Abstract
Objective
To investigate the efficacy of radiofrequency ablation (RFA) as a treatment option for primary hyperparathyroidism (pHPT) and risk factors for postablative eucalcemic parathyroid hormone elevation (ePTH).
Methods
This retrospective study included 51 patients with pHPT who underwent RFA. The patients were divided into the ePTH and normal PTH groups, based on the serum intact parathyroid hormone (iPTH) level one month after ablation. Serum iPTH, calcium, and phosphorus levels, and the volume reduction rates (VRR) of the parathyroid glands were compared between the groups at each follow-up point. Risk factors for ePTH at one month after ablation were examined.
Results
After RFA, one (2%) patient had persistent pHPT, and 50 (98%) patients were cured. The incidence rates of ePTH at 1, 3, 6, and 12 months were 48%, 30%, 20%, and 16%, respectively. Serum iPTH levels in the ePTH group were higher than those in the normal PTH group at each follow-up point (all p < 0.05), except 1 day after ablation (p > 0.05). Serum calcium and phosphorus levels, and the VRR of the glands were comparable in both groups at each follow-up point (all p > 0.05), except for calcium levels 3 days after RFA (p < 0.05). Baseline iPTH (odds ratio, 1.067; p = 0.045) and calcium (odds ratio, 3.923; p = 0.038) levels were independent risk factors for ePTH 1 month after RFA.
Conclusions
RFA is safe and effective for the treatment of pHPT. Moreover, ePTH occurrence after RFA was associated with baseline iPTH and calcium levels and did not increase the risk of recurrent pHPT.
Introduction
Primary hyperparathyroidism (pHPT) is an endocrine disorder associated with excessive secretion of parathyroid hormone (PTH) from one or more parathyroid glands [Citation1]. Classic pHPT characterized by elevated serum calcium and PTH levels [Citation1]. The incidence rates vary from 0.4 to 82 per 100,000 people [Citation2,Citation3]. Approximately 75–85% of patients with pHPT have a single parathyroid adenoma, 2–12% have two adenomas, less than 1–2% have three adenomas, and less than 1–15% have four or more adenomas, and parathyroid carcinoma accounts for <1% of all cases [Citation4]. The current major guidelines recommend parathyroidectomy as the standard treatment method for pHPT [Citation5,Citation6]. For patients with hypercalcemic pHPT, ‘cure’ after parathyroidectomy was defined as maintenance of normal serum calcium concentration for at least six months postoperatively, achieved above 95% of patients with few surgical complications [Citation6]. However, even though the postoperative serum calcium levels remain normal, serum iPTH levels may rise again after returning to the normal range in the weeks following successful parathyroidectomy. This phenomenon is defined as eucalcemic parathyroid hormone elevation (ePTH) [Citation7,Citation8]. The incidence of ePTH after parathyroidectomy has been reported in the range of 10–60% [Citation8–12]. In previous studies, the phenomenon of ePTH after parathyroidectomy have been well described but the exact etiology, prognosis, and risk factors remain controversial.
Recently, thermal ablation of the parathyroid gland is considered as a safe and effective therapy for patients with pHPT who decline surgical treatment or present with contraindications [Citation13,Citation14]. Similarly, radiofrequency ablation (RFA) has high success rates in attaining postoperative normocalcemia and improvement of pHPT-related symptoms [Citation15–20]. Similar to surgery, ePTH have been observed after thermal ablation [Citation21]. This phenomenon is particularly relevant when an increasing clinical application of thermal ablation for pHPT. However, few studies have reported on the prognosis of ePTH after RFA or the risk factors affecting its occurrence. This retrospective study aimed to investigate the efficacy of RFA for pHPT and the risk factors influencing ePTH occurrence after ablation.
Materials and methods
Patients
This retrospective study was approved by the Human Ethics Review Committee of Hangzhou Hospital of Traditional Chinese Medicine (2015LH001) and Zhejiang Provincial People's Hospital (2018JS003). All patients provided written informed consent for treatment prior to undergoing RFA. The informed consent for participation in the current study was waived because of the retrospective nature of this study. Sixty-one patients with pHPT were treated with ultrasound (US)-guided RFA between January 2015 and October 2020 at the two hospitals. Twelve patients were enrolled from Hangzhou Hospital of Traditional Chinese Medicine and 49 patients were enrolled from Zhejiang Provincial People's Hospital. Fifty-one patients were included in the study (), including 21 men and 30 women (age range, 18–87 years; mean age, 57.96 ± 14.34 years).
