Abstract
Objective
Rhabdomyolysis (RM) in exertional heatstroke (EHS) often leads to multiple organ dysfunction including acute kidney injury (AKI). Studies have shown that serum creatine kinase (CK) >1000 U/L as a serological diagnostic criterion for RM does not reflect the risk of AKI or mortality.
Methods
This longitudinal cohort study included all patients with EHS who were admitted to intensive care unit between January 2008 and June 2019. Serum myoglobin (sMb) was studied as the serological marker of RM and compared with CK. Outcome events were AKI and 90-day mortality.
Results
A total of 161 patients were enrolled, of whom 52 (32.3%) had sMb ≥1000 ng/mL. Patients with sMb ≥1000 ng/mL had higher SOFA score, higher APACHE II score, lower GCS score, and higher incidence of disseminated intravascular coagulation, acute myocardial injury, acute liver injury, AKI, and 90-day mortality than patients with sMb <1000 ng/mL. Lymphocytes, neutrophils, D-Dimer were risk factors for AKI in patients with sMb ≥1000 ng/mL. Curve fitting showed a curved relationship between sMb and EHS-induced AKI but not CK. sMb ≥1000 ng/mL showed better predictive ability for AKI (area under curve: 0.786). APACHE II, SOFA, and GCS scores were risk factors for 90-day mortality in patients with sMb ≥1000 ng/mL.
Conclusion
Serum myoglobin is a better predictor of AKI and 90-day mortality than CK in patients with RM after EHS.
Introduction
The Pacific Northwest and western Canada were hit with a record-breaking heat wave in 2021. In late June, there were approximately 600 more deaths in Oregon and Washington than would have been typical for this month. In Canada, at least 486 unexpected deaths were reported during a five-day period, which was nearly three times higher than usual (https://www.nytimes.com/interactive/2021/08/11/climate/deaths-pacific-northwest-heat-wave.html). Heatstroke is an important public health problem and is classified as exertional heatstroke (EHS) and classical heatstroke (CHS) [Citation1]. EHS, defined as an internal body temperature > 40 °C with associated brain impairment, is directly related to strenuous physical activity, especially military training. It is a life-threatening medical emergency that requires prompt recognition and management to ensure survival [Citation1]. EHS is often complicated by rhabdomyolysis (RM), which leads to multiple organ dysfunction including acute kidney injury (AKI) [Citation2]. Approximately 26000 patients develop RM in the United States each year, of whom an estimated 7–10% patients develop AKI [Citation3].
According to the previous studies, the laboratory diagnostic criteria for RM is still serum or plasma creatine kinase (CK) >1000 U/L or 5–10 times the upper limit of normal reference range [Citation4,Citation5]. According to a study, CK >1000 U/L may not be an optimal predictor of organ function and prognosis [Citation6]. In the setting of infection, trauma, crush injury, drug or alcohol abuse, immobilization, and statin use, CK >5000 U/L is generally considered predictive of AKI [Citation7,Citation8]; however, use of this criterion overlooks some patients in the early stages of AKI. Recent evidence suggests a critical role of serum myoglobin (sMb) in the pathogenesis of myoglobinuric AKI, because of its faster kinetics than CK [Citation9]. Therefore, monitoring sMb over time may better reflect the disease activity. It has not been reported whether CK or sMb should be used for the laboratory diagnosis of RM in EHS patients and their specific diagnostic threshold. Therefore, we conducted a retrospective study to investigate the performance of CK and myoglobin as predictors of AKI in a tertiary-care teaching hospital in southern China over a 10-year period. In addition, we analyzed the clinical characteristics, risk factors, and 90-day mortality in EHS patients with RM.
Methods
Study design and participants
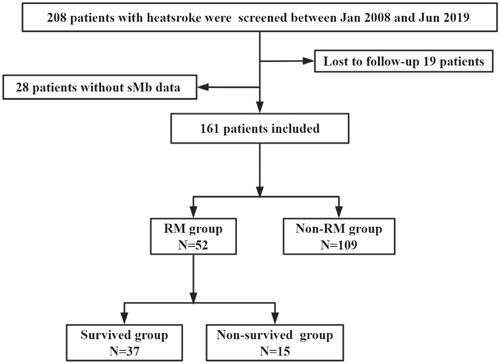
This retrospective cohort study was performed in the intensive care unit (ICU) of the General Hospital of Southern Theater Command of the Peoples Liberation Army from January 2008 to June 2019. The inclusion criterion was: patients with exertional heatstroke caused by strenuous exercise performed in a high-temperature and high-humidity environment. The diagnostic criteria for exertional heatstroke are described elsewhere [Citation2]. The exclusion criteria were as follows: (1) death or discharge within 24 h after admission; (2) incomplete data regarding key indicators; (3) incomplete outcome evaluation data obtained via telephonic follow-up; and (4) a previous history of organ dysfunction, such as chronic kidney disease.
