Abstract
Objective
To evaluate the mid-term symptom improvement of patients with different types of adenomyosis based on magnetic resonance imaging (MRI) classification after ultrasound-guided high intensity focused ultrasound (USgHIFU) treatment.
Materials and methods
A total of 321 patients with adenomyosis who underwent HIFU and completed 18-month follow-up were retrospectively reviewed. Based on the relationship between the adenomyotic lesion and the uterine structural components on T2-weighted imaging (T2WI), adenomyotic lesions were classified as internal, external, full thickness and intramural adenomyosis. Based on the extent of the myometrial involvement, these lesions were further subclassified as asymmetric and symmetric adenomyosis.
Results
All patients completed HIFU ablation in one session. The range of median menstrual pain score in patients with asymmetric internal, symmetric internal, asymmetric external, asymmetric full thickness, symmetric full thickness, and intramural adenomyosis was between 6 and 8 points before HIFU, the median menstrual pain score decreased to 2–4 points 18-month post-HIFU (p < .005). The menstrual pain relief rate was 68.3%, 62.1%, 54.7%, 64.1%, 60%, and 100%, respectively. The median menstrual blood volume score range was between 2 and 4 points in the different groups of patients before HIFU, it decreased to 1–3 points 18-month after HIFU with a relief rate of 68.3%, 51.6%, 51.0%, 55.5%, 57.2%, and 100%, respectively. No serious complication occurred in any of these patients.
Conclusions
Based on our results, USgHIFU is safe and effective in the treatment of patients with different subtypes of adenomyosis with mid-term sustained improvement in symptoms of menstrual pain and menstrual blood volume.
Introduction
Adenomyosis is a common benign disease among women of reproductive age, which is defined by the presence of endometrial glands and stroma within the myometrium [Citation1,Citation2]. The prevalence of adenomyosis varies from 1 to 70% among women of reproductive age, and is frequently reported as 10–30% [Citation3–5].
The common symptoms of adenomyosis include menstrual pain, heavy menstrual blood volume, and a tender, enlarged, and boggy uterus [Citation5–9]. Transvaginal ultrasound (TVUS) is used in the diagnosis of adenomyosis and has a relatively high sensitivity. However, sonographic accuracy appears to be more operators dependent [Citation10–12]. Magnetic resonance imaging (MRI) can clearly show the appearance of adenomyosis with symmetric or asymmetric lesions of internal or external layers of the myometrium. The typical MRI features of adenomyotic lesions are ill-defined low signal intensity areas with hyperintensity foci in the lesions on T2-weighted images [Citation13]. In 2012, Kishi et al. classified adenomyosis as four subtypes based on the relationship between the lesion and the uterine structural components (the endometrium, the junctional zone, the myometrium, and the serosa) revealed by MRI: type I (intrinsic), type II(extrinsicl), type III (intramural) and type IV(indeterminate) [Citation14]. Recently, several other classifications have been proposed, but there remains no consensus classification [Citation10,Citation15].
The treatment for adenomyosis is a major challenge. Hysterectomy remains the only definitive cure for adenomyosis. As the boundary of the adenomyotic lesion is unclear, it is difficult to remove the lesion completely, and the recurrence rate after adenomectomy is very high. which reached 50% in some studies [Citation16].
As a noninvasive treatment, high intensity focused ultrasound (HIFU) ablation has been utilized in the treatment of adenomyosis, and several studies have shown the safety and efficacy [Citation17–21]. A previous study showed that the clinical effective rate was about 80% when the non-perfused volume (NPV, indicative of successful ablation) reached 70% or higher [Citation22]. In the 80% of patients, some reported complete relief of the symptoms, some reported obvious or partial relief. However, about 20% of the patients did not have symptom improvement, it is not clear if the symptom improvement related to NPV ratio of the adenomyotic lesion or subtypes or both. Therefore, the aim of this study was to objectively evaluate the mid-term symptom improvement of patients with different types of adenomyosis based on magnetic resonance imaging (MRI) classification after ultrasound guided high intensity focused ultrasound (USgHIFU) treatment.
Materials and methods
This retrospective study was approved by the ethics committee at our institution (NO. CQMU-20191203), and the requirement for an informed consent to do the research was waived.
