Abstract
Objectives
Recurrent hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI)-positive primary tumor is at high risk of re-recurrence while treated with radiofrequency ablation (RFA). We aimed to investigate whether neoadjuvant conventional transarterial chemoembolization (cTACE) was effective in reducing re-recurrence after RFA for recurrent HCC patients with MVI-positive primary tumors.
Methods
In this retrospective multicenter study, 468 patients with solitary small recurrent HCC (≤3.0cm) underwent RFA alone (n = 322) or with neoadjuvant cTACE (n = 146) between June 2007 and December 2017 were included. Overall survival (OS) and recurrence-free survival (RFS) were compared.
Results
The 1-, 5-year OS rates were 74.8%, 42.5% for RFA with neoadjuvant cTACE group, and 53.5%, 28.7% for RFA group (P < 0.001). The corresponding RFS rates were 51.7%, 24.4% for RFA with neoadjuvant cTACE group, and 36.1%, 9.3% for RFA group (P < 0.001). In subgroup analyses, the OS and RFS for neoadjuvant cTACE group were longer than those for RFA group no matter tumor size > 2cm (HR = 0.52, 95% CI: 0.36–0.77; HR = 0.49, 95% CI: 0.36–0.67) or not (HR = 0.53, 95% CI: 0.32–0.88; HR = 0.65, 95% CI: 0.42–0.98), or the time interval of recurrence from initial treatment ≤ 1 year (HR = 0.53, 95% CI: 0.36–0.77; HR = 0.70, 95% CI: 0.52–0.94) or not (HR = 0.56, 95% CI: 0.34–0.95; HR = 0.39, 95% CI: 0.25–0.62). Multivariable analyses showed that RFA alone (HR = 1.329, P = 0.031; HR = 1.764, P = 0.004) and interval of recurrence from initial treatment > 1 year(HR = 0.642, P = 0.001; HR = 0.298, P = 0.037) were independent prognostic factors of OS and RFS.
Conclusions
Neoadjuvant cTACE could effectively reduce re-recurrence after RFA, and improve the long-term survivals for patients with solitary small recurrent HCC whose primary tumor was MVI-positive.
For recurrent hepatocellular carcinoma (HCC) patients whose primary tumor was positive for microvascular invasion, neoadjuvant conventional transarterial chemoembolization (cTACE) with radiofrequency ablation (RFA) achieved better efficacy.
Multivariable analyses showed that the interval of recurrence from initial treatment > 1 year and RFA alone were independent prognostic factors of overall survival and recurrence-free survival, respectively.
Key points
Introduction
Repeated hepatectomy is regarded to be the first treatment choice for recurrent hepatocellular carcinoma (HCC) because of the confirmed local efficacy [Citation1–3]. Unfortunately, limited by the postoperative adhesion, change of intrahepatic structures and poor liver function reserve due to initial treatment, repeated hepatectomy can be performed in a small proportion of patients with recurrent HCC (10.4%–31%) [Citation1,Citation2]. Radiofrequency ablation (RFA) is as effective as, but less invasive, than repeated hepatectomy in eradicating small recurrent HCC [Citation3–5]. Therefore, RFA is particularly suitable for recurrent HCC treatment considering the small tumor size of recurrent tumors and its least amount of liver function deterioration resulted for patients. However, the re-recurrence rate after RFA can be more than 50%, and the 5-year recurrence-free survival (RFS) is only 20%–30% for some cases [Citation3–6]. Moreover, the post-RFA relapse rate in HCC is higher than that after resection, possibly associated with RFA-induced enhancement of tumor growth, migration and stemness [Citation7–9]. In addition, microvascular invasion (MVI) is a widely acknowledged factor contributing to recurrence after curative treatments [Citation10]. MVI of the primary HCC has been reported to be associated with poorer differentiation and more aggressive behavior of the recurrent HCC [Citation11,Citation12]. Thus, for recurrent HCC patients with MVI-positive primary tumors who underwent RFA, the above dual negative effects may make these patients suffer from a high risk of re-recurrence. In light of this, effective adjuvant/neoadjuvant therapies are urgently needed. Currently, many studies have reported the limited efficacy of adjuvant therapy (i.e., conventional transarterial chemoembolization (cTACE), targeted therapy, systematic chemotherapy and immunotherapy) in reducing HCC recurrence after curative treatment [Citation13–19]. There still lacks an international consensus about the strategy of adjuvant therapy for HCC [Citation20,Citation21]. Considering the limited efficacy of adjuvant cTACE in reducing recurrence after curative treatment in HCC and the potential of neoadjuvant therapy in cancer management, it is worth investigating the role of neoadjuvant cTACE in HCC. For recurrent HCC patients with a high risk of re-recurrence (primary tumor was MVI-positive), we speculated that these patients might probably benefit from neoadjuvant cTACE before RFA.