Patients were included in this study if they met the following criteria: (1) underwent RFA for pHPT; (2) age >18 years; (3) serum iPTH levels higher than 7.3 pmol/l, serum calcium levels higher than 10.2 mg/dl; (4) a single enlarged parathyroid gland identified with US examination; (5) the gland could be detected by technetium 99-m-labeled sestamibi single-photon emission computed tomography (99mTc-sestamibi SPECT) assessment; and (6) refusal or ineligibility for parathyroidectomy.
Patients were excluded from this study if they met the following criteria: (1) two or more enlarged parathyroid glands identified by US or 99mTc-sestamibi SPECT examination; (2) history of thyroid or parathyroid surgery or ablation; (3) inherited parathyroid disease such as multiple endocrine neoplasia (MEN1, MEN2) or familial HPT; (4) other diseases similar to pHPT in biochemical findings such as tertiary hyperparathyroidism postkidney transplant.
Grouping
Patients who were cured after ablation were divided into the ePTH and normal PTH groups based on the occurrence of ePTH one month after treatment (). The occurrence of ePTH was defined as post-ablative iPTH increasing above the normal range (>7.3 pmol/l) again after returning to normal values, given calcium levels within the normal range after successful RFA [Citation22,Citation23]. Cure was defined as calcium levels remaining in the normal range for at least 6 months after ablation [Citation6]. Recurrent pHPT was defined as the recurrence of hypercalcemia after a normocalcemic interval of more than 6 months after ablation. Persistent pHPT was defined as the failure to achieve normocalcemia within 6 months after ablation [Citation6,Citation24].
Data collection and follow-up
Baseline characteristics of interest included age, sex, body mass index (BMI), serum levels of iPTH, calcium, phosphorus, 25 (OH) D3, creatinine, and urea nitrogen. The clinical symptoms recorded included ostealgia, fatigue, fragility fracture, and nephrolithiasis. Parathyroid US and 99mTc-sestamibi SPECT assessments identified the size and position of the parathyroid gland. The ellipsoid formula was used (V = length × width × height × π/6, V, volume; long, maximum diameter; width, and height were the other two vertical diameters) to calculate the volume of each parathyroid gland. Follow-up indicators included serum levels of iPTH, calcium, phosphorus; clinical symptoms, and ablation area volumes. The follow-up times were 1 day, 3 days, 1 month, 3 months, 6 months, and 12 months after RFA. The normal reference ranges for iPTH, calcium, and phosphate levels were 1.4 to 7.3 pmol/l, 8.5 to 10.2 mg/dl, and 0.85 to 1.51 mmol/l, respectively. The volume reduction rate (VRR) was calculated using the following equation: (initial volume - final volume) x 100/initial volume (%). The incidence of complications, including voice change, bleeding, pain, swallowing discomfort, fever, numbness, hypocalcemia (calcium levels of <8.5 mg/dl) and hypoparathyroidism (iPTH levels of <1.4 pmol/l) during the procedure and follow-up period were collected. Patients who presented with ePTH 6 months after ablation were evaluated using the 99mTc-sestamibi SPECT. All patients were followed up for 12 months.
Equipment
US was performed using an iU22 scanner (Philips Amsterdam, Netherlands), a high-frequency line-array probe (L12-5) for conventional US imaging, and a high-frequency line-array probe (L9-3) for contrast-enhanced ultrasound (CEUS). The US contrast agent was Sonovue (Bracco, Milan, Italy). The RFA system including an RF generator (VIVA RF generator, STARmed, Goyang, South Korea), 18-gauge monopolar modified internally cooled radiofrequency electrode with a 7-mm active tip (VIVA, STARmed, Goyang, South Korea) and a water circulation pump (VIVA pump, STARmed, Goyang, South Korea) were used.