Patients
A total of 321 patients with adenomyosis who were treated with HIFU at Chongqing Haifu Hospital between January 2016 and December 2019 were retrospectively reviewed. The medical history of the patients was collected. Following a physical examination, EKG, chest X-ray, routine laboratory tests, ultrasound and MRI examination were performed on all patients. The diagnosis of adenomyosis was suspected by clinical evaluation and confirmed with transvaginal ultrasound and MRI.
Inclusion criteria were as follows: (1) the diagnosis of adenomyosis was made by medical history, transvaginal ultrasound and MRI; (2) all patients presented symptoms and completed HIFU treatment; (3) patients agreed to take pre- and post-HIFU MRI examinations.
Exclusion criteria: (1) patients with suspected or confirmed uterine malignancy; (2) patients with contraindications to MRI; (3) patients with previous surgical scars; (4) patients received hormone therapy after HIFU.
MRI evaluation
All patients underwent pre- and one day post-HIFU MRI evaluation with a 1.5 T MRI (uMR570, United Imaging Company, China). A series of standard T1 weighted images (T1WI), T2 weighted images (T2WI), and contrast enhanced MRI were performed on all patients.
The size and location of the adenomyotic lesions were evaluated on T2WI of MRI. Based on the relationship between the adenomyotic lesions and the endometrium as well as the uterine serosa, the adenomyotic lesions were classified into four types as follows: Internal adenomyosis: the lesion had developed in the thickened junctional zone and that healthy muscular structures were preserved outside the adenomyosis (); external adenomyosis: the lesion was located in the outer myometrium of the uterine wall, the healthy muscular structures were preserved between the adenomyotic lesion and the junctional zone, and the junctional zone was kept intact without aberrancy (); intramural adenomyosis: the lesion was located in the myometrium without any involvement in the junctional zone and the serosa (); full thickness adenomyosis: the lesion occurred in both the uterine inner layer and outer layer ().
Figure 1. Classification of adenomyosis based on MRI: the ill-demarcated low intensity area represents adenomyosis (red arrow heads) that had developed in the thickened junctional zone and that healthy muscular structures were preserved outside the adenomyosis. A. asymetric internal adenomyosis; B. symmetric internal adenomyosis.
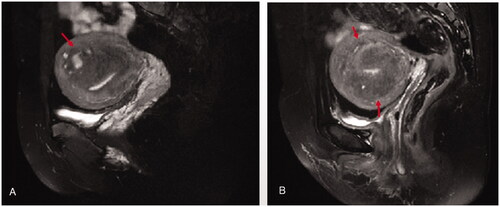
Figure 2. External adenomyosis: the ill-demarcated equal intensity area indicate adenomyosis (red arrow heads) which was located in the outer myometrium of the uterine wall, the healthy muscular structures were preserved in between the adenomyotic lesion and the junctional zone, and the junctional zone was kept intact without aberrancy. A. asymetric external adenomyosis; B. symmetric extenal adenomyosis.
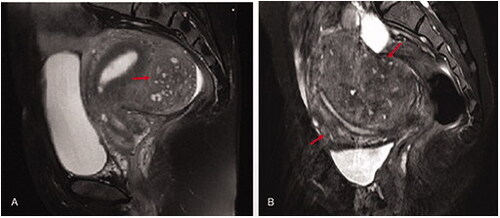
Figure 3. Intramural adenomyosis: the lesion (red arrow heads) was located in the myometrium without any involvement in the junctional zone and the serosa.
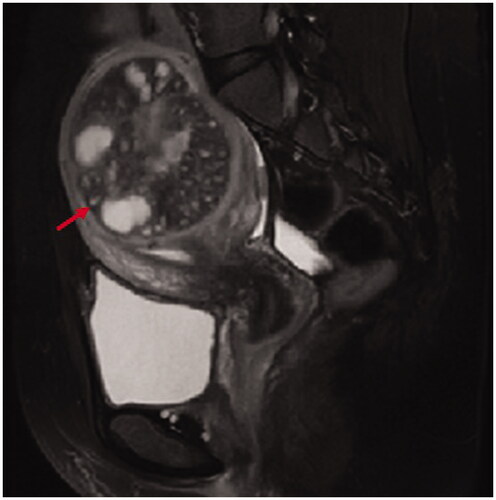
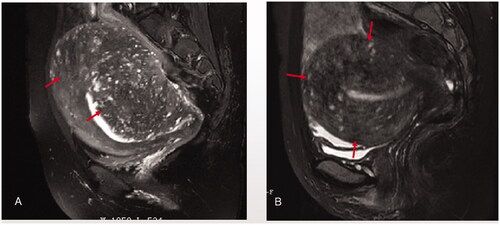
Figure 4. Full thickness adenomyosis: the lesion (red arrow heads) occurred in both the uterine inner layer and outer layer. A. asymetric full thickness adenomyosis; B. symmetric full thickness adenomyosis.