In order to resolve this issue, we performed this retrospective multicenter study to compare the efficacy and safety of RFA with neoadjuvant cTACE and RFA alone for small recurrent HCC ≤ 3.0cm with MVI-positive primary tumor in a large population (n = 468). We aimed to investigate whether neoadjuvant cTACE was effective in reducing re-recurrence after RFA for recurrent HCC patients with MVI-positive primary tumors.
Materials and methods
This is a retrospective multicenter study at three tertiary medical centers. The study was centrally approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (approval number: [2021]378), which was in accordance with the Declaration of Helsinki (1964). The written informed consent was waived because of the retrospective design of this study.
Patients and design
From June 2007 to December 2017, 6277 consecutive patients underwent curative hepatectomy (R0 resection) for primary HCC, and 3904 of them developed recurrence after initial hepatectomy (). Recurrence is diagnosed by either histological confirmation or the non-invasive diagnostic criteria used by the American Association for the Study of Liver Diseases [Citation22]. The patients were included in the analysis according to the following criteria: (1) first intrahepatic recurrence after initial hepatectomy; (2) primary HCC at initial resection was MVI-positive; (3) a solitary recurrent HCC ≤ 3.0cm; (4) absence of macrovascular invasion (radiologic evidence of invasion into a major portal/hepatic vein branches) or extrahepatic metastasis for recurrent tumor; (5) received RFA with neoadjuvant cTACE or RFA alone to treat recurrence; (6) Child-Pugh class A; (7) an Eastern Cooperative Oncology Group performance status score of 0. Patients were excluded if met the following criteria: (1) history of a secondary malignancy; (2) incomplete clinicopathological data including MVI status at primary resection. According to these criteria, 3436 patients were excluded and 468 patients were finally included in the analysis (). Among them, 146 patients received the treatment of RFA with neoadjuvant cTACE, and 322 patients received RFA alone.
Figure 1. Flow chart of the patient selection. HCC: hepatocellular carcinoma; HR: hepatic resection; ECOG: Eastern Cooperative Oncology Group; MVI: microvascular invasion; RFA: radiofrequency ablation; TACE: transarterial chemoembolization.
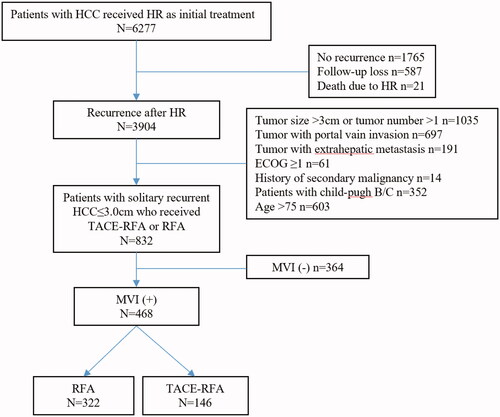
For patients with a high risk of re-recurrence, for example, the recurrent HCC patients whose primary tumor was MVI-positive who underwent RFA in our study, the necessity of cTACE as a neoadjuvant treatment to assist RFA is still controversial and without a universally accepted recommendation. Our multidisciplinary treatment team including interventional radiologists, surgeons, hepatologists and oncologists would fully discuss and decide whether the patients were eligible for RFA with neoadjuvant cTACE or RFA alone in the clinical practice. The criteria for selecting RFA over the combined approach were mainly as followed: tumor size > 2.0cm and the albumin-bilirubin (ALBI) score > −2.60. ALBI was estimated using the following equation: (log10 bilirubin μmol/L × 0.66) + (albumin g/L × −0.085) [Citation23]. But the final decisions were generally made by the patients themselves by informing them of the advantages and disadvantages of treatment choices including treatment efficacy, complications and costs. MVI was defined as positive when the tumor cell clusters within the avascular space of the surrounding hepatic tissue lined by endothelium were visible only on microscopy [Citation10]. MVI status in the resected specimen of primary HCC was evaluated by two senior pathologists in HCC pathology (8, 20 years of experience). Detailed sampling procedure and diagnosis of MVI are reported in the Supplemental Materials.