RFA process
US-guided RFA was performed by a radiologist with over 20 years of experience in interventional ultrasonic treatment. Before ablation, the patient was placed in the supine position. A conventional US examination was performed to determine the location and size of the parathyroid nodule and determine the best puncture path. Next, blood supply to the target nodule was assessed by CEUS. After routine disinfection and local anesthesia administration, a 21-gauge puncture needle was inserted into the area around parathyroid nodule under US guidance. Next, 10–50 ml 5% dextrose solution was injected into the area around the nodule to separate the nodule from adjacent critical structures such as thyroid gland, recurrent laryngeal nerve (RLN), carotid artery, anterior cervical muscles, trachea, esophagus, which constructed a minimum 5-mm ‘liquid isolation zone’. Then, under real-time US guidance, an 18-gauge radiofrequency electrode was introduced into the bottom of the parathyroid nodule and ablated using the ‘moving-shot technique’. Ablation was performed with 30–45 W of output power according to the size of treated nodule. Immediately after ablation, CEUS was performed to evaluate the ablation range of the parathyroid nodule. When CEUS showed no evidence of the parathyroid gland enhancement, ablation was completed. Otherwise, supplemental ablation was performed to help avoid nodule residue.
Statistical analysis
All statistical analyses were performed using SPSS software (Version 26.0, IBM Corp. Armonk, NY, USA). The normality of distribution assumption for all continuous variables was tested using the Kolmogorov–Smirnov test. Continuous variables conforming to the normal distribution are reported as mean ± standard deviation and were compared using the Student’s t-test or the repeated measures analysis of variance. Continuous variables conforming to non-normal distribution are presented as median and interquartile range (Q25-Q75) and were compared using the Mann–Whitney U test or Kruskal–Wallis H test. The categorical variables were expressed as frequency (%) and compared using the Chi-square test or Fisher's exact test. Candidate risk factors were explored in univariate analysis. All variables with p-values of <0.1 in univariate logistic regression analysis were included in subsequent multivariate logistic regression analyses. Two-sided p-values of <0.05 were considered statistically significant.
Results
Therapeutic efficacy
One (2%) patient had persistent pHPT due to recurrence of hypercalcemia one month after RFA. CEUS showed a residue in situ. The remaining 50 (98%) patients met the criteria for cure. The normalization of both serum iPTH and calcium were achieved in 44 out of 51 (86%) patients at 12 months after ablation. At 12 months after ablation, serum iPTH levels decreased from baseline 20.34 (12.51–36.42) to 4.71 (3.81–5.66) pmol/l; serum calcium levels decreased from baseline 11.37 ± 1.02 to 9.37 ± 0.34 mg/dl; serum phosphorus levels increased from 0.85 ± 0.12 at baseline to 1.17 ± 0.12 mmol/l; nodule volume decreased from 0.63 (0.25–1.24) at baseline to 0 (0–0.03) ml. The VRR of the ablation nodule increased 3–6 months after ablation, reaching 100 (98.35–100) at 12 months (all p < 0.001) (). Ostealgia resolved in 10 of 11 patients within a month, and partial remission was observed in a single patient. Fatigue disappeared in 8 of 12 patients within a month and considerably reduced in 4 of 12 patients. Twenty-seven patients who had a history of nephrolithiasis before RFA had no newly developed nephrolithiasis, and two patients who had a history of fragility fractures before RFA had no new fractures during the 1-year follow-up.
Table 1. Changes to the serum levels of iPTH, calcium, phosphorus, and volume of nodule before and after RFA.