Based on the extent of adenomyotic lesion involving the uterus, every type of adenomyosis was further classified into asymmetric (focally) or symmetric subtype. The radiologists manually contoured the adenomyotic lesion, and all the non-enhancing areas on all relevant slices of T1 contrast enhanced post HIFU, then the volume of the targeted adenomyotic lesion and non-perfused volume were calculated by using a software program which was made by Chongqing Haifu [Citation23]. Energy efficiency factor (EEF) was defined as the ultrasound energy delivered for ablating 1mm3 of the adenomyotic lesion (EEF = ηxPxt/V (J/mm3), where η indicates a focusing factor (=0.7), P indicates sonication power (W), t indicates sonication time, and V indicates NPV (mm3) [Citation24]. NPV ratio was defined as (NPV)/(adenomyotic volume) ×100% ().
Figure 5. HIFU treatment for internal and external adenomyosis (red arrow heads). A&B. Pre-HIFU T2WI and contrast enhanced MRI showed an internal adenomyotic lesion located at the anterior wall of the uterus; C. A contrast enhanced MR image obtained 1 day after HIFU showed the internal adenomyotic lesion was ablated. The NPV ratio was 90%. D&E. Pre-HIFU T2WI and contrast enhanced MRI showed an external adenomyotic lesion located at the posterior wall of the uterus. Uterorectal adhesion was observed; F. C. A contrast enhanced MR image obtained 1 day after HIFU showed the enternal adenomyotic lesion was ablated without damaging the serosa of the uterus. The NPV ratio was 60%.
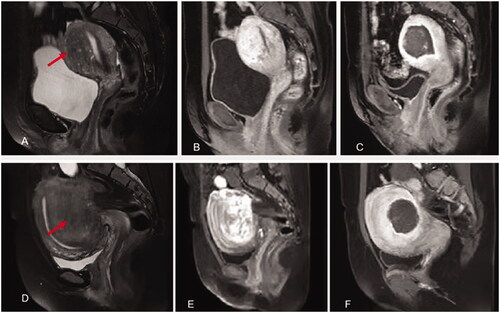
HIFU ablation
HIFU ablation was performed under conscious sedation (fentanyl at 0.8–1 μg/kg administered at 30–40 min intervals; midazolam hydrochloride, at 0.02–0.03 mg/kg, administered at 30–40 min intervals) using the Focused Ultrasound Tumor Therapeutic System (Model-JC200, Chongqing Haifu Medical Technology Co. Ltd., China) equipped with an ultrasound imaging device (MyLab 70, Esaote, Genova, Italy) for real-time guidance during the procedure. The patients were required to report any discomfort during the procedure and the vital signs such as heart rate, blood pressure, respiration and oxygen saturation were monitored.
The patients were placed in a prone position carefully, with the abdominal wall in contact with degassed water. A cold degassed water balloon was placed on the abdominal wall to compress and push the bowel away from the acoustic pathway. The treatment began from the posterior to anterior, from inferior to superior part of the adenomyotic lesion. The focus was kept at least 1.5 cm away from both endometrium and boundary of the adenomyosis. During the procedure, the sonication power was adjusted based on the feedback from the patients and the changed grayscale on the ultrasound imaging. The treatment was terminated when the hyperechoic area covered the entire adenomyosis or the contrast-enhancement ultrasound showed no blood supply in the lesion. The patients were asked to report any adverse effects during the procedure and after HIFU treatment.
Evaluation of clinical symptom
The patients were asked to report their maximum pain score and the average menstruation volume score during menstruation pre- and at 3-, 6-, 12-, and 18-month post-HIFU.
The scoring system for menstrual pain: menstrual pain was scored in accordance with the standards of Visual Analogue Scale (VAS): mild pain (1–3 points), moderate pain (4–6 points), and severe pain (7–10 points).
The scoring system for menstrual blood volume: menstrual blood volume was scored according to patients’ description on a 5-point scale: small (1 point), moderately large (2 points), large (3 points), very large (4 points), and extremely large (5 points).