The most common cause of heatstroke in southern China is strenuous outdoor training. All such patients are treated with cooling immediately on site and are continued to cool down to 39 °C (preferably 38.5 °C to 38.0 °C) after admission. All medical interventions were carried out in accordance with current guidelines [Citation10]. The studies involving human participants were reviewed and approved by the Research Ethics Committee of the General Hospital of Southern Theater Command of the Peoples Liberation Army (HE-2020-09). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Research procedures
Data pertaining to the following basic characteristics of patients were reviewed: age, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, SOFA score, Glasgow Coma Scale (GCS) score, and inflammatory and organ function indicators at admission. The indicators included differential blood counts (white blood cells, neutrophils, lymphocytes, monocytes, and platelets), kidney function markers [blood urea nitrogen (BUN) and serum creatinine (Scr)], liver function markers [total bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST)], C-reactive protein (CRP), procalcitonin (PCT), cardiac markers [CK, MB isoenzyme of creatine kinase (CKMB), sMb, and cardiac troponin I (cTNI)], clotting factors [prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB), and D-dimer], and blood transfusion during treatment. Based on the sMb at admission, patients were assigned to one of the two groups (sMb ≥ 1000 ng/mL group and the sMb < 1000 ng/mL group). Survival time was defined as the duration from onset to death; when the survival time was longer than 90 days, it was recorded as 90 days. The main results, including the 90-day mortality, duration of stay in ICU, and the total cost during hospitalization, were analyzed. Survival curve analysis was performed.
Definitions
(1) Acute myocardial infarction (AMI) [Citation11]: CTN I > 0.1 ng/mL was considered as MI.
(2) Acute liver injury (ALI) [Citation12]: Plasma TBIL ≥34.2 μmol/L and INR ≥1.5, or with any grade of hepatic encephalopathy.
(3) AKI [Citation13]: The Kidney Disease: Improving Global Outcomes (KDIGO) standard: Scr increased to ≥26.5 μmol/L (≥0.3 mg/dl) within 48 h, Scr increased to ≥1.5 times the baseline within 7 days, or urine output <0.5 ml/(kg h) for 6 h.
(4) DIC [Citation14]: International Society for Thrombosis and Haemostasis (ISTH) standard: An ISTH score ≥5 points.
(5) Lymphocytopenia [Citation15]: absolute lymphocytes less than 0.8 × 109/L.
Statistical analysis
Non-normally distributed continuous variables are presented as median and interquartile range (IQR), and the categorical variables are presented as frequency (percentage). Continuous variables were compared using the independent two-sample t-test or Mann–Whitney U-test. Categorical variables were compared using the Chi-Squared test or Fisher’s exact test. Risk factors for AKI were identified using univariate analysis. Indicators associated with a p value < .1 in univariate analysis were included in the multivariate logistic regression model: odds ratio (OR) and 95% confidence interval level (95% CI) was used to assess the predictive ability of each variable for AKI. The ability of each variable to predict 90-day mortality was assessed using Cox proportional hazards model [hazard ratio (HR) and 95% CI]. The predictive ability of APACHE II score, SOFA score, GCS score for 90-day mortality were assessed using the area under the receiver operating characteristic (AU-ROC) curve and the optimal cutoff value was determined by Youden’s index. Ninety-day mortality in the sMb ≥ 1000 ng/mL group and the sMb < 1000 ng/mL group was analyzed using the Kaplan–Meier’s method and the between-group differences were assessed using the log-rank test. Statistical analyses were performed using the IBM SPSS Windows version 23.0 (IBM Corp., Armonk, NY, USA), Empower (R) (http://www.empowerstats.com, X&Y solutions, Inc., Boston, MA, USA), and R (http://www.R-project.org) software. Two-tailed p values less than .05 were considered indicative of statistical significance.