Treatment scheme
In the group of RFA with neoadjuvant cTACE, neoadjuvant TACE was performed within 2 weeks before RFA (median, 7 days; range, 3–14 days). Contrast-enhanced computed tomography (CECT) was performed within 2 weeks before cTACE to assess the tumor condition and liver condition. Before RFA, contrast-enhanced ultrasound (CEUS) was conducted to evaluate the liver tumor burden after neoadjuvant cTACE. In the group of RFA alone, RFA was performed within 2 weeks after full evaluation of the tumor condition by both CECT and CEUS. In both groups, RFA was performed with a curative intention.
TACE
cTACE was performed by two experienced radiologists with over 10 years of experience in interventional therapy at each center [Citation24–26]. At the beginning of cTACE, visceral angiography via a superior mesenteric artery or common hepatic artery was conducted to evaluate arterial blood supply of the liver and to confirm patency of the portal vein by combining the pre-operational CECT images. Then, a microcatheter was inserted into the segmental or subsegmental tumor-feeding arteries. cTACE was performed using an emulsion of mixtures of lipiodol (3–5mL) and chemotherapeutic agents according to the practice of each center such as epirubicin, cisplatin or oxaliplatin. Subsequently, embolization was finally performed with absorbable gelatin sponge particles (1–2 mm in diameter) until the blood flow was static for more than 10 successive heartbeats. After embolization, angiography was performed to confirm the extent of vascular occlusion and to assess blood flow in other arterial vessels.
RFA
RFA was performed percutaneously by two ablation experts with over 10 years of experience under real-time ultrasound guidance according to previous literature at each center [Citation24,Citation27]. Treatment was performed under conscious analgesic sedation and local anesthesia. A commercially available Cool-tipTM RFA system (Valleylab, Boulder, CO, USA) with 17-gauge internally cooled electrodes of 2–3 cm, active tip length was used for ablation. The electrode was inserted into the lesion under the guidance of ultrasound with an aim to generate an ablative zone covering an area larger than 1 cm around HCC. The number of electrodes used in the ablation session was determined by the tumor size and location. Generally, Generally, one electrode was used for tumors measuring ≤2.0 cm in diameter, and two electrodes for tumors measuring 2.1–3.0 cm in diameter with an interelectrode distance of 1.0–2.0 cm. At the end of the procedure, the needle tract was ablated with the electrode being retracted by 1cm increments to prevent bleeding and tumor seeding. The technical success of ablation was evaluated by immediate CEUS after RFA. If residual unabated tumor was detected, an additional RFA was performed on the same day.
Follow-up
For all the patients, CECT and CEUS were performed 4 weeks after RFA to assess the technique efficacy. Thereafter, the patients were followed up on the schedule: every 3 months for the first two years and every 6 months later on [Citation28]. At each follow-up visit, CEUS and blood tests including liver function tests and α-fetoprotein were performed. CECT or magnetic resonance imaging (MRI) was performed every half a year. Chest radiography, chest CT, and bone scintigraphy were performed when clinically suspicious of extrahepatic metastasis. When local tumor progression [Citation29], intrahepatic distant recurrence or extrahepatic recurrence was detected, patients were recommended for secondary appropriate treatments according to the characteristics of the recurrent tumors, and the patients’ general condition and liver function. The adverse events were evaluated by the Society of Interventional Radiology classification [Citation30,Citation31].
The major complications were defined as clinical events requiring major therapeutic interventions with prolonged hospitalization, unplanned increase in the level of care, permanent adverse sequelae and death [Citation31]. Minor complications were defined as clinical events requiring no therapy or overnight admission for observation only. Overall survival (OS) was defined as the time interval between the recurrent HCC was detected and the time of death or the last follow-up. RFS was defined as the time interval between the initial diagnosis of recurrent HCC and the date of recurrence or death. The follow-up was censored on July 31, 2019.