Prognosis of ePTH
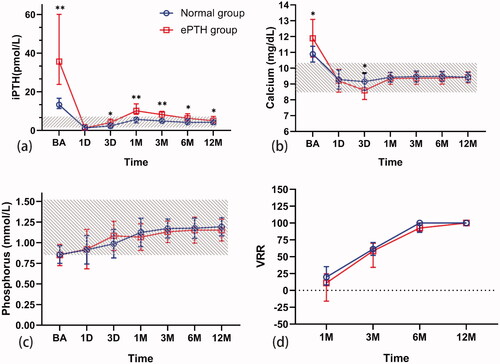
The incidence rates of ePTH at 1, 3, 6, and 12 months after ablation were 48% (24/50), 30% (15/50), 20% (10/50), and 16% (8/50), respectively. None of these patients took additional treatment for ePTH during the follow-up. Comparisons of serum iPTH, calcium, and phosphorus levels, and the VRR of the ablation zone between groups at each follow-up point are shown in . Serum iPTH levels in the ePTH group were higher than those in the normal PTH group at each follow-up point (all p < 0.05) except 1 day after RFA (p > 0.05). In the ePTH group, iPTH values at baseline, 1 day, 3 days, 1 month, 3 months, 6 months, and 12 months were 35.66 (23.79–60.04) pmol/l, 1.33 (0.53–3.13) pmol/l, 4.12 (2.16–5.96) pmol/l, 10.13 (8.36–13.71) pmol/l, 8.20 (6.09–9.86) pmol/l, 6.33 (4.92–8.62) pmol/l, and 5.03 (4.34–7.25) pmol/l, respectively. In the ePTH group (n = 24), serum iPTH levels spontaneously returned to normal 12 months after ablation in 18 patients. Serum calcium levels at 3 days after RFA in the ePTH group were significantly lower than those in the normal PTH group (p = 0.001), but there was no significant difference between the groups at other follow-up points (all p > 0.05). There was no difference in serum phosphorus levels or the VRR of the ablation zone between the ePTH and normal PTH groups (all p > 0.05). No new parathyroid lesions were found in patients with ePTH after ablation on follow-up US and 99mTc-sestamibi SPECT evaluations.
Figure 2. Comparison of serum iPTH, calcium, phosphorus levels, and VRR of parathyroid nodule between the groups. Changes to the level of serum (a) iPTH, (b) calcium, and (c) phosphorus before and after radiofrequency ablation (RFA), and (d) VRR of parathyroid nodule after RFA in the two groups. Calcium and phosphorus values are presented as mean ± standard deviation; iPTH values and VRR of parathyroid nodule are presented as median and interquartile range. The normal range is depicted in gray color. *p < 0.05; **p < 0.001. iPTH: intact parathyroid hormone; VRR: volume reduction rate; RFA: radiofrequency ablation; BA: baseline; 1D: 1 day; 3D: 3 days; 1M: 1 month; 3M: 3 months; 6M: 6 months; 12M: 12 months.
Risk factors associated with ePTH
Between-group comparisons of baseline characteristics are presented in . The baseline iPTH levels in the ePTH group were higher than those in the normal PTH group (p < 0.001). The baseline calcium levels in the ePTH group were higher than those in the normal PTH group (p = 0.001). There was no significant difference in age, sex, BMI, ALP, 25(OH)D3, BUN, creatinine level, incidence of clinical symptoms, parathyroid gland volume, or tumor location between the two groups (all p > 0.05).
Table 2. Comparison of baseline variables between ePTH group and normal PTH group.
shows factors associated with ePTH occurrence a month after ablation. Both baseline serum iPTH (odds ratio [OR] = 1.067, 95% confidence interval [CI]: 1.001–1.137, p = 0.045) and calcium (OR = 3.923, 95% CI: 1.078–14.280, p = 0.038) levels were significantly and positively associated with ePTH a month after ablation.
Table 3. Logistic regression analysis of risk factors affecting ePTH occurrence at 1 month after RFA.
Complications
All 51 patients tolerated the RFA well. Three (5.9%) patients had slightly lower voices after than before the procedure. All patients recovered completely within 2–3 months. Nine (17.6%) patients experienced slight pain at the puncture site or discomfort while swallowing, and all recovered within 1–3 days without persistent pain or discomfort. Four (7.8%) patients developed numbness, eight (15.7%) patients developed hypocalcemia, and 27 (52.94%) patients developed transient hypoparathyroidism on the first day after RFA, and all recovered within 1 month without any specific therapy. There was no case of skin burns, hematoma, infection, or permanent RLN injury during or after RFA.