Results
Clinical characteristics of patients with different sMb values following EHS
A total of 161 male patients were enrolled, of whom 52 (32.3%) developed RM using sMb ≥ 1000 ng/mL as the serological diagnostic criteria for RM (). Patients with sMb ≥ 1000 ng/mL were significantly older [25.0 vs 20.0 (years), p = .005], and had higher APACHE II score (15.0 vs 10.0, p = .002), higher SOFA score (5.0 vs 3.0, p < .001), and lower GCS score (8.0 vs 12.0, p = .023) than patients with sMb < 1000 ng/mL. RM patients had worse coagulation indices, including significantly decreased platelet count and FIB, prolonged PT, INR, and D-dimer (all p < .05), which implied a significantly higher incidence of DIC (54.8% vs 28.1%, p = .003). Moreover, these patients had significantly higher incidence of AMI (76.9% vs 40.4%, p < .001), ALI (32.7% vs 10.2%, p < .001), and AKI (73.1% vs 29.0%, p < .001), and 90-day mortality (28.8% vs 6.4%, p < .001) as well as higher total cost of hospitalization [96749.7 vs 33504.1 (RMB), p = .002] ().
Table 1. Comparisons of clinical characteristics between MB < 1000 ng/ml and MB ≥ 1000 ng/ml with EHS.
Risk factors for AKI after EHS
On univariate analysis, neutrophils, PT, D-dimer, sMb ≥ 1000 ng/mL and DIC showed a significant association with post-EHS AKI (p < .05 for all). On multivariate logistics analysis, lymphocyte (OR 2.07, 95% CI 1.24–3.44, p = .005), neutrophil (OR 1.14, 95% CI 1.02–1.27, p = .024), D-dimer (OR 1.08, 95% CI 1.01–1.17, p = .034), and sMb ≥ 1000 ng/mL (OR 6.51, 95% CI 2.22–19.11, p < .001) were risk factors for AKI in EHS patients, while CK ≥ 1000 U/L (p = .421) and DIC (p = .067) were not ().
Table 2. Risk factors for AKI induced by EHS.
Comparisons of survivors and nonsurvivors with sMb ≥ 1000 ng/mL after EHS
Among the patients with sMb ≥ 1000 ng/mL after EHS, 37 survived (71.2%) and 15 died (28.8%). The survivor group had significantly lower APACHE II scores (14.0 vs 23.0, p < .001), lower SOFA scores (4.0 vs 13.0, p < .001), and higher GCS scores (10.0 vs 3.0, p = .007) than the non-survivor group. In the survivor group, patients had better blood coagulation indices (platelets, PT, INR, APTT, FIB and D-dimer; p < .05 for all), so the incidence of DIC was significantly lower (37.9% vs 92.3%, p < .001). Moreover, Scr [149.0 vs 233.0 (µmol/L)] and cystatin C [1.0 vs 1.5 (mg/L)] were lower, and the incidence of AKI and AMI was lower (64.9% vs 93.3%, p = .036; 69.4% vs 100.0%, p = .012, respectively). However, there were no significant differences with respect to CK [2918.0 vs 5060.0 (U/L), p = .808] and ALI (27.0% vs 46.7%, p = .171). The total hospitalization costs were significantly lower in the survivor group [69584.4 vs 157620.0 (RMB), p = .004] ().
Table 3. Comparisons of clinical characteristics between survivors and non-survivors with sMb ≥ 1000 ng/ml induced by EHS.
Diagnostic value of sMb and CK in predicting EHS-induced AKI
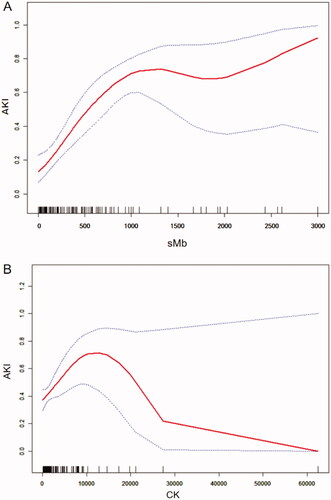
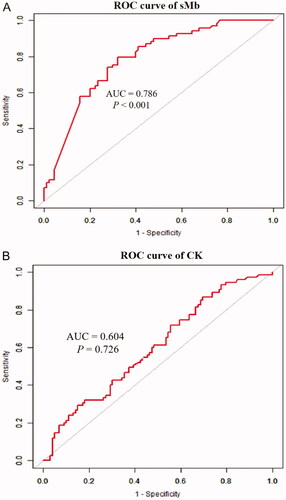
The curve fitting showed a curve relationship between sMb and EHS-induced AKI. The association between sMb and AKI, CK and AKI at admission both showed a curving relationship (). On ROC curve analysis, use of sMb ≥ 1000 ng/mL had a 79.1% sensitivity and 67.8% specificity for the prediction of AKI [AUC: 0.786 (95% CI 0.716–0.856, p < .001)] (). However, the predictive value of CK ≥ 1000 U/L for AKI was less than that of sMb [AUC: 0.604 (95% CI 0.520–0.688, p = .726)] ().