The relief ratio of scores of menstrual pain and menstrual blood volume before and after HIFU were compared to determine whether the symptoms were alleviated using the following criteria: (1) complete relief: symptoms are completely relieved; (2) obvious relief: the relief ratio is over 50%; (3) partial relief: the relief ratio is less than 50%; (4) ineffective: post-operative scores increase or equal to pre-operative. Clinical relief included obvious relief, partial relief, and complete relief.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL). Normal distribution data was indicated as mean ± SD. The abnormal distribution data were reported as median and interquartile range. One-way ANOVA analysis and Kruskal–Wallis test were applied when comparing variables between the groups, when a statistical difference was found, post-hoc test with Dunnett’s was performed to check within and among subgroups. The Chi-square test was applied for the analysis of the enumerated data. When p value was less than .05, the difference was considered as statistically significant.
Results
Patients and lesions
A total of 321 patients who met the inclusion criteria and exclusion criteria and completed 18 months follow-up were enrolled in this study. Among them, 315 patients had menstrual pain, only 277 patients complained heavy menstrual blood volume before HIFU. Based on the features on MRI, 45 patients were classified as asymmetric internal adenomyosis, 29 were classified as symmetric internal adenomyosis; 75 patients were classified as asymmetric external adenomyosis, no symmetric external adenomyosis was found in the subjects; 93 patients were classified as asymmetric full thickness adenomyosis, 76 were symmetric full thickness adenomyosis; three patients were classified as intramural adenomyosis.
The baseline characteristics of the patients were shown in . The average age in patients with asymmetric external adenomyosis was significantly younger than those with internal adenomyosis (p < .05). The asymmetric external adenomyosis was more often seen in the posterior wall of the uterus (p < .05). The volume of adenomyotic lesion was significant lower in patients with asymmetric adenomyotic lesion than the patients with symmetric adenomyotic lesion (p < .05). No other significant difference in baseline characteristics was observed between the patients with different type of adenomyosis.
Table 1. Comparison of baseline characteristics of patients with different types of adenomyosis.
Peri-HIFU procedure evaluation
As shown in , no significant difference was found between patients with different subtypes of adenomyosis in HIFU treatment settings and procedures (p > .05). No significant difference occurred even when corrected for adenomyotic lesion size. Each group of patients with adenomyosis was treated using similar sonication power, sonication time and treatment time and similar NPV ratio was achieved. The EEF in patients with asymmetric external adenomyosis was significantly higher than that in the other groups (p < .05).
Table 2. Comparison of HIFU treatment results between different types of adenomyosis.
Adverse effects and complications
During the procedure, the common adverse effects included transient leg pain, sciatic or buttock pain, skin discomfort, lower abdominal pain and groin pain. These pains were mild and often disappeared after sonication was terminated. We compared the incidence rate of adverse effects between different groups. The percentage of sciatic or buttock pain was 31.1%, 27.6%, 56%, 41.9% and 40.8% in in patients with asymmetric internal, symmetric internal, asymmetric external, asymmetric full thickness, and symmetric full thickness, respectively (). The incidence of sciatic or buttock pain in patients with asymmetric external adenomyosis was significantly higher than that in the other groups of patients (p < .05). No other significant difference was observed between the subgroups (p > .05) (). After HIFU treatment, mild pain in the treated region and sacrum was observed in some cases; the pain subsided within 3 days. No any major post-HIFU complication was observed in any of these patients.
Table 3. Comparison of adverse effects among patients with different types of adenomyosis.
Improvement of menstrual pain
Among the 321 patients, 315 reported menstrual pain before HIFU treatment. Four patients with asymmetric internal adenomyosis, one patient with asymmetric full thickness adenomyosis, and one patient with symmetric full thickness adenomyosis who did not have menstrual pain before HIFU were not included in statistical analysis. The pre-HIFU menstrual pain score in patients with asymmetric internal adenomyosis was significantly lower than those with other types of adenomyosis. After HIFU treatment, the pain score significantly decreased in every group. The symptom relief sustained over 18-month after HIFU treatment. The patients with internal adenomyosis had the lowest menstrual pain score, while the patients with asymmetric external adenomyosis had the highest menstrual pain score at 18 months after HIFU (, p < .05). The clinical effectiveness rate of HIFU treatment for patients with asymmetric internal adenomyosis, symmetric internal adenomyosis, asymmetric external adenomyosis, asymmetric full thickness adenomyosis, symmetric full thickness adenomyosis, and intramural adenomyosis in alleviating menstrual pain at 18-month follow-up was 68.3%, 62.1%, 54.7%, 64.1%, 60% and 100%, respectively. No significant difference was found between patients with different subtype adenomyosis in clinical effectiveness rate (, p > .05).