Discussion
RFA is relatively noninvasive, does not lead to scar formation, and has good repeatability and effectiveness at reducing nodule volume and normalizing calcium and iPTH levels in most patients. It may serve as a therapeutic alternative for patients with pHPT [Citation16,Citation18,Citation20]. In this study, 51 patients with pHPT treated with RFA were followed up for 12 months. One patient had persistent pHPT, and six patients had elevated PTH levels after 12 months. The cure rate at 12 months was 98%. The rate of normalized serum iPTH and calcium levels at 12 months was 86%.
Previous studies have shown that serum iPTH and calcium levels in patients with pHPT decreased rapidly on the first day after operation [Citation25]. However, iPTH levels began to rebound several days or weeks after a successful operation, sometimes exceeding the normal value range, while serum calcium levels remained within the normal range [Citation7,Citation26]. We found that iPTH decreased significantly on day 1 after ablation, compared to pre-ablation levels, but began to increase on day 3 after RFA in the cured patients, peaking 1 month after the ablation, although it remained below its baseline value and then naturally decreased. Serum calcium levels were normal throughout the follow-up period. The incidence rates of ePTH at 1, 3, 6, and 12 months after ablation were 48%, 30%, 20%, and 16%, respectively, consistent with values previously reported for surgical procedures [Citation22,Citation27–29].
It remains unclear whether ePTH is a transient phenomenon after RFA in the treatment of pHPT or if it predisposes patients to recurrent disease. Herein, among 24 patients who developed ePTH a month after ablation, 18 returned to normal PTH levels at 12 months. Serum calcium and phosphorus levels were normal at each follow-up point after the ablation. Most patients in the two groups had no symptoms of ostealgia or fatigue, and no new case of nephrolithiasis or fractures occurred. Recurrent pHPT is an unlikely diagnosis, given the clinical, biochemical, and imaging findings. These findings are consistent with those of previous studies on surgical cohorts, showing that ePTH after RFA is a dynamic, reversible, and temporary clinical process and does not increase the risk of recurrent pHPT [Citation10,Citation30].
Previous studies have reported several factors associated with ePTH, including higher preoperative iPTH levels, lower preoperative 25(OH)D3 levels, heavier adenoma, advanced age, and lower creatinine clearance rate [Citation7,Citation23,Citation31–33]. Our study found that baseline iPTH and calcium levels were independent risk factors associated with ePTH after RFA. The risk of ePTH after ablation was 1.067 per pmol/l change in baseline iPTH level and 3.923 per mg/dl change in baseline serum calcium level, which may be related to the high bone turnover rate in these patients. Previous studies have reported that bone turnover is dominated by anabolism at low iPTH levels. In contrast, bone anabolism and catabolism are vigorous at high iPTH levels. With catabolism being dominant, whereby calcium is freed from the bone and released into the blood to increase serum calcium levels [Citation34,Citation35]. After the operation, the serum iPTH level decreased rapidly. The rate of bone catabolism rapidly decreased; however, that of anabolism remained stable, resulting in a short period of decreased serum calcium levels. Subsequently, as calcium levels decreased, the iPTH secreted by the residue parathyroid gland increased, causing a rebound in iPTH levels [Citation30,Citation36].
Voice change is a serious complication of RFA in patients with both benign thyroid nodules and recurrent thyroid cancer. It is usually caused by thermal injury to the RLN. Most patients recover within 1–3 months after RFA [Citation37]. Herein, 3 (5.9%) patients had transient low voice. This proportion is comparable to that reported for parathyroidectomy (6.1%), but slightly higher than that reported for thermal ablation of thyroid nodules (1.5%) [Citation38,Citation39]. This finding may be attributed to the anatomical location of the parathyroid gland, which is near the RLN. The RLN is very sensitive to thermal stimulation. The ‘hydrodissection technique’ make the nodules island, which can effectively reduce the thermal stimulation of RLN. In addition, precise puncture, relatively low power and ablation monitored by US can avoid the damage to the RLN [Citation15].