Statistical analysis
A normal distribution test was performed for continuous variables. Continuous variables following normal distribution were presented as means ± SD and others as median and quartile. Categorical variables were presented as numbers and percentages. Differences between the groups of RFA with neoadjuvant cTACE and RFA alone were compared with the t-test or Mann-Whitney test for continuous variables and with χ2 test for categorical variables. Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. The potential survival predictors were analyzed by univariable and multivariable Cox proportional hazard regression models. Independent prognostic factors were identified through stepwise selection in a Cox regression model. Added variables that were significantly related to survival on the univariable analysis (P < 0.05) were subsequently included in the multivariable Cox model. The above-included survival predictors were predefined according to both the medical relevance and previously published data [Citation24,Citation28]. In order to adjust the influence of the variables (ALBI, tumor size, tumor number) that might affect the selection between these two treatments, we first compared these variables in , performed subgroup analysis stratified by these variables, and included them in the univariable and multivariable Cox regression analyses when evaluating the prognostic value of treatment modality. Statistical significance was considered as a two-sided P value of less than 0.05. The above statistical analysis was performed with SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Table 1. Baseline characteristics of recurrent HCC patients with MVI-positive primary tumor who had undergone neoadjuvant TACE with RFA or RFA alone.
Results
Patient characteristics and technical success
The baseline characteristics of recurrent HCC patients in the group of RFA with neoadjuvant cTACE (n = 146) and the group of RFA alone (n = 322) were described in . There was no significant difference in all the listed variables between these two groups (all P > 0.05). The mean age and gender distribution were both similar between the groups of RFA with neoadjuvant cTACE (54.8 ± 8.9 years, 89.7% being male) and RFA alone (54.9 ± 8.5, 89.1% being male). For both treatment groups, all the patients achieved technical success after a single treatment session of RFA.
Comparison of the Survival Outcomes between Groups of RFA with Neoadjuvant cTACE and RFA Alone
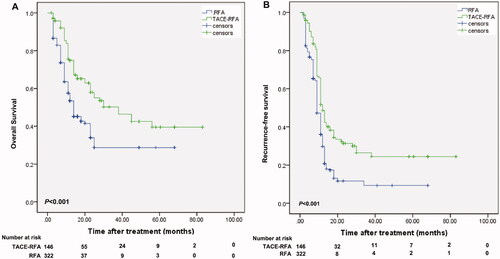
The median follow-up duration was 36.2 (range, 14–87) months in the group of RFA with neoadjuvant cTACE, and 36.5 (range, 13–87) months in the group of RFA alone. At the time of censoring, 59 patients in the group of RFA with neoadjuvant cTACE and 157 patients in the group of RFA alone died; and 63 patients in the group of RFA with neoadjuvant cTACE and 190 patients in the group of RFA alone developed recurrence. The 1-, 3-, and 5-year OS rates were 74.8%, 50.3%, and 42.5% for the group of RFA with neoadjuvant cTACE, and 53.5%, 28.7%, and 28.7% for the group of RFA alone (; P < 0.001). Correspondingly, the 1-, 3-, and 5-year RFS rates were 51.7%, 26.5%, and 24.4% for the group of RFA with neoadjuvant cTACE, and 36.1%, 9.3% and 9.3% for the group of RFA alone (; P < 0.001).
Figure 2. Kaplan-Meier curves of the survival outcomes of patients with solitary small recurrent HCC with MVI-positive primary tumor who were treated with neoadjuvant TACE and RFA or RFA alone. Kaplan-Meier curves of overall survival (A) and recurrence-free survival (B) for patients who underwent neoadjuvant TACE and RFA or RFA alone. HCC: hepatocellular carcinoma; MVI: microvascular invasion; TACE: transarterial chemoembolization; RFA: radiofrequency ablation.