Table 4. Comparison of menstrual pain score before and 18 months after HIFU treatment.
Table 5. Evaluation of treatment efficacy in 315 patients with menstrual pain 18 months after HIFU treatment.
Improvement of menstruation blood volume
Among the 321 patients who had completed 18-month follow-up, 277 had varying degrees of heavy menstrual blood volume before HIFU treatment. Four patients with asymmetric internal adenomyosis, 24 patients with asymmetric external adenomyosis, 10 patients with asymmetric full thickness adenomyosis, and six patients with symmetric full thickness adenomyosis who did not have heavy menstrual blood volume before HIFU were not included for statistical analysis. The patients with internal adenomyosis had higher menstrual blood volume score than that of patients with external asymmetric adenomyosis and full thickness asymmetric adenomyosis. The clinical effectiveness rate of HIFU treatment for patients with asymmetric internal adenomyosis, symmetric internal adenomyosis, asymmetric external adenomyosis, asymmetric full thickness adenomyosis, symmetric full thickness adenomyosis, and intramural adenomyosis in improving menstrual blood volume at 18-month follow-up was 68.3%, 51.7%, 50.9%, 55.4%, 57.1% and 100%, respectively. The relief rate of heavy menstrual blood volume in patients with asymmetric external adenomyosis was significantly lower than that of the other groups ( and , p < .005).
Table 6. Comparison of menstrual blood volume scores before and 18-month after HIFU treatment.
Table 7. Evaluation of treatment efficacy in 277 patients with heavy menstrual blood volume after HIFU treatment.
Discussion
Several studies have demonstrated that adenomyosis consists of multiple subtypes with various patterns in MRI [Citation14]. Kishi et al. reported that the internal, external, intramural and indeterminate subtypes of adenomyosis (subtypes I–IV) consisted of 59(38.8%), 51(33.6%), 22(14.5%), and 20(13.1%) patients, respectively [Citation14]. Our study showed that the internal adenomyosis (Kishi type I) was observed in 74 of the 321 (23.1%) patients, the external adenomyosis (Kishi type II) was observed in 75 of the 321 (23.4%) patients, only 3 patients had intramural adenomyosis (Kishi type III), while the full thickness adenomyosis (Kishi type IV) was observed in 169 of the 321 (52.6%) patients. Kishi et al. reported that anterior wall involvement of adenomyosis is more frequently seen in internal adenomyosis. External adenomyosis is frequently seen in posterior wall of the uterus [Citation14]. In our study, we observed that in patients with asymmetric adenomyosis, either internal or external, or full thickness, the adenomyotic lesion was more frequently seen in the posterior wall of the uterus ().
HIFU treatment was successfully performed in all the patients in the current study. Our results showed that the similar NPV ratio was achieved in every subtype using similar treatment power and spending similar treatment time. In order to avoid damage to the endometrium and serosa, we didn’t pursue a high NPV ratio in the current study. In comparison with the previous studies, we found that the short-term symptom relief is not related to NPV ratio, but the mid-term results are related to NPV ratio [Citation22,Citation25]. Therefore, we speculated that achieving a high NPV ratio safely is important in long-term symptom relief.
However, EEF achieved in external asymmetric adenomyosis was significantly higher than that in the other subtypes (). This higher EEF indicated that it needed more acoustic energy to ablate the same volume of adenomyotic lesion in external adenomyosis than that in other subtypes of adenomyosis. We cannot explain this phenomenon clearly. One possible explanation was that most of the external adenomyotic lesions were adjacent to the sacrum and many were accompanied by pelvic endometriosis and had uterorectal adhesion, a higher percentage of patients had obvious sciatic or buttock pain during the procedure (). Therefore, we had to slow down the treatment, and the treatment intensity (the sonication time in every hour, calculate as sonication time/hour) was lower than that of other subtypes. Low treatment intensity decreased the involvement of energy in the adenomyotic lesion. However, we did not find a significant difference between the different types in treatment intensity (p = .091). We further compared the variables between patients with external asymmetric adenomyosis and patients with other types of adenomyosis and found that the volume of adenomyotic lesions in patients with external asymmetric adenomyosis was significantly smaller than that of patients with other types of adenomyosis (). Therefore, we speculated that the greater EEF achieved in external asymmetric adenomyosis was related to the small volume of external asymmetric adenomyotic lesions. This phenomenon may be explained by ‘damage–damage interference effects’ because the expansion of the necrotic area and the ascent of temperature on the focal point will significantly influence the acoustic environment of surrounding focus tissue and contribute to the ultrasonic energy deposition [Citation26]. An in vitro experimental study demonstrated that the EEF mass was less than the EEF slice, while the EEF slice was less than the EEF fascicle, which also could be used to explain this result [Citation27].
