Baltimore). 2017; 96(30):e7687.
- Zhu X, Deng X, Xue M. Clinical study of high-intensity focused ultrasound combined with suction curettage in the treatment of cesarean scar pregnancy. Chin J Pract Gynecol Obstet. 2018; 34(05):563–565. (in Chinese)
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Yang WW, Zhu BR, Li J, et al. Analysis of complications of high intensity focused ultrasound in treatment of uterine leiomyoma. Zhonghua Fu Chan Ke Za Zhi. 2010;45(12):913–916. (in Chinese)