This study had some limitations. First, this was a retrospective study with relatively small sample. In this study, no significant positive findings were found for ePTH risk factors reported in previous studies such as 25(OH)D3, adenoma volume, age, and creatinine clearance; these relationships should be assessed in larger studies. Second, the influence mechanism of baseline iPTH and calcium levels on ePTH found in this study is still only theoretical speculation. An experimental research basis and prospective studies are needed. Third, the present study was based on data from two centers; multicenter studies are required to validate these findings.
In conclusion, RFA is safe and effective in treating pHPT and may be considered an alternative treatment option when parathyroidectomy is contraindicated or declined by patients. Moreover, ePTH at 1 month after RFA was associated with baseline iPTH and calcium levels and did not increase the risk of pHPT recurrence.
Author contributions
Study design and conduct: LT. R. and CZ. P. Data acquisition: CZ. P., HH. C., ZX. Z., QH. H. and HS. P. Data analysis: HH. C. Data interpretation: all authors. Manuscript drafting: CZ. P., HH. C., Z. Z. and AL. C. Revising manuscript: LT. R., CZ. P., HH. C., Z. Z., ZX. Z. and QH. H. Approving the final version of the manuscript: all authors.
Disclosure summary
The authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Additional information
Funding
References
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet (London, England. 2018;391(10116):168–178.
- Wermers RA, Khosla S, Atkinson EJ, et al. Incidence of primary hyperparathyroidism in rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21(1):171–177.
- Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metabolism. 2013;98(3):1122–1129.
- Fraser WD. Hyperparathyroidism. Lancet (London, England). 2009;374(9684):145–158.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569.
- Wilhelm SM, Wang TS, Ruan DT, et al. The american association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968.
- Wang TS, Ostrower ST, Heller KS. Persistently elevated parathyroid hormone levels after parathyroid surgery. Surgery. 2005;138(6):1130–1135. discussion 5-6.
- Lang BH, Wong IY, Wong KP, et al. Eucalcemic parathyroid hormone elevation after parathyroidectomy for primary sporadic hyperparathyroidism: risk factors, trend, and outcome. Ann Surg Oncol. 2012;19(2):584–590.
- Biskobing DM. Significance of elevated parathyroid hormone after parathyroidectomy. Endocrine practice: official journal of the american college of endocrinology and the american association of. Endocr Pract. 2010;16(1):112–117.
- Solorzano CC, Mendez W, Lew JI, et al. Long-term outcome of patients with elevated parathyroid hormone levels after successful parathyroidectomy for sporadic primary hyperparathyroidism. Arch Surg. 2008;143(7):659–663; discussion 63.
- Cano-Valderrama O, Ochagavía S, Sanabria C, et al. How should we define cure after parathyroidectomy for normocalcemic primary hyperparathyroidism? A retrospective cohort study. Updates Surg. 2021;73(6):2293–2299.
- Denizot A, Pucini M, Chagnaud C, et al. Normocalcemia with elevated parathyroid hormone levels after surgical treatment of primary hyperparathyroidism. Am J Surg. 2001;182(1):15–19.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. International journal of hyperthermia: the official journal of european society for hyperthermic oncology. Int J Hyperthermia. 2019;36(1):835–840.
- Wei Y, Peng L, Li Y, et al. Clinical study on safety and efficacy of microwave ablation for primary hyperparathyroidism. Korean J Radiol. 2020;21(5):572–581.
- Wei Y, Peng CZ, Wang SR, et al. Microwave ablation versus radiofrequency ablation for primary hyperparathyroidism: a multicenter retrospective study. International journal of hyperthermia: the official journal of european society for hyperthermic oncology. Int J Hyperthermia. 2021;38(1):1023–1030.
- Hussain I, Ahmad S, Aljammal J. Radiofrequency ablation of parathyroid adenoma: a novel treatment option for primary hyperparathyroidism. AACE Clin Case Rep. 2021;7(3):195–199.
- Sormaz IC, Poyanlı A, Açar S, et al. The results of Ultrasonography-Guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602.
- Ha EJ, Baek JH, Baek SM. Minimally invasive treatment for benign parathyroid lesions: Treatment efficacy and safety based on nodule characteristics. Korean J Radiol. 2020;21(12):1383–1392.