In this study, 315 patients had menstrual pain before HIFU treatment. Our results showed that the pre-HIFU menstrual pain score was lower in patients with internal adenomyosis than that of patients with other types of adenomyosis. The menstrual pain score significantly decreased in every group of patients with different subtypes of adenomyosis after HIFU and sustained for 18 months, but the patients with internal adenomyosis had a higher percentage decrease in pain score. This phenomenon may be explained by that the internal adenomyosis is less associated with pelvic endometriosis. The early studies showed that the incidence of adenomyosis combined with endometriosis is 65%, and outer myometrium lesions are more likely to be combined with endometriosis, especially deep endometriosis [Citation9]. The stretch of these deeply infiltrating endometriotic nodules may exacerbate painful symptoms and the menstrual pain are more likely to occur in patients with deep endometriosis [Citation28–Citation29].
Our study also showed that patients with internal adenomyosis had higher pre-HIFU menstrual blood volume score, patients with external adenomyosis had lower pre-HIFU menstrual blood volume score than the other subtypes. After HIFU treatment, the menstrual blood volume scores all decreased in patients with different subtypes of adenomyosis. The clinical relief rate is consistent with earlier studies [Citation30], which suggested that HIFU is effective in relieving heavy menstrual blood volume and menstrual pain. In this study, we didn’t observe a significant difference in the clinical effective rate of menstrual pain between patients with different subtype of adenomyosis. However, a significantly lower clinical relief rate of heavy menstrual blood volume was found in patients with asymmetric external adenomyosis at 18-month after ultrasound-guided HIFU treatment when compared with other groups of patients with adenomyosis, even if with similar immediately NPV ratio ( and ). These phenomena might be explained by the fact that during HIFU procedure, a safe distance should be kept at least 1.5 cm away from focus to the endometrium and serum of the uterus, thus may lead to incomplete ablation of adenomyotic lesion (). Meanwhile patients with external adenomyosis mainly manifest as menstrual pain while the symptom of heavy menstrual blood volume is not obviously before treatment (), even if the score of menstrual blood volume decreased at 18-month after treatment, the relief rate of heavy menstrual blood volume was significantly lower than that in the other groups of patients. Therefore, our results may indicate that except from NPV ratio, the different subtype of adenomyosis maybe another important factor that influencing the clinical efficacy.
The present study also indicated that HIFU can be safely used to treat different subtypes of adenomyosis. In this study, patients often complained of mild lower abdominal local pain owing to uterine contraction after HIFU treatment; sciatic or buttock pain was also a frequent adverse effect when the lesions were located at the posterior wall of the uterus, but such pain was mild. The pain subsided in 1–3 days without any specific treatment. Our results showed that the ratio of sciatic or buttock pain in the group of patients with external adenomyosis was much higher than that of patients with other subtypes of adenomyosis because of the location of the lesions. No any major complication occurred in this study.
This study is limited because it is a retrospective study, all patients were from a single center and some bias may occur because the enrolled patients were those who didn’t receive hormone therapy. This study is also limited because of the relatively small number in some subtypes. In addition, we used the same HIFU treatment protocol for different subtypes of adenomyosis that may have also influenced the results. Future multicenter studies or prospective randomized controlled trials with large subjects are required to address the difference of HIFU for different subtypes of adenomyosis. It is also required to further optimize individual HIFU treatment protocol for patients with different subtypes of adenomyosis.
Conclusions
HIFU is a promising technique for treating patients with adenomyosis. Although further studies are needed for optimization of indications, the present results showed that HIFU can be used to treat all subtypes of adenomyosis safely to alleviate the symptoms of heavy menstrual blood volume and menstrual pain.