Subgroup analysis of survival outcomes
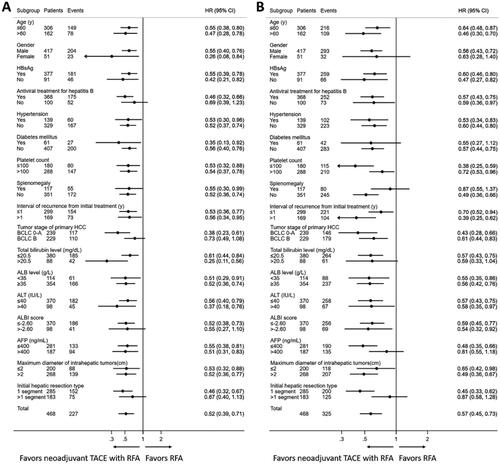
In order to further investigate the efficacy of neoadjuvant cTACE with RFA versus RFA alone in different patient populations, we performed multiple subgroup analyses stratified by the clinical variables listed in and . As shown in , neoadjuvant cTACE with RFA consistently achieved longer OS than RFA alone in nearly all the subgroups except for the subgroup of no antiviral treatment for hepatitis B (Hazard ratio (HR) = 0.69, 95% CI: 0.39–1.23), the subgroup of tumor stage of primary HCC at BCLC stage B (HR = 0.73, 95% CI: 0.49–1.08), the subgroup of ALBI score > −2.60 (HR = 0.55, 95% CI: 0.27–1.10) and the subgroup of initial hepatic resection type >1 segment (HR = 0.67, 95% CI: 0.40–1.13). For the subgroup analyses about RFS, neoadjuvant cTACE with RFA also yielded significantly longer RFS than RFA alone in most of the patient subgroups except in those of female gender (HR = 0.63, 95% CI: 0.28–1.40), presence of diabetes mellitus (HR = 0.55, 95% CI: 0.27–1.12), presence of splenomegaly (HR = 0.87, 95% CI: 0.55–1.37), AFP > 400 ng/mL (HR = 0.81, 95% CI: 0.55–1.18) and initial hepatic resection type > 1 segment (HR = 0.87, 95% CI: 0.58–1.28).
Figure 3. The hazard ratio of overall survival and recurrence-free survival for patients with solitary small recurrent HCC with MVI-positive primary tumor who were treated with neoadjuvant TACE and RFA or RFA alone in different subgroups stratified by clinical parameters. (A) for overall survival; (B) for recurrence-free survival. HBsAg: hepatitis B surface antigen; ALT: alanine aminotransferase; ALB: albumin; ALBI: albumin bilirubin; AFP: alpha fetoprotein; BCLC: Barcelona Clinic Liver Cancer; TACE: transarterial chemoembolization; RFA: radiofrequency ablation.
Univariable and multivariable analyses of long-term survivals
Univariable and multivariable analyses showed that the interval of recurrence from initial treatment> 1 year (HR = 0.642, 95% CI: 0.495–0.833, P = 0.001) and treatment allocation (RFA vs. RFA with neoadjuvant cTACE) (HR = 1.329, 95% CI: 1.024–1.633, P = 0.031) were independent prognostic factors of OS (). Similarly, univariable and multivariable analyses presented that the interval of recurrence from initial treatment > 1 year (HR = 0.298, 95% CI: 0.095–0.932, P = 0.037) and treatment allocation (RFA vs. RFA with neoadjuvant cTACE) (HR = 1.764, 95% CI: 1.445–2.083, P = 0.004) were also independent prognostic factors of RFS ().
Table 2. Univariable and multivariable analyses of predictors of overall survival and recurrence-free survival for recurrent HCC patients with MVI-positive primary tumor.
Recurrence and treatment for subsequent recurrent HCC
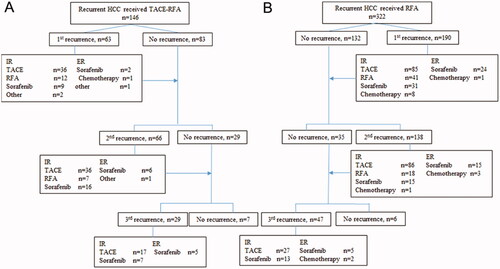
During the follow-up, recurrence occurred in 63 of 146 (43.2%) patients in the group of RFA with neoadjuvant cTACE, and 190 of 322 (59.0%) patients in the group of RFA alone (P = 0.075) (). Among 63 patients who developed recurrence after RFA with neoadjuvant cTACE, 59 patients (93.7%) experienced intrahepatic recurrence and were mostly treated by cTACE subsequently. Among 190 patients who developed recurrence after RFA alone, 165 patients (86.8%) experienced intrahepatic recurrence and were mostly treated by cTACE. The details of subsequent recurrent HCC were summarized in .
Figure 4. Recurrences after the treatments for recurrent HCC patients with MVI-positive primary tumor, and treatment modalities for subsequent recurrences in patients with solitary small recurrent hepatocellular carcinoma. (A) for patients who underwent adjuvant TACE and RFA; (B) for patients who underwent RFA alone. HCC: hepatocellular carcinoma; TACE: transarterial chemoembolization; RFA: radiofrequency ablation; ER: extrahepatic recurrence, IR: intrahepatic recurrence.