Risk factors for 90-day mortality in EHS complicated with sMb ≥ 1000 ng/mL
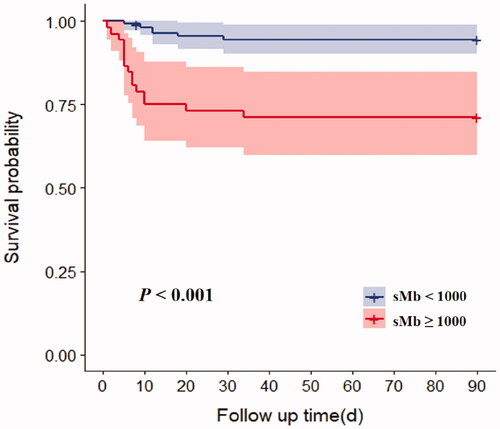
On multivariate logistic regression, APACHE II score (HR 1.6, 95% CI 1.1–2.3, p = .016), SOFA score (HR 2.2, 95% CI 1.3–4.0, p = .006), and GCS score (HR 2.0, 95% CI 1.1–3.7, p = .019) were independent risk factors for 90-day mortality in patients with sMb ≥ 1000 ng/mL following EHS (). The AUCs for the prediction of mortality based on APACHE II, SOFA, and GCS scores were 0.901 (95% CI 0.716–1.000), 0.960 (95% CI 0.894–1.000), and 0.827 (95% CI 0.645–1.000), respectively. The optimal cutoff score levels were 21.5, 9.5, 6.5 points, respectively (sensitivity: 90.9%, 95.5%, 77.3%; specificity: 87.5%, 87.5%, 75%, respectively) (p < .01 for all) (). In addition, patients with sMb ≥ 1000 ng/mL after EHS had significantly higher 90-day mortality (p < .001) ().
Figure 4. ROC curves of APACHE II、SOFA、GCS in predicting 90-day mortality with sMB ≥ 1000 ng/ml induced by EHS.
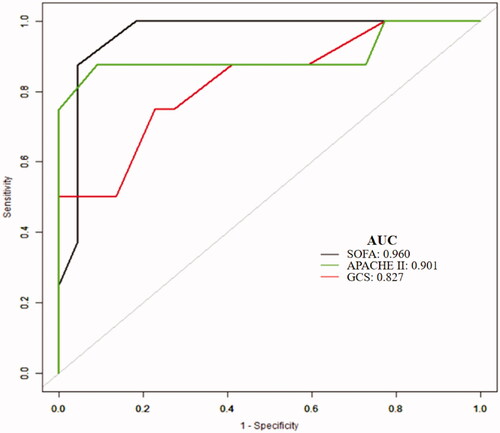
Table 4. Risk factors for 90-day mortality with sMb ≥ 1000 ng/ml induced by EHS.
Discussion
This retrospective study included 161 patients with EHS. To the best of our knowledge, this is the first study to report that sMb ≥ 1000 ng/mL may be a better diagnostic criterion for RM than CK ≥ 1000 U/L for the purpose of predicting the risk of AKI after EHS. In addition, this is the first study to identify APACHE II score, SOFA score, and GCS score at admission as independent risk factors for 90-day mortality in patients with sMb ≥ 1000 ng/mL following EHS. Our findings suggest that wider use of sMb may facilitate early intervention in patients with suspected RM, and aid in the timely prevention of impending AKI after EHS.