Disclosure statement
Zhibiao Wang and Lian Zhang are senior consultants to Chongqing Haifu. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
References
- Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best practice & research. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):511–521.
- Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98(3):572–579.
- Yu O, Schulze-Rath R, Grafton J, et al. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-2015. Am J Obstet Gynecol. 2020;223(1):94.e1–e10.
- Di Donato N, Montanari G, Benfenati A, et al. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;181:289–293.
- Eisenberg V, Arbib N, Schiff E, et al. Sonographic signs of adenomyosis are prevalent in women undergoing surgery for endometriosis and may suggest a higher risk of infertility. Biomed Res Int. 2017;2017:8967803.
- Puente J, Fabris A, Patel J, et al. Adenomyosis in infertile women: prevalence and the role of 3D ultrasound as a marker of severity of the disease. Reprod Biol Endocrinol. 2016;14(1):60.
- Pinzauti S, Lazzeri L, Tosti C, et al. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound in obstetrics & gynecology: the official journal of the international society of. Ultrasound Obstet Gynecol. 2015;46(6):730–736.
- Pervez S, Javed K. Adenomyosis among samples from hysterectomy due to abnormal uterine bleeding. J Ayub Med Coll Abbottabad. 2013;25(1-2):68–70.
- Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32(7):1393–1401.
- Bazot M, Dara ?E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109(3):389–397.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Mer Ed Ith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. Am J Obstet Gynecol. 2009;201(1):107.e1–e6.
- Sudderuddin S, Helbren E, Telesca M, et al. MRI appearances of benign uterine disease. Clin Radiol. 2014;69(11):1095–1104.
- Kishi Y, Suginami H, Kuramori R, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207(2):114.e1–114.e7.
- Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril. 2018;109(3):380–388.
- Younes G, Tulandi T. Conservative surgery for adenomyosis and results: a systematic review. J Minim Invasive Gynecol. 2018;25(2):265–276.
- Du C, Wang Y, Qu D, et al. Magnetic resonance imaging T2WI hyperintense foci number and the prognosis of adenomyosis after high-intensity focused ultrasound treatment. Int J Gynaecol Obstet. 2021;154(2):241–247.
- Li W, Mao J, Liu Y, et al. Clinical effectiveness and potential long-term benefits of high-intensity focused ultrasound therapy for patients with adenomyosis. J Int Med Res. 2020;48(12):300060520976492.
- Jeng C, Ou K, Long C, et al. 500 Cases of high-intensity focused ultrasound (HIFU) ablated uterine fibroids and Adenomyosis. Taiwan J Obstet Gynecol. 2020;59(6):865–871.
- Lee J, Hong G, Lee K, et al. Safety and efficacy of Ultrasound-Guided High-Intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221.
- Keserci B, Duc NM. The role of T1 perfusion-based classification in predicting the outcome of magnetic resonance-guided high-intensity focused ultrasound treatment of adenomyosis. Int J Hyperthermia. 2018;34(3):306–314.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis . Eur J Radiol. 2012;81(11):3624–3630.
- Li F, Wang Z, Du Y, et al. Study on therapeutic dosimetry of HIFU ablation tissue. J Biomed Eng. 2006;23(4):839–843.
- Keserci B, Duc N. Magnetic resonance imaging features influencing high-intensity focused ultrasound ablation of adenomyosis with a nonperfused volume ratio of ≥90% as a measure of clinical treatment success: retrospective multivariate analysis. Int J Hyperthermia. 2018;35(1):626–636.
- Chen L, ter Haar G, Hill CR. Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol. 1997;23(6):921–931.
- Li F, Wang Z, Du Y, et al. Study on therapeutic dosimetry of HIFU ablation tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2006;23:839–843.
- Vercellini P, Trespidi L, De Giorgi O, et al. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996;65(2):299–304.
- Kor E, Mostafavi SRS, Mazhin ZA, et al. Relationship between the severity of endometriosis symptoms with the spread of the disease on ultrasound. BMC Res Notes. 2020;13(1):546.
- Haiyan S, Lin W, Shuhua H, et al. High-intensity focused ultrasound (HIFU) combined with gonadotropin-releasing hormone analogs (GnRHa) and levonorgestrel-releasing intrauterine system (LNG-IUS) for adenomyosis: a case series with long-term follow up. International journal of hyperthermia: the official journal of european society for hyperthermic oncology. Int J Hyperthermia. 2019;36(1):1179–1185.