- Khandelwal AH, Batra S, Jajodia S, et al. Radiofrequency ablation of parathyroid adenomas: Safety and efficacy in a study of 10 patients. Indian J Endocrinol Metab. 2020;24(6):543–550.
- Korkusuz H, Wolf T, Grünwald F. Feasibility of bipolar radiofrequency ablation in patients with parathyroid adenoma: a first evaluation. International journal of hyperthermia: the official journal of european society for hyperthermic oncology. Int J Hyperthermia. 2018;34(5):639–643.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Carsello CB, Yen TW, Wang TS. Persistent elevation in serum parathyroid hormone levels in normocalcemic patients after parathyroidectomy: does it matter? Surgery. 2012;152(4):575–581; discussion 81-3.
- Nordenström E, Westerdahl J, Bergenfelz A. Long-term follow-up of patients with elevated PTH levels following successful exploration for primary hyperparathyroidism. World J Surg. 2004;28(6):570–575.
- Guerin C, Paladino NC, Lowery A, et al. Persistent and recurrent hyperparathyroidism. Updates Surg. 2017;69(2):161–169.
- Debruyne F, Delaere P, Ostyn F, et al. Daily follow-up of serum parathyroid hormone and calcium after surgery for primary hyperparathyroidism. J Otolaryngol. 1999;28(6):305–308.
- Duke WS, Kim AS, Waller JL, et al. Persistently elevated parathyroid hormone after successful parathyroid surgery. Laryngoscope. 2017;127(7):1720–1723.
- Bergenfelz A, Valdemarsson S, Tibblin S. Persistent elevated serum levels of intact parathyroid hormone after operation for sporadic parathyroid adenoma: evidence of detrimental effects of severe parathyroid disease. Surgery. 1996;119(6):624–633.
- Yamashita H, Noguchi S, Moriyama T, et al. Reelevation of parathyroid hormone level after parathyroidectomy in patients with primary hyperparathyroidism: importance of decreased renal parathyroid hormone sensitivity. Surgery. 2005;137(4):419–425.
- Oltmann SC, Maalouf NM, Holt S. Significance of elevated parathyroid hormone after parathyroidectomy for primary hyperparathyroidism. Endocrine practice: official journal of the american college of endocrinology and the american association of. Endocr Pract. 2011; 17 Suppl 1:57–62.
- Tisell LE, Jansson S, Nilsson B, et al. Transient rise in intact parathyroid hormone concentration after surgery for primary hyperparathyroidism. Br J Surg. 2005;83(5):665–669.
- Westerdahl J, Valdemarsson S, Lindblom P, et al. Postoperative elevated serum levels of intact parathyroid hormone after surgery for parathyroid adenoma: sign of bone remineralization and decreased calcium absorption. World J Surg. 2000;24(11):1323–1329.
- Pathak PR, Holden SE, Schaefer SC, et al. Elevated parathyroid hormone after parathyroidectomy delays symptom improvement. J Surg Res. 2014;190(1):119–125.
- Mizrachi A, Gilat H, Bachar G, et al. Elevated parathyroid hormone levels after parathyroidectomy for primary hyperparathyroidism. Head Neck. 2009;31(11):1456–1460.
- Guo CY, Holland PA, Jackson BF, et al. Immediate changes in biochemical markers of bone turnover and circulating interleukin-6 after parathyroidectomy for primary hyperparathyroidism. Eur J Endocrinol. 2000; 142:451–459.
- Zeng Z, Peng CZ, Liu JB, et al. Efficacy of ultrasound-guided radiofrequency ablation of parathyroid hyperplasia: Single session vs. Two-Session for effect on hypocalcemia. Sci Rep. 2020;10(1):6206.
- Mandal AK, Udelsman R. Secondary hyperparathyroidism is an expected consequence of parathyroidectomy for primary hyperparathyroidism: a prospective study. Surgery. 1998;124(6):1021–1026.
- Baek JH, Lee JH, Sung JY, Korean Society of Thyroid Radiology, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Joliat GR, Guarnero V, Demartines N, et al. Recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Incidence and postoperative evolution assessment. Medicine (Baltimore)). 2017;96(17):e6674.
- Kim JH, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.