In a previous study, use of CK ≥ 1000 U/L as the serological diagnostic criterion for RM was not found to fully reflect the degree of organ injury and predict the prognosis [Citation6]. Due to the influence of heat injury, EHS-induced RM is significantly different from RM occurring after normal exercise. In the context of infection, trauma, crush injury, drug and alcohol abuse, immobilization, and statin use, CK value of > 5000 U/L is generally considered as a predictor of AKI [Citation7,Citation16]. In another study [Citation17], CK > 10,000 U/L (> 50 times the normal upper value) was found useful in predicting renal failure in patients with EHS. However, this value was too high and not conducive to early detection of high-risk patients. In a multicenter retrospective clinical study of 387 patients with severe RM (CK > 5000 U/L), sMb > 8000 U/L at admission was associated with increased risk of stage 2–3 AKI [Citation18]. Several studies have suggested that peak myoglobin levels in serum or plasma may be more predictive of AKI than CK [Citation19–22]. In addition, the half-life of CK in blood is 1.5 days, while that of myoglobin is 2–4 h; in addition, the myoglobin level does not return to baseline until 6–8 h after the event [Citation4,Citation23]. Therefore, dynamic monitoring of sMb levels over time may better reflect the disease activity and treatment efficacy. Collectively, we believe that the threshold value of CK > 5000 U/L is too high and patients with stage 1–2 AKI are liable to be overlooked using this threshold. Therefore, establishment of sMb as a biomarker of RM and determining its optimal cutoff level in patients with EHS is a key imperative.
RM releases myoglobin that can be decomposed into myosin, which plays an important role in the coagulation cascades, including both coagulation factors and fibrinolysis [Citation24]. In addition, some nuclear proteins are released after muscle cell injury, such as histone 3 [Citation25,Citation26] and HMGB1 [Citation27]. These proteins can induce activation of platelets, leading to the occurrence of DIC and ALI. However, mounting evidence suggests that the role of sMb in predicting AKI in patients with RM should be reassessed [Citation28,Citation29]. In this study, on multivariate analysis, sMb ≥ 1000 ng/mL was a risk factor for AKI, while CK ≥ 1000 U/L was not. On ROC curve analysis, sMb ≥ 1000 ng/mL showed a better sensitivity and specificity for predicting AKI than CK ≥ 1000 U/L, which was different from some studies [Citation30,Citation31].
Several hypotheses may potentially explain the better predictive ability of sMb for AKI than CK. Firstly, it is reported that sMb rather than CK is a direct mediator of RM-induced AKI [Citation32]. Therefore, sMb may more accurately reflect the renal toxicity of RM than CK. Secondly, unlike CK, the release of sMb is not dependent on lymphatic transport and it is released more rapidly into the bloodstream, which makes it more accurate as an early marker for risk stratification of RM-induced AKI [Citation33]. Thirdly, because of its direct renal toxicity, sMb may also reflect the extent of tissue injury which leads to systemic inflammation and ultimately causes renal function impairment. In addition, patients with sMb ≥ 1000 ng/mL after EHS showed significantly higher 90-day mortality. Therefore, in-depth study of the pathological mechanism linking sMb and kidney is required to provide theoretical basis for developing therapeutic strategy based on reducing sMb for RM-induced AKI following EHS.
On multivariate logistic regression analysis, APACHE II score, SOFA score, and GCS score were all identified as independent risk factors for 90-day mortality in EHS patients. However, SOFA score had the best AUC for the prediction of mortality in patients with sMb ≥ 1000 ng/mL and not APACHE II score. The APACHE II score is an important scoring system for prognostic assessment of critically ill patients, which factors age and chronic health. However, our patients were previously healthy and the median age in our cohort was 21 years. In addition, because the APACHE II score excludes some vital acute organ functions including coagulation function and liver function, it is not as comprehensive as the SOFA score. The optimal cutoff level of SOFA score for predicting 90-day mortality was 9.5 points (95.5% sensitivity and 87.5% specificity). This suggests that the follow-up treatment with the primary aim of protecting key organ function, especially kidney, is an important way to reduce mortality.
Some limitations of our study should be considered while interpreting the results. This was a retrospective study with a relatively small number of cases. In addition, our cohort exclusively comprised of relatively young, male patients with EHS. The results may not be generalizable to patients with other forms of heatstroke. Larger prospective studies are required to obtain more definitive evidence.
Conclusions
Use of sMb ≥ 1000 ng/mL as the diagnostic criterion for RM may allow better stratification of EHS patients with respect to the risk of development of AKI and mortality. More widespread use of sMb may contribute to early intervention in suspected RM and facilitate timely prevention of impending AKI after EHS.