Complications
No unexpected complications or treatment-related deaths occurred in our study (). The leading three common complications in both groups were vomiting, pain and fever, all of which were manageable with conservative treatments. All the listed complications were not significantly different between groups of RFA with neoadjuvant cTACE and RFA alone (all P > 0.05). Regarding major complications, the incidence was 12.3% (18/146) in the group of RFA with neoadjuvant cTACE and 9.6% (31/322) in the group of RFA alone, without significant difference (P = 0.428).
Table 3. Complications after treatment.
Discussion
In this large multicenter study with 468 patients with solitary small recurrent HCC whose primary tumor was MVI-positive, we found that RFA with neoadjuvant cTACE yielded significantly higher 5-year OS (42.5% vs. 28.7.4%) and RFS rates (24.4% vs. 9.3%) than RFA alone. These findings indicated that for patients with a high-risk of re-recurrence, such as recurrent HCC whose primary tumor was positive for MVI, neoadjuvant cTACE was effective in reducing the recurrence after RFA even the recurrent HCC was solitary and no larger than 3 cm.
The survival benefit of neoadjuvant cTACE for recurrent HCC patients with MVI-positive primary tumor who underwent RFA might be explained as followed. First, the presence of MVI for the primary HCC may indicate aggressive behavior of the recurrent HCC [Citation11,Citation12]. For these patients, the prevalence of micrometastasis might be high. Although the analyzed tumors in our study were solitary and small, a previous study showed that even for these solitary small tumors, micrometastases (i.e., MVI) spread along [Citation32]. It was reported that a certain proportion of MVI could be found distantly in 1–2cm away from the tumor [Citation10,Citation31]. Neoadjuvant TACE could occlude the hepatic arterial flow and reduce the portal venous flow, which helped reduce the heat-sink effect of the subsequent RFA and enhance the ablation efficacy [Citation24]. Therefore, for our patients whose primary lesions were MVI-positive, neoadjuvant cTACE could assist RFA to enlarge the ablation zone (up to 7 cm), and then improve the possibility of clearance of micrometastasis and therefore help in reducing the risk of recurrence [Citation6,Citation24]. Second, as the first-line treatment for intermediate-stage HCC, neoadjuvant cTACE could also treat pre-existing microscopic tumor lesions or occult intrahepatic multi-nodules that traditional imaging devices fail to detect before treatment [Citation13]. And this efficacy of cTACE was correlated to the unique rich microcirculation of visible/invisible early micro-lesions that resulted in the retention of lipiodol to cause tumor necrosis or suppression. The above two advantages of neoadjuvant cTACE contributed to reducing the tumor recurrence after RFA, and therefore achieving a longer long-term survival for patients with a high risk of re-recurrence.
Our large-scale multicentric study found that neoadjuvant cTACE might be an appropriate therapy to assist RFA to reduce recurrence and prolong survivals for solitary small recurrent HCC patients whose primary tumors were MVI-positive. We thought this finding was of great clinical significance from the following perspectives. First, due to the regular follow-up after initial treatment, recurrent tumors are usually detected at a small size, in which condition, micro-invasive treatment such as RFA is always the choice. But the major obstacle limiting the efficacy of RFA is the high recurrence rate. Therefore, effectively reducing the re-recurrence risk after RFA with neoadjuvant cTACE is important because it retains the unique characteristics of micro-invasiveness for RFA while improving its efficacy. Second, it has been reported that HCC at second or later recurrence is three times as prone to subsequent recurrence as is primary HCC [Citation33]. Curative eradication of the primary tumor and the first recurrent tumor is critical to prolonging patients’ RFS. Therefore, for recurrent HCC patients with MVI-positive primary lesions who are at high risk of re-recurrence, satisfactory tumor control should be achieved for the first recurrence in order to avoid subsequent frequent recurrences. Given this, RFA with neoadjuvant cTACE, a treatment modality that could better control the tumor, is needed. Third, we found that neoadjuvant cTACE could confer survival benefit to HCC patients in selective patient populations, and identified a suitable subpopulation that could benefit from neoadjuvant cTACE in the treatment of RFA.