Author’s contributions
Ming Wu and Zhifeng Liu contributed to the study concept and design. Ming Wu, Conglin Wang and Li Zhong collected the data. Ming Wu and Conglin Wang performed the statistical analysis. Ming Wu and Conglin Wang drafted the manuscript. All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Epstein Y, Yanovich R. Heatstroke. N Engl J Med. 2019;380(25):2449–2459.
- Wu M, Wang C, Liu Z, et al. Clinical characteristics and risk factors associated with acute kidney injury inpatient with exertional heatstroke: an over 10-Year intensive care survey. Front Med (Lausanne). 2021;8:678434.
- Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emerg Med. 2007;2(3):210–218.
- Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis - an overview for clinicians. Crit Care. 2005;9(2):158–169.
- Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651–659.
- Wu M, Wang C, Liu Z, et al. Sequential organ failure assessment score for prediction of mortality of patients with rhabdomyolysis following exertional heatstroke: a longitudinal cohort study in Southern China. Front Med (Lausanne). 2021;8:724319.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. CDS. 2009;4(3):181–187.
- Rodríguez E, Soler MJ, Rap O, et al. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8(12):e82992.
- Lippi G, Plebani M. Serum myoglobin immunoassays: obsolete or still clinically useful? Clin Chem Lab Med. 2016;54(10):1541–1543.
- Liu SY, Song JC, Mao HD, et al. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 2020;7:1.
- Zhong L, Ji J, Wang C, et al. Clinical characteristics and risk factors of male exertional heatstroke in patients with myocardial injury: an over 10-year retrospective cohort study. Int J Hyperthermia. 2021;38(1):970–975.
- Ji J, Gao J, Wang C, et al. Characteristics and outcome of exertional heatstroke patients complicated by acute hepatic injury: a cohort study. J Clin Transl Hepatol. 2021;9(5):655–660.
- Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830.
- Taylor FJ, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(11):1327–1330.
- Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821–1828.
- Scalco RS, Snoeck M, Quinlivan R, et al. Exertional rhabdomyolysis: physiological response or manifestation of an underlying myopathy? BMJ Open Sport Exerc Med. 2016;2(1):e151.
- Candela N, Silva S, Georges B, et al. Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;10:27.
- Kasaoka S, Todani M, Kaneko T, et al. Peak value of blood myoglobin predicts acute renal failure induced by rhabdomyolysis. J Crit Care. 2010;25(4):601–604.
- El-Abdellati E, Eyselbergs M, Sirimsi H, et al. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care. 2013;3(1):8.
- Premru V, Kovač J, Ponikvar R. Use of myoglobin as a marker and predictor in myoglobinuric acute kidney injury. Ther Apher Dial. 2013;17(4):391–395.
- Tarazona V, Figueiredo S, Hamada S, et al. Admission serum myoglobin and the development of acute kidney injury after major trauma. Ann Intensive Care. 2021;11(1):140.
- Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81-82:209–230.
- Deguchi H, Morla S, Griffin JH. Novel blood coagulation molecules: skeletal muscle myosin and cardiac myosin. J Thromb Haemost. 2021;19(1):7–19.
- Li Y, Liu Z, Shi X, et al. Prognostic value of plasma exosomal levels of histone H3 protein in patients with heat stroke. Exp Ther Med. 2021;22(3):922.
- Ito T, Totoki T, Yokoyama Y, et al. Serum histone H3 levels and platelet counts are potential markers for coagulopathy with high risk of death in septic patients: a single-center observational study. j Intensive Care. 2019;7(1):63.
- Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5(2):611–647.
- Maffulli N, Oliva F, Frizziero A, et al. ISMuLT guidelines for muscle injuries. Muscles Ligaments Tendons J. 2013;3(4):241–249.
- Chen CY, Lin YR, Zhao LL, et al. Clinical factors in predicting acute renal failure caused by rhabdomyolysis in the ED. Am J Emerg Med. 2013;31(7):1062–1066.
- Safari S, Yousefifard M, Hashemi B, et al. The value of serum creatine kinase in predicting the risk of rhabdomyolysis-induced acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. 2016;20(2):153–161.
- Assanangkornchai N, Akaraborworn O, Kongkamol C, et al. Characteristics of creatine kinase elevation in trauma patients and predictors of acute kidney injury. J Acute Med. 2017;7:54–60.
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62–72.
- Sayers SP, Clarkson PM. Short-term immobilization after eccentric exercise. Part II: creatine kinase and myoglobin. Med Sci Sports Exerc. 2003;35:762–768.