There are several limitations to our study. First, it is a retrospective study with inevitable selection bias (i.e., there are some factors influencing the selection between these two treatments), but our study is based on multicentric data with relatively large sample size. Second, we did not perform a biopsy of the recurrent lesions for all the patients who received RFA with neoadjuvant cTACE or RFA alone. Third, the number of patients in the TACE/RFA group was limited (n = 146) when compared to the control RFA-only group (n = 322), which could influence the validity of our results to some extent. In conclusion, neoadjuvant cTACE could effectively reduce the tumor recurrence after RFA, and improve the long-term survival outcomes for patients with solitary small recurrent HCC whose primary tumor was MVI-positive.
Ethics approval and consent to participate
The study was centrally approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (approval number: [2021]378), Guangzhou, China, which was in accordance with the Declaration of Helsinki (1964). The written informed consent was waived because of the retrospective design of this study.
Author Contributions
Dr Chen and Dr Peng had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SL Chen, ZW Peng, M Kuang. Acquisition, analysis, or interpretation of data: SL Chen, ZW Peng, XX Wu, JP Li, YJ Zhang, H Pang, MX Lin, ZG Wang, H Xiao, MS Chen, ST Feng, M Kuang. Drafting of the manuscript: SL Chen, ZW Peng. Critical revision of the manuscript for important intellectual content: SL Chen, ZW Peng, M Kuang. Statistical analysis: ZW Peng, SL Chen, XX Wu, B Li. Obtained funding: SL Chen, ZW Peng, M Kuang. Administrative, technical, or material support: JP Li, XX Wu, MS Chen, ST Feng, ZW Peng, M Kuang
Supplemental Material
Download PDF (73.1 KB)Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Chan AC, Chan SC, Chok KS, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013;19:411–419.
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955.
- Lu MD, Yin XY, Xie XY, et al. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg. 2005;92:1393–1398.
- Choi D, Lim HK, Rhim H ,et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319–2329.
- Song KD, Lim HK, Rhim H, et al. Repeated hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma after hepatic resection: a propensity score matching study. Radiology. 2015;275:599–608.
- Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700.
- Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40.
- Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37:3514–3527.
- Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58:1667–1680.
- Roayaie S, Blume IN, Thung SN et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855.
- Hou YF, Li B, Wei YG et al. Second hepatectomy improves survival in patients with microvascular invasive hepatocellular carcinoma meeting the Milan criteria. Medicine. 2015;94:e2070.
- Meniconi RL, Komatsu S, Perdigao F, et al. Recurrent hepatocellular carcinoma: a western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157:454–462
- Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24:2074–2081.
- Ren ZG, Lin ZY, Xia JL, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;10:2791–2794.
- Chen X, Zhang B, Yin X, et al. Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection. J Cancer Res Clin Oncol. 2013;139:773–781.
- Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e1386.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology. 1996;43:1405–1409.
- Chua TC, Saxena A, Chu F, et al. Hepatic resection with or without adjuvant iodine-131-lipiodol for hepatocellular carcinoma: a comparative analysis. Int J Clin Oncol. 2011;16:125–132.
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33;550–558.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432.
- Shao G, Zou Y, Lucatelli P, et al. Chinese expert consensus on technical recommendations for the standard operation of drug-eluting beads for transvascular embolization. Ann Transl Med. 2021;9(8):714.
- Lucatelli P, Burrel M, Guiu B, et al. CIRSE standards of practice on hepatic transarterial chemoembolisation. Cardiovasc Intervent Radiol. 2021;44(12):1851–1867.
- Puijk RS, Ahmed M, Adam A, et al. Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. 2021;301(3):533–540.
- Chen S, Peng Z, Lin M, et al. Combined percutaneous radiofrequency ablation and ethanol injection versus hepatic resection for 2.1-5.0 cm solitary hepatocellular carcinoma: a retrospective comparative multicentre study. Eur Radiol. 2018;28:3651–3660.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Leoni CJ, Potter JE, Rosen MP, et al. Classifying complications of interventional procedures: a survey of practicing radiologists. J Vasc Interv Radiol. 2001;12(1):55–59.
- Shi M, Zhang CQ, Zhang YQ, et al. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381.
- Yamashiki N, Yoshida H, Tateishi R, et al. Recurrent hepatocellular carcinoma has an increased risk of subsequent recurrence after curative treatment. J Gastroenterol Hepatol. 2007;22:2155–2160.
